The Influence of Non-Optimal Rearing Conditions and Substrates on the Performance of the Black Soldier Fly (Hermetia illucens)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Larvae Rearing and Sample Collection
2.2. Substrate Preparation
2.3. Experimental Design and Larval Performance
2.4. Waste Reduction and Conversion Efficiency
2.5. Statistical Analysis
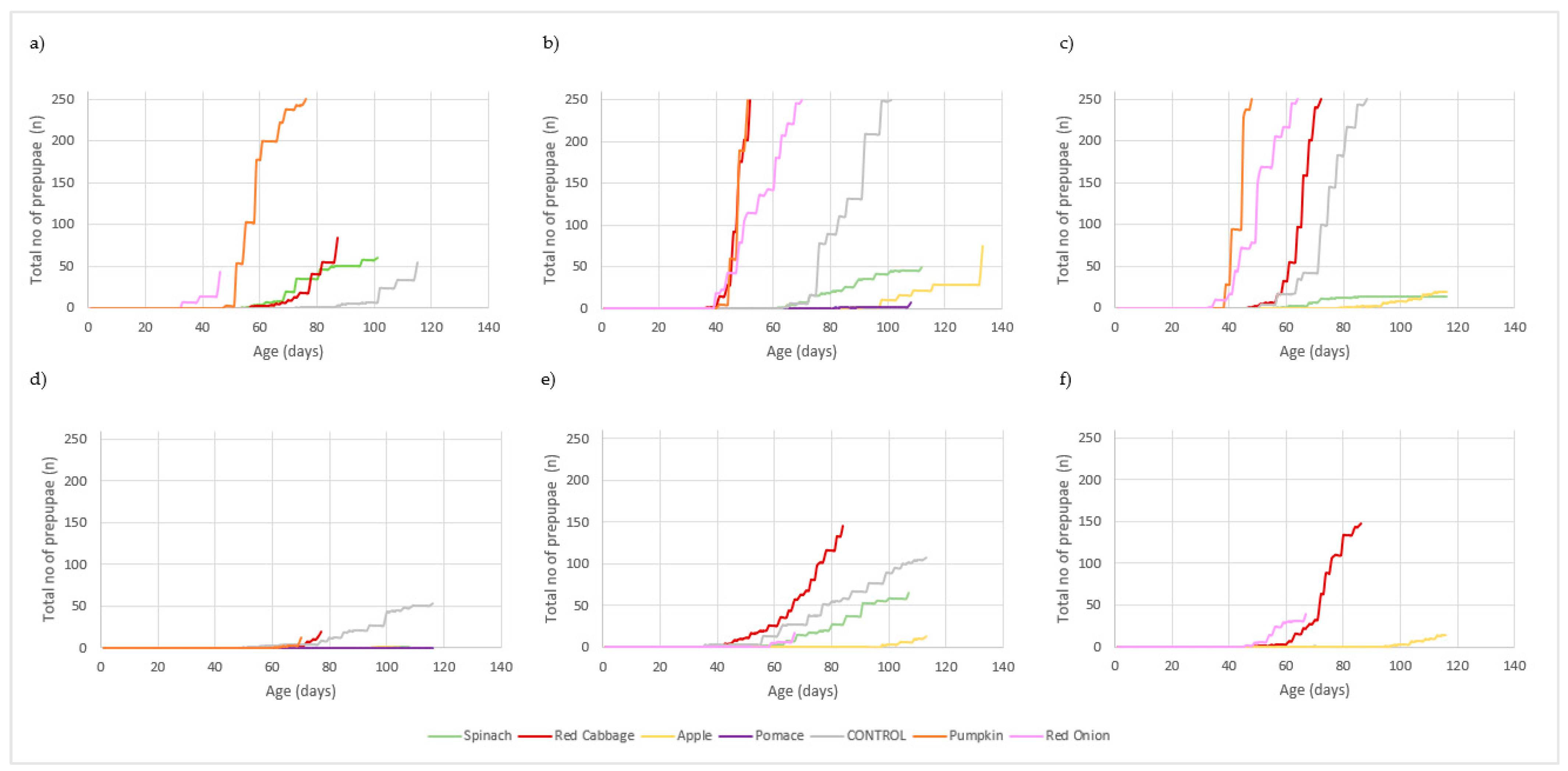
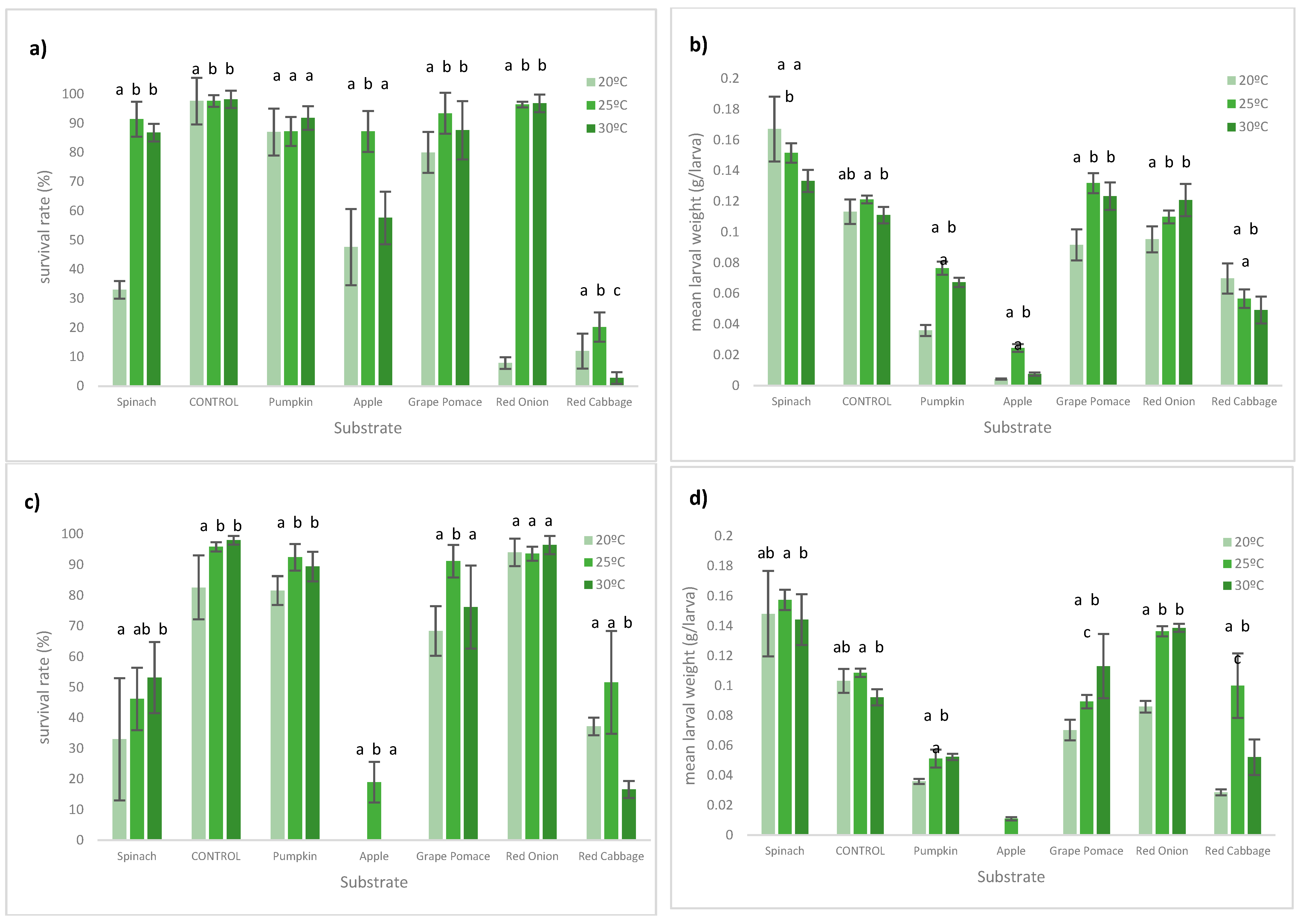
3. Results
3.1. Larval Performance
3.2. Waste Reduction and Conversion Efficiency
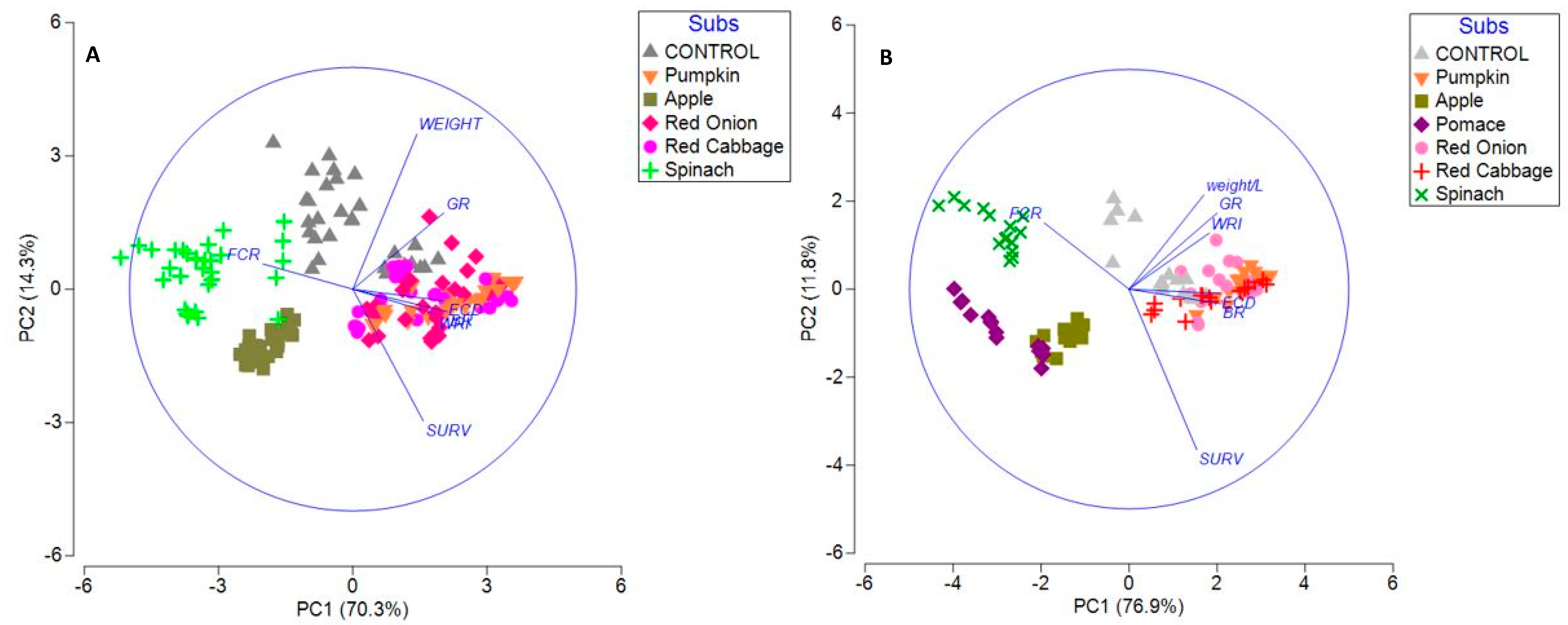
3.3. Principal Component Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Webb, R.; Buratini, J. Global Challenges for the 21st Century: The Role and Strategy of the Agri-Food Sector. Anim. Reprod. 2016, 13, 133–142. [Google Scholar] [CrossRef]
- Ameixa, O.M.C.C.; Duarte, P.M.; Rodrigues, D.P. Insects, Food Security and Sustainable Aquaculture. In Zero Hunger; Leal Filho, W., Azul, A.M., Brandli, L., Özuyar, P.G., Wall, T., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–11. [Google Scholar] [CrossRef]
- Pinotti, L.; Giromini, C.; Ottoboni, M.; Tretola, M.; Marchis, D. Review: Insects and Former Foodstuffs for Upgrading Food Waste Biomasses/Streams to Feed Ingredients for Farm Animals. Animal 2019, 13, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Ankers, P. State-of-the-Art on Use of Insects as Animal Feed. Anim. Feed Sci. Technol. 2014, 197, 1–33. [Google Scholar] [CrossRef]
