Reproductive Apparatus, Gonadic Maturation, and Allometry of Cyclocephala barrerai Martínez (Coleoptera: Melolonthidae: Dynastinae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cyclocephala barrerai Insects
2.2. Reproductive Apparatus Description
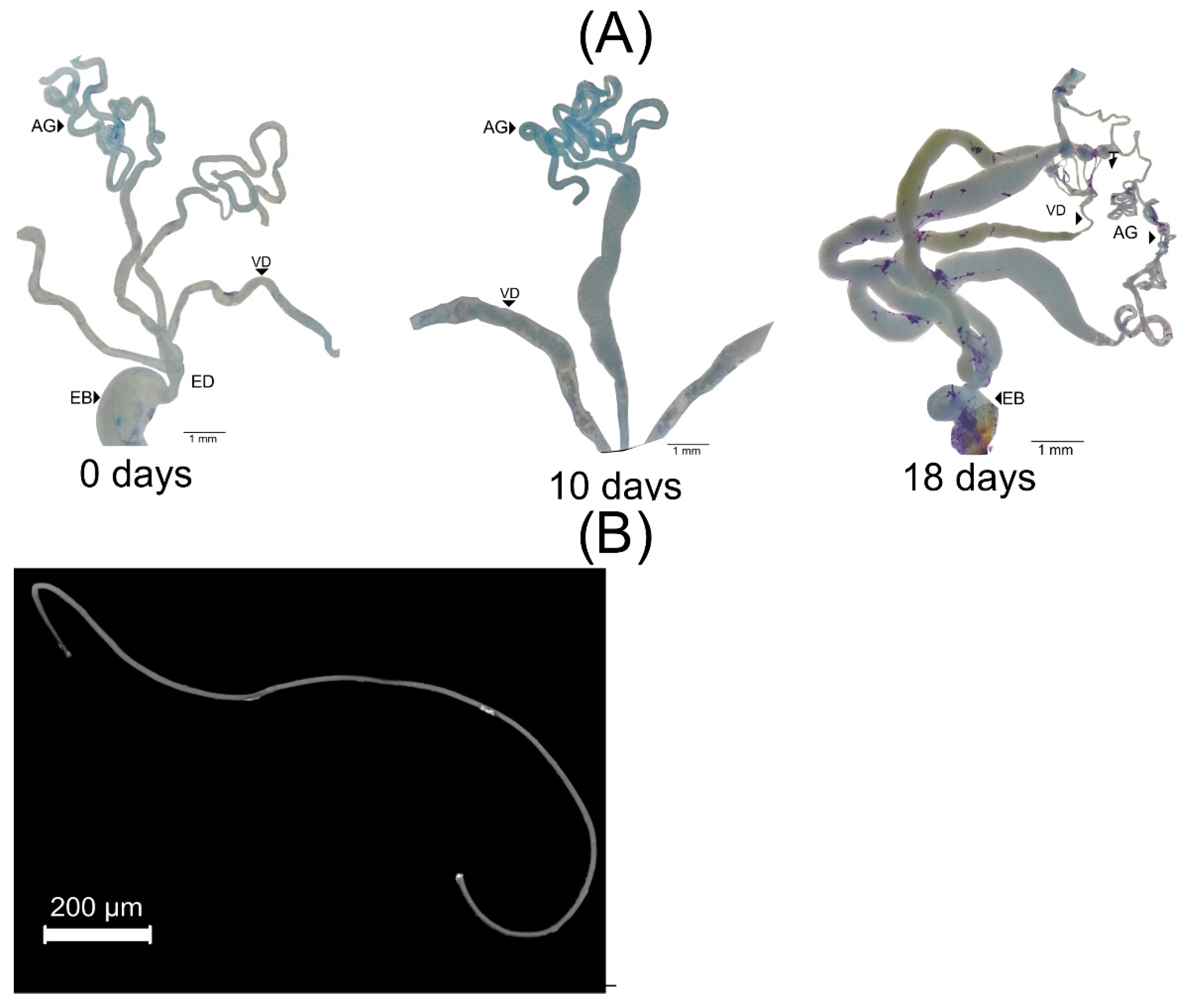
