Risk Assessment of the Worldwide Expansion and Outbreak of Massicus raddei (Blessig) (Coleoptera: Cerambycidae) Based on Host Plant and Climatic Factors
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
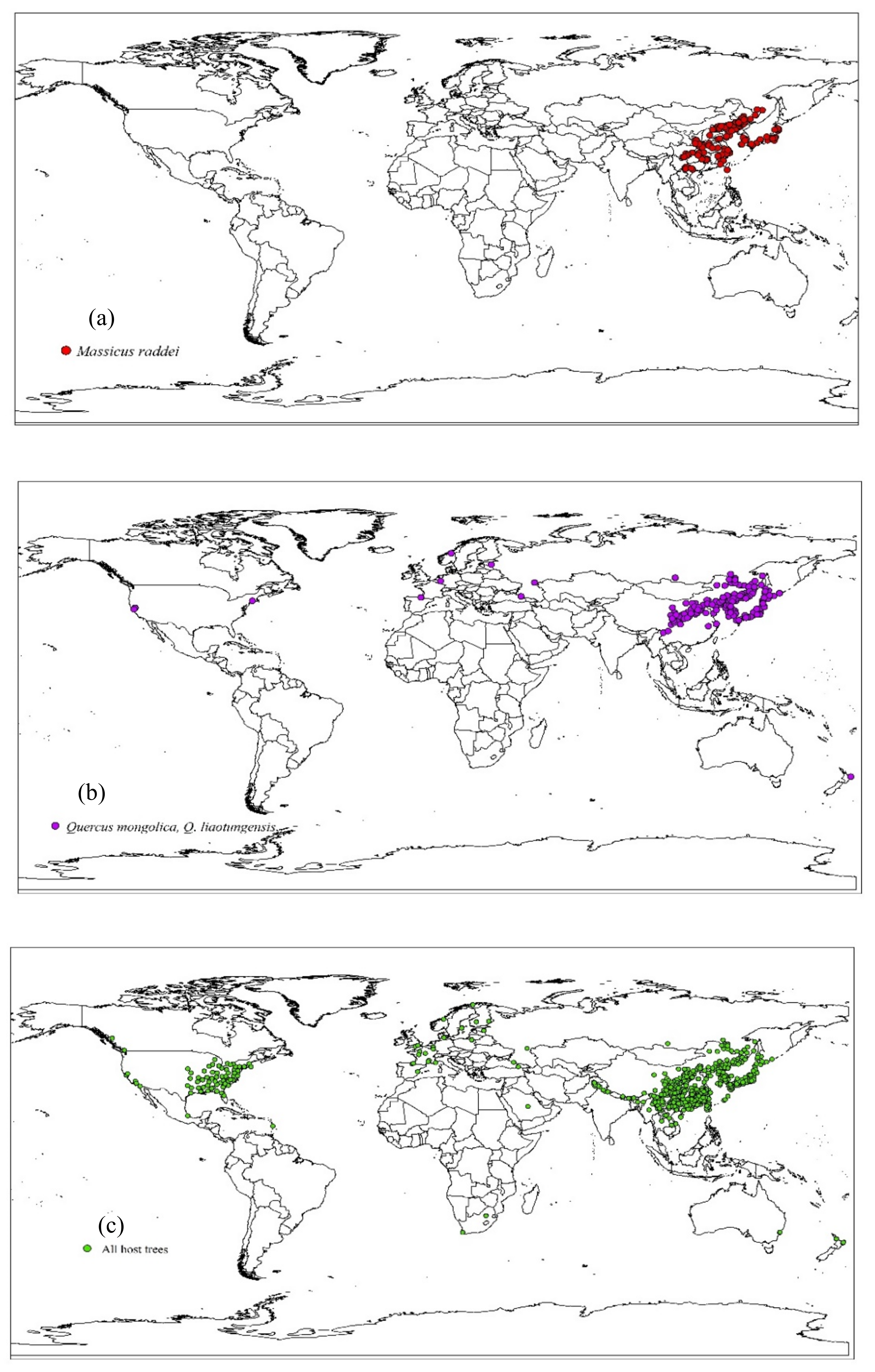
2.1. Actual Distributions of M. raddei and Its Hosts
2.2. Environmental Variables
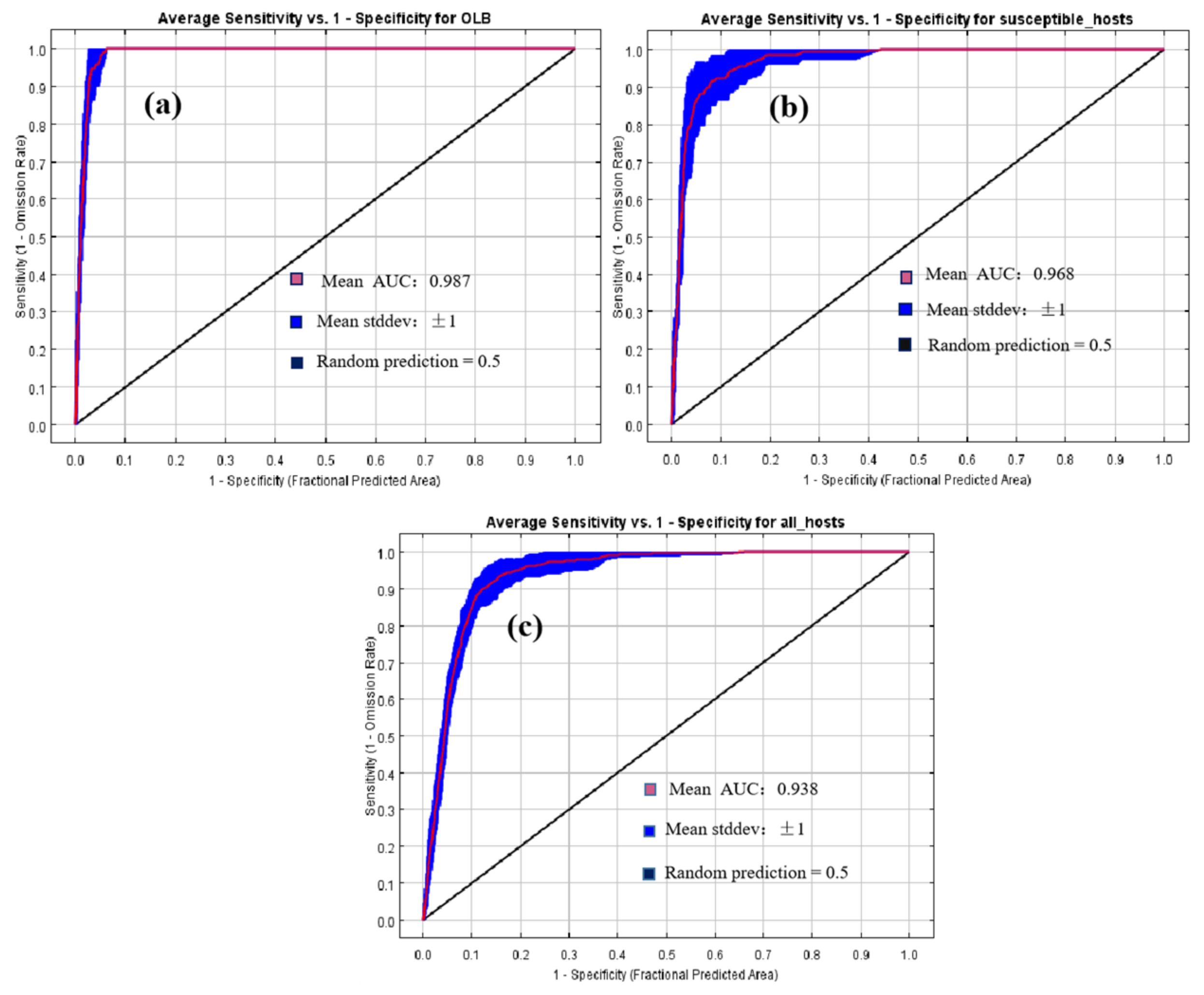
2.3. Model Development and Accuracy Evaluation
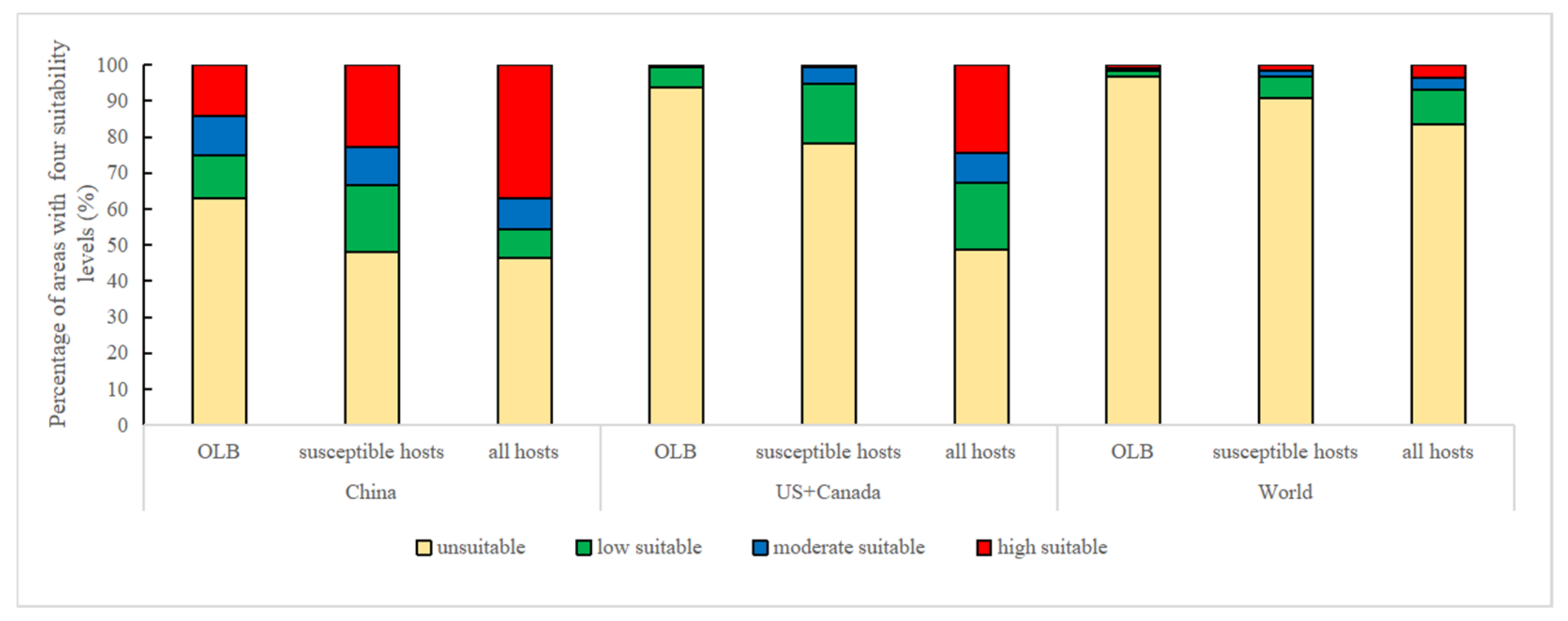
2.4. Classification and Calculation of Suitable Areas
3. Results
3.1. Current Distributions of M. raddei and Its Hosts
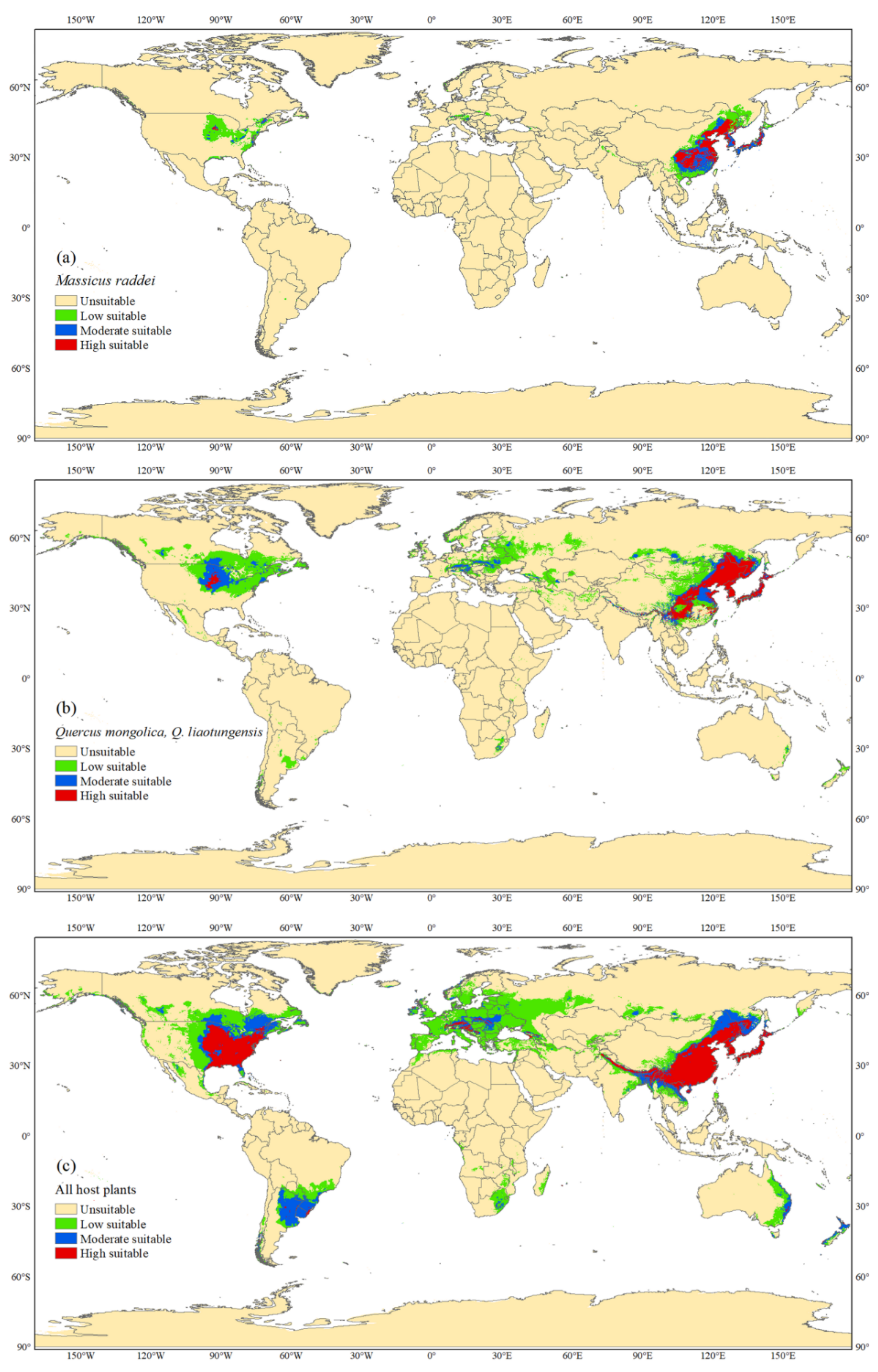
3.2. Global Potential Distribution of M. raddei
3.3. Global Potential Distributions of Hosts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, S.X.; Xie, Y.Z.; Deng, G.F. Economic Insect Fauna of China, Coleoptera: Cerambycidae; Science Press: Beijing, China, 1959; Volume 1, p. 43. [Google Scholar]
- Sun, Y.P. Control Techniques of Massicus raddei (Blessig); Liaoning Science and Technology Publishing House: Shenyang, China, 2001. [Google Scholar]