- Tomberlin, J.K.; Sheppard, D.C.; Joyce, J.A. Selected Life-History Traits of Black Soldier Flies (Diptera: Stratiomyidae) Reared on Three Artificial Diets. Ann. Entomol. Soc. Am. 2002, 95, 379–386. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; Volk, N.; Diehl, J.J.E.; van Loon, J.J.A.; Belušič, G. Photoreceptor Spectral Sensitivity of the Compound Eyes of Black Soldier Fly (Hermetia illucens) Informing the Design of LED-Based Illumination to Enhance Indoor Reproduction. J. Insect Physiol. 2016, 95, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Tschirner, M.; Simon, A. Influence of Different Growing Substrates and Processing on the Nutrient Composition of Black Soldier Fly Larvae Destined for Animal Feed. J. Insects Food Feed. 2015, 1, 249–259. [Google Scholar] [CrossRef]
- Lalander, C.; Diener, S.; Zurbrügg, C.; Vinnerås, B. Effects of Feedstock on Larval Development and Process Efficiency in Waste Treatment with Black Soldier Fly (Hermetia illucens). J. Clean. Prod. 2019, 208, 211–219. [Google Scholar] [CrossRef]
- Dzepe, D.; Nana, P.; Kuietche, H.M.; Kimpara, J.M.; Magatsing, O.; Tchuinkam, T.; Djouaka, R. Feeding Strategies for Small-Scale Rearing Black Soldier Fly Larvae (Hermetia illucens) as Organic Waste Recycler. SN Appl. Sci. 2021, 3, 252. [Google Scholar] [CrossRef]
- Mutafela, R.N. High Value Organic Waste Treatment via Black Soldier Fly Bioconversion. Master’ Thesis, Royal Institute of Technology, Stockholm, Sweden, 2015. [Google Scholar]
- Leni, G.; Caligiani, A.; Sforza, S. Killing Method Affects the Browning and the Quality of the Protein Fraction of Black Soldier Fly (Hermetia illucens) Prepupae: A Metabolomics and Proteomic Insight. Food Res. Int. 2019, 115, 116–125. [Google Scholar] [CrossRef]
- Kasmaei, K.M.; Gonda, H.; Udén, P.; Knicky, M.; Vidakovic, A. Ensiling as a Method for Storage and Processing of Black Soldier Fly Larvae for Use as Animal Feed; Research Report from Swedish Energy Agency: Eskilstuna, Sweden, 2018. [Google Scholar]
- Supriyatna, A.; Kurrahman, O.T.; Cahyanto, T.; Yuliawati, A.; Kulsum, Y. The Potency of Black Soldier Larvae (Hermetia illucens L.) as a Source of Protein for Livestock Feed. Biosaintifika J. Biol. Biol. Educ. 2018, 10, 449–455. [Google Scholar] [CrossRef][Green Version]
- Nguyen, T.T.X.; Tomberlin, J.K.; Vanlaerhoven, S. Ability of Black Soldier Fly (Diptera: Stratiomyidae) Larvae to Recycle Food Waste. Environ. Entomol. 2015, 44, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Bloukounon-Goubalan, A.Y.; Saïdou, A.; Chrysostome, C.A.A.M.; Kenis, M.; Amadji, G.L.; Igué, A.M.; Mensah, G.A. Physical and Chemical Properties of the Agro-Processing by-Products Decomposed by Larvae of Musca Domestica and Hermetia illucens. Waste Biomass Valorization 2020, 11, 2735–2743. [Google Scholar] [CrossRef]
- European Commission. COMMISSION REGULATION (EU) 2017/893 of 24 May 2017 Amending Annexes I and IV to Regulation (EC) No 999/2001 of the European Parliament and of the Council and Annexes X, XIV and XV to Commission Regulation (EU) No 142/2011 as Regards the provisions on processed animal protein. Off. J. Eur. Union 2017, 2017, 1–25. [Google Scholar]
- European Commission. Regulation (EC) No 999/2001 of the European Parliment and of the Council of 22 May Laying down Rules for the Prevention, Control and Eradication of Certain Transmissible Spongiform Encephalopathies (31/5/2001). Off. J. Eur. Communities 2001, L 147, 1–69.