- Cho, Y.H.; Kim, Y.J.; Han, Y.G.; Cha, J.Y.; Jeong, J.C.; Seo, J.K.; Nam, S.H. A faunistic study of insects on Mt. Juwang National Park. J. Natl. Park Res. 2010, 1, 225–253. [Google Scholar]
- Kim, H.; Oh, H.-W.; Kim, J.-A.; Park, D.-S.; Park, H.-M.; Bae, K.S. Luteimicrobium xylanilyticum sp. nov., isolated from the gut of a long-horned beetle, Massicus raddei. Int. J. Syst. Evol. Microbiol. 2014, 64, 1401–1405. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Jung, S.-Y.; Lim, J.-S.; Jang, J.; Kim, K.-M.; Lee, Y.-M.; Lee, B.-W. A review of host plants of Cerambycidae (Coleoptera: Chrysomeloidea) with new host records for fourteen cerambycids, including the Asian longhorn beetle (Anoplophora glabripennis Motschulsky), in Korea. Korean J. Appl. Entomol. 2014, 53, 111–133. [Google Scholar] [CrossRef]
- Kojima, T. Further investigation on the immature stages of some Japanese Cerambyeid-beetles, with notes of their habits. J. Coll. Agric. Imp. Univ. Tokyo 1931, 11, 263–308. [Google Scholar]
- Leksono, A.S.; Takada, K.; Nakagoshi, N.; Nakamura, K. Species composition of Modellidae and Cerambycidae (Coleoptera) in a coppice woodland. J. For. Res. 2006, 11, 61–64. [Google Scholar] [CrossRef]