- FAO. Food Loss and Waste | Climate Change|Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/in-action/seeking-end-to-loss-and-waste-of-food-along-production-chain/en/ (accessed on 10 January 2022).
- Drewery, M.L.; Liu, X.; Wickersham, T.A. Black Soldier Fly Larvae (BSFL) as a Feed for Beef Cattle: A Hedonic Pricing Model. J. Insects Food Feed. 2022, 1–10. [Google Scholar] [CrossRef]
- Smetana, S.; Spykman, R.; Heinz, V. Environmental Aspects of Insect Mass Production. J. Insects Food Feed. 2021, 7, 553–571. [Google Scholar] [CrossRef]
- Halloran, A.; Roos, N.; Eilenberg, J.; Cerutti, A.; Bruun, S. Life Cycle Assessment of Edible Insects for Food Protein: A Review. Agron. Sustain. Dev. 2016, 36, 57. [Google Scholar] [CrossRef]
- Ameixa, O.M.C.C.; Rebelo, J.; Silva, H.; Pinto, D.C.G.A. Gall Midge Baldratia Salicorniae Kieffer (Diptera: Cecidomyiidae) Infestation on Salicornia europaea L. Induces the Production of Specialized Metabolites with Biotechnological Potential. Phytochemistry 2022, 200, 113207. [Google Scholar] [CrossRef]
- Boate, U.R.; Abalis, O.R. Review on the Bio-Insecticidal Properties of Some Plant Secondary Metabolites: Types, Formulations, Modes of Action, Advantages and Limitations. Asian J. Res. Zool. 2020, 3, 27–60. [Google Scholar] [CrossRef]
- Waldbauer, G.P.; Friedman, S. Self-Selection of Optimal Diets by Insects. Annu. Rev. Entomol. 1991, 36, 43–63. [Google Scholar] [CrossRef]
- Boyer, J.; Liu, R.H. Apple Phytochemicals and Their Health Benefits. Nutr. J. 2004, 3, 1. [Google Scholar] [CrossRef]
- Bolarinwa, I.F.; Oke, M.O.; Olaniyan, S.A.; Ajala, A.S. A Review of Cyanogenic Glycosides in Edible Plants. In Toxicology—New Aspects to This Scientific Conundrum; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef]
- Cruz, A.B.; da Pitz, H.S.; Veber, B.; Bini, L.A.; Maraschin, M.; Zeni, A.L.B. Assessment of Bioactive Metabolites and Hypolipidemic Effect of Polyphenolic-Rich Red Cabbage Extract. Pharm. Biol. 2016, 54, 3033–3039. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Toledo, E.; Delgado-Rodríguez, M.; Gaforio, J.J. Naturally Lignan-Rich Foods: A Dietary Tool for Health Promotion? Molecules 2019, 24, 917. [Google Scholar] [CrossRef] [PubMed]
- Lobo, M.G.; Hounsome, N.; Hounsome, B. Biochemistry of Vegetables: Secondary Metabolites in Vegetables-Terpenoids, Phenolics, Alkaloids, and Sulfur-Containing Compounds. In Handbook of Vegetables and Vegetable Processing, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; pp. 1–2, 47–82. [Google Scholar] [CrossRef]
- Slimestad, R.; Fossen, T.; Vågen, I.M. Onions: A Source of Unique Dietary Flavonoids. J. Agric. Food Chem. 2007, 55, 10067–10080. [Google Scholar] [CrossRef] [PubMed]
- Salamatullah, A.M.; Uslu, N.; Özcan, M.M.; Alkaltham, M.S.; Hayat, K. The Effect of Oven Drying on Bioactive Compounds, Antioxidant Activity, and Phenolic Compounds of White and Red-Skinned Onion Slices. J. Food Process. Preserv. 2021, 45, e15173. [Google Scholar] [CrossRef]
- Popova, A.; Mihaylova, D. Antinutrients in Plant-Based Foods: A Review. Open Biotechnol. J. 2019, 13, 68–76. [Google Scholar] [CrossRef]
- Zdunić, G.M.; Menković, N.R.; Jadranin, M.B.; Novaković, M.M.; Šavikin, K.P.; Živković, J. Phenolic Compounds and Carotenoids in Pumpkin Fruit and Related Traditional Products. Chem. Ind. 2016, 70, 429–433. [Google Scholar] [CrossRef]
- Vincenzo, L.; Lattanzio, V.M.T.; Cardinali, A. Role of Phenolics in the Resistance Mechanisms of Plants against Fungal Pathogens and Insects. In Phytochemistry; Imperato, F., Ed.; Research Signpost: Trivandrum, India, 2015; Volume 661, pp. 23–67. [Google Scholar]
- Yilmaz, Y.; Toledo, R.T. Major Flavonoids in Grape Seeds and Skins: Antioxidant Capacity of Catechin, Epicatechin, and Gallic Acid. J. Agric. Food Chem. 2004, 52, 255–260. [Google Scholar] [CrossRef]
- Sinha, K.; Khare, V. Review on: Antinutritional Factors in Vegetable Crops. Pharma Innov. J. 2017, 6, 353–358. [Google Scholar]
- Diener, S.; Studt Solano, N.M.; Roa Gutiérrez, F.; Zurbrügg, C.; Tockner, K. Biological Treatment of Municipal Organic Waste Using Black Soldier Fly Larvae. Waste Biomass Valorization 2011, 2, 357–363. [Google Scholar] [CrossRef]
- Nana, P.; Kimpara, J.M.; Tiambo, C.K.; Tiogue, C.T.; Youmbi, J.; Choundong, B.; Fonkou, T. Black Soldier Flies (Hermetia illucens Linnaeus) as Recyclers of Organic Waste and Possible Livestock Feed. Int. J. Biol. Chem. Sci. 2019, 12, 2004. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Neveu, V.; Vos, F.; Scalbert, A. Identification of the 100 Richest Dietary Sources of Polyphenols: An Application of the Phenol-Explorer Database. Eur. J. Clin. Nutr. 2010, 64, S112–S120. [Google Scholar] [CrossRef] [PubMed]
- Dortmans, B.; Diener, S.; Verstappen, B.; Zurbrügg, C. Black Soldier Fly Biowaste Processing—A Step-by Step Guide, 2nd ed.; Eawag—Swiss Federal Institute of Aquatic Science and Technology: Dübendorf, Switzerland, 2021. [Google Scholar]
- Heuzé, V.; Tran, G. Grape pomace. Feedipedia, A Programme by INRAE, CIRAD, AFZ and FAO. Available online: https://www.feedipedia.org/node/691 (accessed on 20 May 2021).