- Nga, C.T.Q.; Long, K.D.; Thinh, T.H. New records of the tribe Cerambycini (Coleoptera: Cerambycidae: Cerambycinae) from Vietnam. Tap. Chi. Sinh. Hoc. 2014, 36, 28–443. [Google Scholar]
- Anisimov, N.S.; Bezborodov, V.G. On the northern border of the distribution of Neocerambyx raddei (Coleoptera, Cerambycidae) in east Asia. Far East. Entomol. 2017, 332, 22–24. [Google Scholar]
- Koshkin, E.S. New data on the distribution of some longhorn beetles (Coleoptera, Cerambycidae) in Khabarovskii Krai of Russia. Euroasian Èntomol. J. 2021, 20, 216–220. [Google Scholar] [CrossRef]
- EPPO (European and Mediterranean Plant Protection Organization). Pest Risk Analysis for Massicus raddei (Coleoptera: Cerambycidae), Oak Longhorn Beetle; EPPO: Paris, France, 2018. [Google Scholar]
- Epanchin-Niell, R.S. Economics of invasive species policy and management. Biol. Invasions 2017, 19, 3333–3354. [Google Scholar] [CrossRef]
- Li, Y.; Johnson, A.J.; Gao, L.; Wu, C.; Hulcr, J. Two new invasive Ips bark beetles (Coleoptera: Curculionidae) in mainland China and their potential distribution in Asia. Pest Manag. Sci. 2021, 77, 4000–4008. [Google Scholar] [CrossRef]
- Haack, R.A.; Hérard, F.; Sun, J.; Turgeon, J.J. Managing invasive populations of Asian longhorned beetle and citrus longhorned beetle: A worldwide perspective. Annu. Rev. Entomol. 2010, 55, 521–546. [Google Scholar] [CrossRef]
- Brabbs, T.; Collins, D.; Hérard, F.; Maspero, M.; Eyre, D. Prospects for the use of biological control agents against Anoplophora in Europe. Pest Manag. Sci. 2015, 71, 7–14. [Google Scholar] [CrossRef]
- Peterson, A.T.; Scachetti-Pereira, R. Potential geographic distribution of Anoplophora glabripennis (Coleoptera: Cerambycidae) in North America. Am. Midl. Nat. 2004, 151, 170–178. [Google Scholar] [CrossRef]
- Rank, A.; Ramos, R.S.; da Silva, R.S.; Soares, J.R.S.; Picanço, M.C.; Fidelis, E.G. Risk of the introduction of Lobesia botrana in suitable areas for Vitis vinifera. J. Pest Sci. 2020, 93, 1167–1179. [Google Scholar] [CrossRef]
- Shim, J.Y.; Jung, J.M.; Byeon, D.H.; Jung, S.; Lee, W.H. Evaluation of the spatial distribution of Anoplophora glabripennis (Motschulsky) (Coleoptera: Cerambycidae) in South Korea combining climate and host plant distribution. J. Asia-Pac. Èntomol. 2020, 23, 646–652. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Dang, Y.Q.; Wang, X.Y. Risk analysis of dispersal and outbreak of Massicus raddei (Blessig) (Coleoptera: Cerambycidae) in China based on climate and host distribution. Sci. Silvae Sin. 2022, 58, 118–132. (in press). [Google Scholar]
- Martin, A.; Simon, C. Temporal variation in insect life cycles. BioScience 1990, 40, 359–367. [Google Scholar] [CrossRef]
- Williams, K.S.; Simon, C. The ecology, behavior, and evolution of periodical cicadas. Annu. Rev. Entomol. 1995, 40, 269–295. [Google Scholar] [CrossRef]
- Chejara, V.K.; Kriticos, D.J.; Kristiansen, P.; Sindel, B.M.; Whalley, R.D.B.; Nadolny, C. The current and future potential geographical distribution of Hyparrhenia hirta. Weed Res. 2010, 50, 174–184. [Google Scholar] [CrossRef]
- Phillips, S.J. A Brief Tutorial on Maxent. 2017. Available online: http://Biodiversityinformatics.amnh.org/open_source/maxent/ (accessed on 3 May 2022).