- USDA Agricultural Research Service. FoodData Central. Available online: https://fdc.nal.usda.gov/ (accessed on 21 May 2021).
- Diener, S.; Zurbrügg, C.; Tockner, K. Conversion of Organic Material by Black Soldier Fly Larvae: Establishing Optimal Feeding Rates. Waste Manag. Res. 2009, 27, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Banks, I.J. To Assess the Impact of Black Soldier Fly (Hermetia illucens) Larvae on Faecal Reduction in Pit Latrines. Ph.D. Thesis, London School of Hygiene & Tropical Medicine, London, UK, 2014. No. 2014. pp. 1–231. [Google Scholar] [CrossRef]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ Primer V7: User Manual; Prime Ltd.: Plymouth, UK, 2008; p. 93. [Google Scholar]
- Barragan-Fonseca, K.B.; Dicke, M.; van Loon, J.J.A. Influence of Larval Density and Dietary Nutrient Concentration on Performance, Body Protein, and Fat Contents of Black Soldier Fly Larvae (Hermetia illucens). Entomol. Exp. Appl. 2018, 166, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Purkayastha, D.; Sarkar, S. Sustainable Waste Management Using Black Soldier Fly Larva: A Review. Int. J. Environ. Sci. Technol. 2021, 1–26. [Google Scholar] [CrossRef]
- Barragan-Fonseca, K.B.; Dicke, M.; van Loon, J.J.A. Nutritional Value of the Black Soldier Fly (Hermetia illucens L.) and Its Suitability as Animal Feed—A Review. J. Insects Food Feed. 2017, 3, 105–120. [Google Scholar] [CrossRef]
- Fatchurochim, S.; Geden, C.J.; Axtell, R.C. Filth Fly (Diptera) Oviposition and Larval Development in Poultry Manure of Various Moisture Levels. J. Entomol. Sci. 1989, 24, 224–231. [Google Scholar] [CrossRef]
- Cammack, J.A.; Tomberlin, J.K. The Impact of Diet Protein and Carbohydrate on Select Life-History Traits of the Black Soldier Fly Hermetia illucens (L.) (Diptera: Stratiomyidae). Insects 2017, 8, 56. [Google Scholar] [CrossRef]
- Su, C.H.; Nguyen, H.C.; Pham, U.K.; Nguyen, M.L.; Juan, H.Y. Biodiesel Production from a Novel Nonedible Feedstock, Soursop (Annona muricata L.) Seed Oil. Energies 2018, 11, 2562. [Google Scholar] [CrossRef]
- Environmental Working Group. EWG’s 2022 Shopper’s Guide to Pesticides in ProduceTM. Available online: http://www.ewg.org/foodnews/ (accessed on 12 September 2021).
- Bajwa, U.; Sandhu, K.S. Effect of Handling and Processing on Pesticide Residues in Food- A Review. J. Food Sci. Technol. 2014, 51, 201–220. [Google Scholar] [CrossRef]
- Thompson, B.M.; Reddy, G.V.P. Effect of Temperature on Two Bio-Insecticides for the Control of Confused Flour Beetle (Coleoptera: Tenebrionidae). Fla. Entomol. 2016, 99, 67–71. [Google Scholar] [CrossRef]
- Macaulay, S.J.; Buchwalter, D.B.; Matthaei, C.D. Water Temperature Interacts with the Insecticide Imidacloprid to Alter Acute Lethal and Sublethal Toxicity to Mayfly Larvae. N. Z. J. Mar. Freshw. Res. 2020, 54, 115–130. [Google Scholar] [CrossRef]
- Lalander, C.; Ermolaev, E.; Wiklicky, V.; Vinnerås, B. Process Efficiency and Ventilation Requirement in Black Soldier Fly Larvae Composting of Substrates with High Water Content. Sci. Total Environ. 2020, 729, 138968. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T. Influence and Diet on Black Soldier Fly (Hermetia illucens Linneaus) (Diptera: Stratiomyidae) Life History Traits. Electronic Thesis, University of Windsor, Windsor, ON, Canda, 2010. [Google Scholar]
- Broeckx, L.; Frooninckx, L.; Slegers, L.; Berrens, S.; Noyens, I.; Goossens, S.; Verheyen, G.; Wuyts, A.; Van Miert, S. Growth of Black Soldier Fly Larvae Reared on Organic Side-Streams. Sustainability 2021, 13, 12953. [Google Scholar] [CrossRef]
- Liland, N.S.; Biancarosa, I.; Araujo, P.; Biemans, D.; Bruckner, C.G.; Waagbø, R.; Torstensen, B.E.; Lock, E.J. Modulation of Nutrient Composition of Black Soldier Fly (Hermetia illucens) Larvae by Feeding Seaweed-Enriched Media. PLoS ONE 2017, 12, e0183188. [Google Scholar] [CrossRef]
- Meneguz, M.; Schiavone, A.; Gai, F.; Dama, A.; Lussiana, C.; Renna, M.; Gasco, L. Effect of Rearing Substrate on Growth Performance, Waste Reduction Efficiency and Chemical Composition of Black Soldier Fly (Hermetia illucens) Larvae. J. Sci. Food Agric. 2018, 98, 5776–5784. [Google Scholar] [CrossRef]
- Danieli, P.P.; Lussiana, C.; Gasco, L.; Amici, A.; Ronchi, B. The Effects of Diet Formulation on the Yield, Proximate Composition, and Fatty Acid Profile of the Black Soldier Fly (Hermetia illucens L.) Prepupae Intended for Animal Feed. Animals 2019, 9, 178. [Google Scholar] [CrossRef]
- Scala, A.; Cammack, J.A.; Salvia, R.; Scieuzo, C.; Franco, A.; Bufo, S.A.; Tomberlin, J.K.; Falabella, P. Rearing Substrate Impacts Growth and Macronutrient Composition of Hermetia illucens (L.) (Diptera: Stratiomyidae) Larvae Produced at an Industrial Scale. Sci. Rep. 2020, 10, 19448. [Google Scholar] [CrossRef]
- Klammsteiner, T.; Walter, A.; Bogataj, T.; Heussler, C.D.; Stres, B.; Steiner, F.M.; Schlick-Steiner, B.C.; Insam, H. Impact of Processed Food (Canteen and Oil Wastes) on the Development of Black Soldier Fly (Hermetia illucens) Larvae and Their Gut Microbiome Functions. Front. Microbiol. 2021, 12, 619112. [Google Scholar] [CrossRef]
- Nyakeri, E.M.; Sijabat, T.W.S. Optimization of Production of Black Soldier Fly Larvae (Hermetia illucens, L) for Fish Feed Formulation. Ph.D. Thesis, Jaramogi Oginga Odinga University of Science and Technology, Bondo, Kenya, 2018. [Google Scholar]
- Kaur, R.; Shekhar, S.; Prasad, K. Secondary Metabolites of Fruits and Vegetables with Antioxidant Potential. In Secondary Metabolites—Trends and Reviews [Working Title]; IntechOpen: London, UK, 2022; p. 13. [Google Scholar] [CrossRef]
- Arias, A.; Feijoo, G.; Moreira, M.T. Exploring the Potential of Antioxidants from Fruits and Vegetables and Strategies for Their Recovery. Innov. Food Sci. Emerg. Technol. 2022, 77, 102974. [Google Scholar] [CrossRef]