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmannn, F.; Leathwick, J.R.; Lehmannn, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Ramos, R.S.; Kumar, L.; Shabani, F.; Picanço, M.C. Mapping global risk levels of Bemisia tabaci in areas of suitability for open field tomato cultivation under current and future climates. PLoS ONE 2018, 13, e0198925. [Google Scholar] [CrossRef]
- Zhu, G.; Bu, W.; Gao, Y.; Liu, G. Potential geographic distribution of brown marmorated stink bug invasion (Halyomorpha halys). PLoS ONE 2012, 7, e31246. [Google Scholar] [CrossRef]
- Dang, Y.Q.; Zhang, Y.L.; Wang, X.Y.; Xin, B.; Quinn, N.F.; Duan, J.J. Retrospective analysis of factors affecting the distribution of an invasive wood-boring insect using native range data: The importance of host plants. J. Pest Sci. 2021, 94, 981–990. [Google Scholar] [CrossRef]
- EPPO (Europrean Mediterranean Plant Protection Organization). Massicus raddei, EPPO Alert List. 2015. Available online: https://www.eppo.int/QUARANTINE/Alert_List/insects/Massicus_raddei.htm (accessed on 3 March 2015).
- USDA_APHIS (U.S. Department of Agriculture, Animal Plant Health Inspection Service, Plant Protection and Quarantine). New Pest Response Guidelines: Massicus raddei (Blessig) (Coleoptera: Cerambycidae), Mountain Oak Longhorned Beetle. 2015. Available online: http://www.aphis.usda.gov/import_export/plants/manuals/online_manuals.shtml (accessed on 20 January 2015).
- Wang, Z.C. Primary Illustrated Handbook of Insects of Northeastern China, II (Cerambycidae); Jilin Science and Technology Publishing House: Jilin, China, 2003; p. 198. [Google Scholar]
- FDPC (Forest Disease and Pest Control Station of National Forestry Administration). A General Survey of Forest Insect Pests in China (2003–2007); China Forestry Publishing House: Beijing, China, 2008.
- FDPC (Forest Disease and Pest Control Station of National Forestry Administration). Calendar of Forest Pest Control; China Forestry Publishing House: Beijing, China, 2010.
- Xu, Z.H.; Guo, S.B.; Guo, J.Y. Fauna of Insects from Xiaowutai Mountain; China Forestry Press: Beijing, China, 2013; pp. 201–202. [Google Scholar]
- Wang, Z.C. Monographia of Original Colored Longicorn Beetles of China; Scientific and Technical Documentation Press: Beijing, China, 2014; pp. 393–394. [Google Scholar]
- FGDPC (Forest and Grassland Disease and Pest Control Station of National Forestry and Grassland Administration). A General Survey of Forest Insect Pests in China (2014–2017); China Forestry Publishing House: Beijing, China, 2019.