- Rima, M.; Chbani, A.; Roques, C.; El Garah, F. Comparative Study of the Insecticidal Activity of a High Green Plant (Spinacia Oleracea) and a Chlorophytae Algae (Ulva Lactuca) Extracts against Drosophila Melanogaster Fruit Fly. Ann. Pharm. Fr. 2021, 79, 36–43. [Google Scholar] [CrossRef]
- Cosme, F.; Gonçalves, B.; Ines, A.; Jordão, A.M.; Vilela, A. Grape and Wine Metabolites: Biotechnological Approaches to Improve Wine Quality. In Grape and Wine Biotechnology; InTech: London, UK, 2016; p. 13. [Google Scholar] [CrossRef]
| Wheat Bran | Apple | Grape Pomace | Pumpkin | Red Cabbage | Red Onion | Spinach | ||
|---|---|---|---|---|---|---|---|---|
| Plant-based substrate composition (g/100 g wet basis) | Moisture | 9.9 | 81.2 | 49.5 | 86.47 | 88.9 | 83.5 | 92 |
| Protein | 15.1 | 0.3 | 5.8 | 1 | 1.4 | 0.9 | 2.9 | |
| Carbohydrate | 61.6 | 13.8 | 13.0 | 10.5 | 7.4 | 9.9 | 2.6 | |
| Fat | 2.73 | 0.2 | 2.8 | 0.1 | 0.2 | 0.1 | 0.6 | |
| Fibre | 10.4 | 2.4 | 12.2 | 2 | 2.1 | 2.2 | 1.6 | |
| TRIAL | ||||||||
| NAT SM (g/100 g wet basis) | Protein | 1.7 | 0.3 | 5.8 | 1 | 1.4 | 0.9 | 2.9 |
| Carbohydrate | 6.8 | 13.8 | 13.0 | 10.5 | 7.4 | 9.9 | 2.6 | |
| Fat | 0.3 | 0.2 | 2.8 | 0.1 | 0.2 | 0.1 | 0.6 | |
| Fibre | 1.2 | 2.4 | 12.2 | 2 | 2.1 | 2.2 | 1.6 | |
| Moisture | 90 | 81.2 | 49.5 | 86.47 | 88.9 | 83.5 | 92 | |
| 70% SM (g/100 g wet basis) | Protein | 5 | 0.5 | 3.4 | 2.2 | 3.8 | 1.7 | 10.8 |
| Carbohydrate | 20.5 | 22 | 7.7 | 23.2 | 20.1 | 18 | 9.7 | |
| Fat | 0.9 | 0.3 | 1.7 | 0.2 | 0.5 | 0.2 | 2.2 | |
| Fibre | 3.5 | 3.8 | 7.3 | 4.4 | 5.7 | 4.4 | 6.0 | |
| Temp. (°C) | Substrate Moisture (%) | Control | Apple | Grape Pomace | Pumpkin | Red Cabbage | Red Onion | Spinach |
|---|---|---|---|---|---|---|---|---|
| 20 | 70 | C7020 | A7020 | Pom7020 | P7020 | RC7020 | RO7020 | S7020 |
| 25 | C7025 | A7025 | Pom7025 | P7025 | RC7025 | RO7025 | S7025 | |
| 30 | C7030 | A7030 | Pom7030 | P7030 | RC7030 | RO7030 | S7030 | |
| 20 | NAT | CN20 | AN20 | PomN20 | PN20 | RCN20 | RON20 | SN20 |
| 25 | CN25 | AN25 | PomN25 | PN25 | RCN25 | RON25 | SN25 | |
| 30 | CN30 | AN30 | PomN30 | PN30 | RCN30 | RON25 | SN30 |
| Substrate | Temp. (°C) | SM (%H2O) | Feed 1 (mg/larva.d) | WRI 2 | FCR 2 | BR 2 (%) | ECD 2 (%) |
|---|---|---|---|---|---|---|---|
| Control | 20 | 70 | 2.890 ± 0.003 | 0.54 a ± 0.03 | 12.0 a ± 1.7 | 5.1 a ± 0.9 | 8.5 a ± 1.3 |
| Pumpkin | 4.537 ± 0.341 | 1.28 b ± 0.50 | 5.0 b ± 0.4 | 11.8 b ± 0.9 | 20.0 b ± 1.5 | ||
| Apple | 2.281 ± 0.012 | 0.47 a ± 0.03 | 23.9 c ± 2.2 | 3.1 a ± 0.4 | 4.2 c ± 0.4 | ||
| Grape Pomace | 1.471 ± 0.004 | 0.41 c ± 0.01 | 225.8 d ± 40.9 | 0.4 c ± 0.1 | 0.5 d ± 0.1 | ||
| Red Onion | 2.437 ± 0.007 | 1.18 b ± 0.01 | 5.2 b ± 0.5 | 12.6 b ± 0.9 | 19.3 e ± 1.6 | ||
| Red Cabbage | 2.497 ± 0.003 | 0.79 d ± 0.05 | 6.1 e ± 0.6 | 11.3 b ± 1.3 | 16.5 ae ± 1.7 | ||
| Spinach | 2.047 ± 0.137 | 0.67 a ± 0.11 | 71.9 f ± 7.0 | 1.3 d ± 0.1 | 1.4 f ± 0.1 | ||
| Control | 25 | 4.709 ± 0.051 | 0.73 a ± 0.01 | 5.6 a ± 0.3 | 9.3 a ± 0.6 | 18.0 a ± 1.0 | |
| Pumpkin | 6.904 ± 0.009 | 1.56 b ± 0.02 | 4.0 b ± 0.1 | 11.2 b ± 0.4 | 25.0 b ± 0.6 | ||
| Apple | 3.797 ± 0.002 | 0.57 c ± 0.01 | 16.0 c ± 0.6 | 4.0 c ± 0.1 | 6.2 c ± 0.2 | ||
| Grape Pomace | 1.709 ± 0.007 | 0.50 d ± 0.01 | 24.7 d ± 1.2 | 3.4 d ± 0.2 | 4.1 d ± 0.2 | ||
| Red Onion | 4.742 ± 0.033 | 1.36 b ± 0.06 | 4.2 e ± 0.4 | 12.5 b ± 0.6 | 24.0 e ± 2.3 | ||
| Red Cabbage | 4.820 ± 0.168 | 1.65 b ± 0.06 | 4.6 a ± 0.3 | 13.6 b ± 0.6 | 21.8 a ± 1.3 | ||
| Spinach | 2.835 ± 0.152 | 0.68 a ± 0.06 | 60.0 d ± 10.9 | 1.2 c ± 0.2 | 1.7 d ± 0.3 | ||