- Yu, G.Y. Photographic Atlas of Beijing Beetles; Technology Press: Beijing, China, 2020; pp. 245–250. [Google Scholar]
- Aiello-Lammens, M.E.; Boria, R.A.; Radosavljevic, A.; Vilela, B.; Anderson, R.P. SpThin: An R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography 2015, 38, 541–545. [Google Scholar] [CrossRef]
- Boria, R.A.; Olson, L.E.; Goodman, S.M.; Anderson, R.P. Spatial filtering to reduce sampling bias can improve the performance of ecological niche models. Ecol. Model. 2014, 275, 73–77. [Google Scholar] [CrossRef]
- Daly, C. Guidelines for assessing the suitability of spatial climate data sets. Int. J. Climatol. 2010, 26, 707–721. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; García-Marquéz, J.R.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Peterson, A.T.; Soberón, J.; Pearson, R.G.; Anderson, R.P.; Martínez-Meyer, E.; Nakamura, M.; Araújo, M.B. Ecological Niches and Geographical Distribution; Princeton University Press: Princeton, NJ, USA, 2011. [Google Scholar]
- Arthur, F.H.; Morrison, W.R.; Morey, A.C. Modeling the potential range expansion of larger grain borer, Prostephanus truncatus (Coleoptera: Bostrichidae). Sci. Rep. 2019, 9, 6862. [Google Scholar] [CrossRef]
- Tang, Y.L. Studies on the Ecology of Massicus raddei (Coleoptera: Cerambycidae) and Its Biological Control. Ph.D. Thesis, Chinese Academy of Forestry, Beijing, China, 2011. [Google Scholar]
- Yang, Z.Q.; Wang, X.Y.; Wang, B.; Yu, Y.P.; Dong, Z.M.; Mu, Z.H.; Wang, H.S. Relationship between adult emergence of Massicus raddei (Celeoptera: Cerambycidae) and temperature and relative humidity. Acta. Ecol. Sin. 2011, 31, 7486–7491. [Google Scholar]
- Yin, X.J.; Zhou, G.S.; Sui, X.H.; He, Q.J.; Li, R.P. Potential geographical distribution of Quercus wutaishanica forest and its dominant factors. Sci. Silvae Sin. 2013, 49, 10–14. [Google Scholar]
- Li, T.; He, X.Y.; Chen, Z.J. Tree-ring growth responses of mongolian oak (Quercus mongolica) to climate change in southern northeast: A case study in Qianshan mountains. Chin. J. Appl. Ecol. 2014, 25, 1841–1848. [Google Scholar]
- Zhang, Y.; Manzoor, A.; Wang, X. Mitochondrial DNA analysis reveals spatial genetic structure and high genetic diversity of Massicus raddei (Blessig) (Coleoptera: Cerambycidae) in China. Ecol. Evol. 2020, 10, 11657–11670. [Google Scholar] [CrossRef] [PubMed]
- Gómez, S.R.; Gil-Tapetado, D.; García-Gila, J.; Blasco-Aróstegui, J.; Polidori, C. The leaf beetle Labidostomis lusitanica (Coleoptera: Chrysomelidae) as an Iberian pistachio pest: Projecting risky areas. Pest Manag. Sci. 2021, 78, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Nebraska Statewide Arboretum. Trees for Western Nebraska; Nebraska Statewide Arboretum: Lincoln, NE, USA, 2008. [Google Scholar]
- Nebraska Statewide Arboretum. Liaoning Oak (Quercus liaotungensis); Nebraska Statewide Arboretum: Lincoln, NE, USA, 2013. [Google Scholar]
- North Dakota Tree Information Center. Mongolian Oak (Quercus mongolica); North Dakota Tree Information Center: Watford City, ND, USA, 2014. [Google Scholar]
- Feng, Y.; Xu, L.; Li, W.; Xu, Z.; Cao, M.; Wang, J.; Tao, J.; Zong, S. Seasonal change in supercooling capacity and major cryoprotectants of overwintering Asian longhorned beetle (Anoplophora glabripennis) larvae. Agric. For. Entomol. 2016, 18, 302–312. [Google Scholar] [CrossRef]
- Luo, Y.Q.; Lu, C.K.; Chen, H.J.; Niu, J.F.; Yuan, F.Q. More attention should be paid to an important forest pest: Massicus raddei (Blessig). Plant Quar. 2005, 19, 354–356. [Google Scholar]
- Eyles, A.; Jones, W.; Riedl, K.; Cipollini, D.; Schwartz, S.; Chan, K.; Herms, D.A.; Bonello, P. Comparative phloem chemistry of manchurian (Fraxinus mandshurica) and two North American ash species (Fraxinus americana and Fraxinus pennsylvanica). J. Chem. Ecol. 2007, 33, 1430–1448. [Google Scholar] [CrossRef] [PubMed]
- Rebek, E.J.; Herms, D.A.; Smitley, D.R. Interspecific variation in resistance to emerald ash borer (Coleoptera: Buprestidae) among North American and Asian Ash (Fraxinus spp.). Environ. Entomol. 2008, 37, 242–246. [Google Scholar] [CrossRef]
- Zhao, S.C.; State Forestry and Grassland Administration of the People’s Republic of China. Strictly Protect Whole Natural Forest. 2015. Available online: http://www.forestry.gov.cn/main/195/content-742128.html (accessed on 26 February 2015).