| Control | 30 | 5.322 ± 0.055 | 0.96 a ± 0.05 | 9.9 a ± 0.6 | 8.2 a ± 0.4 | 10.1 a ± 0.7 | |
| Pumpkin | 7.090 ± 0.066 | 1.75 b ± 0.04 | 5.7 b ± 0.2 | 11.0 b ± 0.4 | 17.5 b ± 0.8 | ||
| Apple | 4.023 ± 0.004 | 0.55 c ± 0.01 | 22.8 c ± 0.7 | 3.5 c ± 0.1 | 4.4 c ± 0.1 | ||
| Grape Pomace | 2.021 ± 0.009 | 0.45 d ± 0.02 | 44.0 d ± 6.4 | 0.6 d ± 0.1 | 0.7 d ± 0.1 | ||
| Red Onion | 5.368 ± 0.084 | 1.54 b ± 0.04 | 7.0 b ± 0.5 | 10.9 b ± 0.8 | 14.4 b ± 1.1 | ||
| Red Cabbage | 4.270 ± 0.110 | 1.21 e ± 0.03 | 6.3 b ± 0.5 | 12.6 b ± 1.4 | 16.0 b ± 1.5 | ||
| Spinach | 2.990 ± 0.166 | 0.78 ac ± 0.07 | 960.0 e ± 763.6 | 0.2 d ± 0.1 | 0.2 d ± 0.2 | ||
| Control | 20 | NAT | 0.731 ± 0.086 | 0.51 a ± 0.03 | 16.0 a ± 14.4 | 1.4 a ± 0.2 | 9.1 a ± 4.4 |
| Pumpkin | 2.006 ± 0.002 | 1.28 b ± 0.01 | 6.10 b ± 0.4 | 6.9 b ± 0.0 | 16.6 b ± 1.1 | ||
| Apple | 1.405 ± 0.003 | 0.55 c ± 0.01 | 26.7 c ± 1.7 | 3.1 c ± 0.0 | 3.8 c ± 0.2 | ||
| Grape Pomace | 2.595 ± 0.266 | 0.46 ± 0.05 | - | - | - | ||
| Red Onion | 1.454 ± 0.002 | 1.46 d ± 0.02 | 6.8 d ± 0.4 | 8.8 d ± 0.3 | 8.7 a ± 0.3 | ||
| Red Cabbage | 1.388 ± 0.004 | 0.96 e ± 0.02 | 8.9 a ± 0.2 | 7.7 e ± 0.2 | 7.8 a ± 0.2 | ||
| Spinach | 0.624 ± 0.004 | 0.49 a ± 0.03 | 68.9 e ± 8.8 | 1.2 f ± 0.0 | 1.5 d ± 0.2 | ||
| Control | 25 | 1.285 ± 0.085 | 0.57 a ± 0.01 | 10.0 a ± 2.4 | 5.9 a ± 0.4 | 10.4 a ± 2.1 | |
| Pumpkin | 2.359 ± 0.001 | 1.31 b ± 0.01 | 4.0 b ± 0.2 | 7.7 b ± 0.0 | 25.0 b ± 1.0 | ||
| Apple | 2.265 ± 0.010 | 0.53 c ± 0.01 | 17.7 c ± 2.1 | 2.6 c ± 0.0 | 5.7 c ± 0.7 | ||
| Grape Pomace | 2.591 ± 0.006 | 0.40 ± 0.01 | 203.6 ± 68.6 | 0.4 ± 0.0 | 0.5 ± 0.2 | ||
| Red Onion | 2.419 ± 0.003 | 1.52 d ± 0.00 | 4.8 d ± 0.2 | 9.0 d ± 0.0 | 21.0 d ± 0.9 | ||
| Red Cabbage | 2.212 ± 0.003 | 1.07 e ± 0.00 | 8.6 a ± 0.2 | 7.9 e ± 0.0 | 11.6 a ± 0.3 | ||
| Spinach | 1.057 ± 0.002 | 0.65 f ± 0.03 | 21.9 e ± 2.7 | 3.0 f ± 0.0 | 4.6 e ± 0.5 | ||
| Control | 30 | 1.599 ± 0.001 | 0.61 a ± 0.02 | 19.9 a ± 4.8 | 4.3 a ± 0.0 | 5.2 a ± 1.0 | |
| Pumpkin | 2.399 ± 0.003 | 1.26 b ± 0.02 | 11.1 b ± 0.8 | 7.0 b ± 0.0 | 9.1 b ± 0.6 | ||
| Apple | 2.652 ± 0.002 | 0.59 a ± 0.01 | 31.0 c ± 1.3 | 2.5 c ± 0.0 | 3.2 c ± 0.1 | ||
| Grape Pomace | 3.344 ± 0.239 | 0.57 ± 0.03 | - | - | - | ||
| Red Onion | 2.665 ± 0.005 | 1.49 c ± 0.02 | 9.0 d ± 0.5 | 8.4 d ± 0.0 | 0.111 d ± 0.6 | ||
| Red Cabbage | 2.401 ± 0.003 | 1.15 d ± 0.01 | 11.9 b ± 0.3 | 7.6 e ± 0.0 | 0.084 b ± 0.2 | ||
| Spinach | 1.069 ± 0.002 | 0.51 e ± 0.02 | 147 e ± 44.3 | 0.7 f ± 0.0 | 0.7 e ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, N.; Costa, R.; Ameixa, O.M.C.C. The Influence of Non-Optimal Rearing Conditions and Substrates on the Performance of the Black Soldier Fly (Hermetia illucens). Insects 2022, 13, 639. https://doi.org/10.3390/insects13070639
Ribeiro N, Costa R, Ameixa OMCC. The Influence of Non-Optimal Rearing Conditions and Substrates on the Performance of the Black Soldier Fly (Hermetia illucens). Insects. 2022; 13(7):639. https://doi.org/10.3390/insects13070639
Chicago/Turabian StyleRibeiro, Nuno, Rui Costa, and Olga M. C. C. Ameixa. 2022. "The Influence of Non-Optimal Rearing Conditions and Substrates on the Performance of the Black Soldier Fly (Hermetia illucens)" Insects 13, no. 7: 639. https://doi.org/10.3390/insects13070639
APA StyleRibeiro, N., Costa, R., & Ameixa, O. M. C. C. (2022). The Influence of Non-Optimal Rearing Conditions and Substrates on the Performance of the Black Soldier Fly (Hermetia illucens). Insects, 13(7), 639. https://doi.org/10.3390/insects13070639