- Ding, Y.Y. The general office of the CPC central committee and the general office of the state council issued the plan for the protection and restoration system of natural forests to protect whole natural forests. Environ. Econ. 2019, 14, 31–33. [Google Scholar]
- Tang, J.; Li, J.; Lu, H.; Lu, F.; Lu, B. Potential distribution of an invasive pest, Euplatypus parallelus, in China as predicted by Maxent. Pest Manag. Sci. 2019, 75, 1630–1637. [Google Scholar] [CrossRef]
- Stockwell, D.R.B.; Beach, J.H.; Stewart, A.; Vorontsov, G.; Vieglais, D.; Pereira, R.S. The use of the GARP genetic algorithm and internet grid computing in the Lifemapper world atlas of species biodiversity. Ecol. Model. 2006, 195, 139–145. [Google Scholar] [CrossRef]
- Wang, X.Y.; Huang, X.L.; Jiang, L.Y.; Qiao, G.X. Predicting potential distribution of chestnut phylloxerid (Hemiptera: Phylloxeridae) based on GARP and Maxent ecological niche models. J. Appl. Entomol. 2010, 134, 45–54. [Google Scholar] [CrossRef]

| Environmental Variables | Percent Contribution/% | Accumulated Percent Contribution/% |
|---|---|---|
| Precipitation of the warmest quarter; Bio18 | 53.6 | 53.6 |
| Isothermality; Bio03 | 25.6 | 79.2 |
| Mean temperature of the wettest quarter; Bio08 | 14.5 | 93.7 |
| Annual mean temperature; Bio01 | 4.4 | 98.1 |
| Temperature seasonality; Bio04 | 1.1 | 99.2 |
| Maximum temperature of the warmest month; Bio05 | 0.8 | 100.0 |
| Environmental Variables | Percent Contribution/% | Accumulated Percent Contribution/% |
|---|---|---|
| Precipitation of the warmest quarter; Bio18 | 43.0 | 43.0 |
| Annual mean temperature; Bio01 | 30.9 | 73.9 |
| Maximum temperature of the warmest month; Bio05 | 13.5 | 87.4 |
| Isothermality; Bio03 | 6.0 | 93.4 |
| Precipitation seasonality; Bio15 | 5.1 | 98.5 |
| Mean diurnal range; Bio02 | 1.5 | 100.0 |
| Environmental Variables | Percent Contribution/% | Accumulated Percent Contribution/% |
|---|---|---|
| Precipitation of the warmest quarter; Bio18 | 49.9 | 49.9 |
| Isothermality; Bio03 | 20.7 | 70.6 |
| Annual mean temperature; Bio01 | 13.9 | 84.5 |
| Mean temperature of the warmest quarter; Bio10 | 7.9 | 92.4 |
| Annual precipitation; Bio12 | 6.7 | 99.1 |
| Mean temperature of the wettest quarter; Bio08 | 0.9 | 100.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Dang, Y.; Wang, X. Risk Assessment of the Worldwide Expansion and Outbreak of Massicus raddei (Blessig) (Coleoptera: Cerambycidae) Based on Host Plant and Climatic Factors. Insects 2022, 13, 730. https://doi.org/10.3390/insects13080730
Zhang Y, Dang Y, Wang X. Risk Assessment of the Worldwide Expansion and Outbreak of Massicus raddei (Blessig) (Coleoptera: Cerambycidae) Based on Host Plant and Climatic Factors. Insects. 2022; 13(8):730. https://doi.org/10.3390/insects13080730
Chicago/Turabian StyleZhang, Yufan, Yingqiao Dang, and Xiaoyi Wang. 2022. "Risk Assessment of the Worldwide Expansion and Outbreak of Massicus raddei (Blessig) (Coleoptera: Cerambycidae) Based on Host Plant and Climatic Factors" Insects 13, no. 8: 730. https://doi.org/10.3390/insects13080730
APA StyleZhang, Y., Dang, Y., & Wang, X. (2022). Risk Assessment of the Worldwide Expansion and Outbreak of Massicus raddei (Blessig) (Coleoptera: Cerambycidae) Based on Host Plant and Climatic Factors. Insects, 13(8), 730. https://doi.org/10.3390/insects13080730