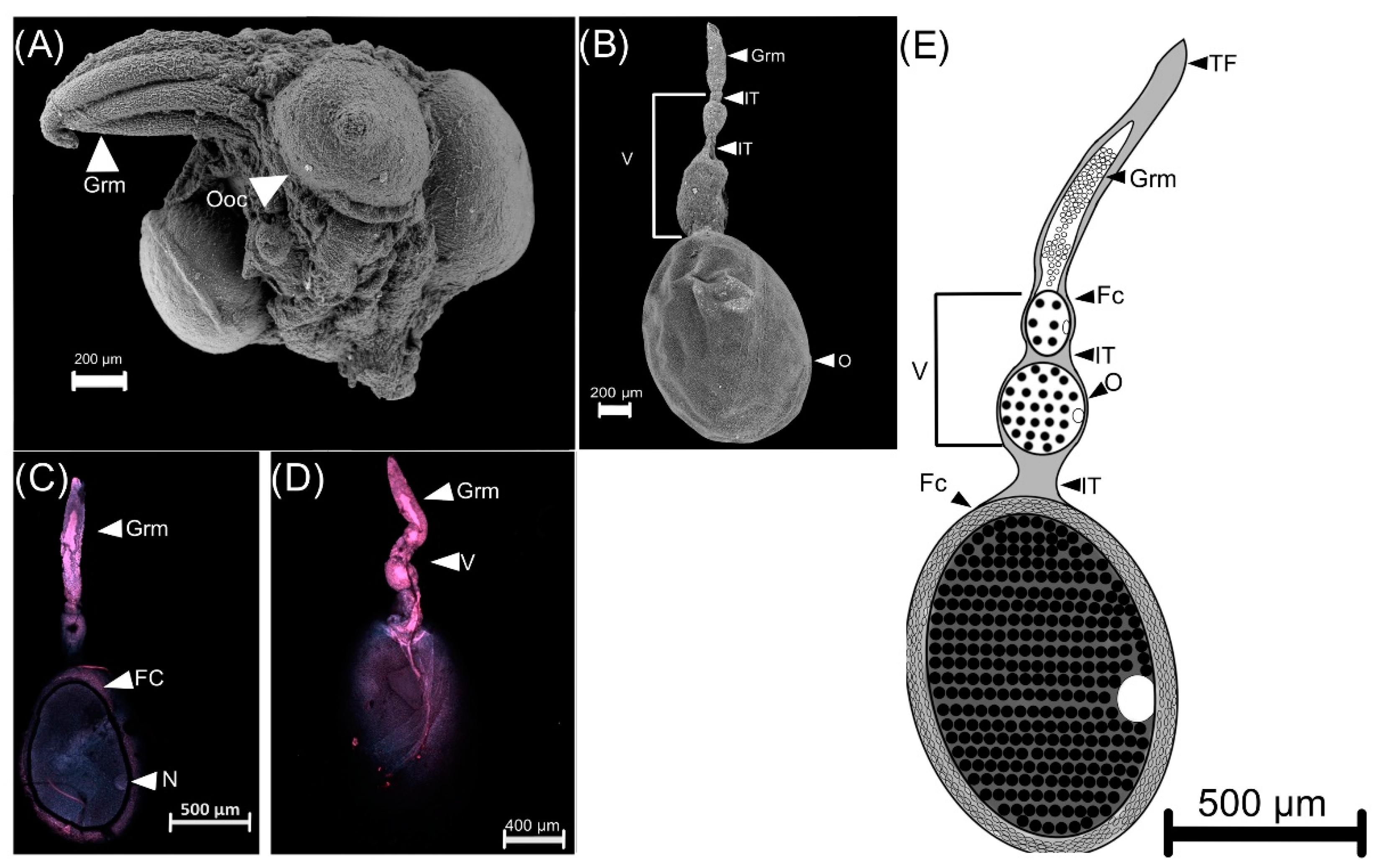
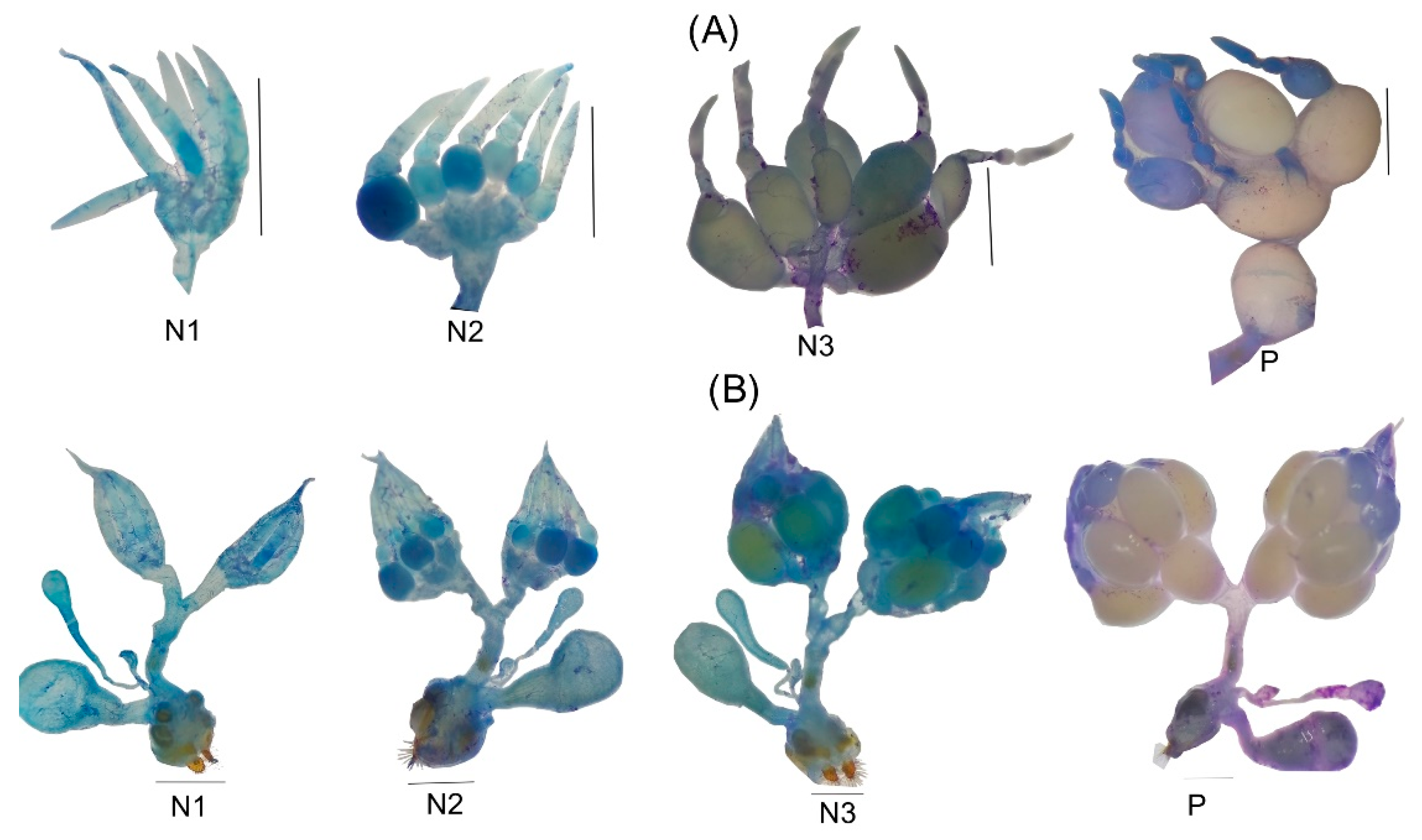
2.3. Gonadic Maturation
2.4. Allometry
Statistical Analysis
3. Results
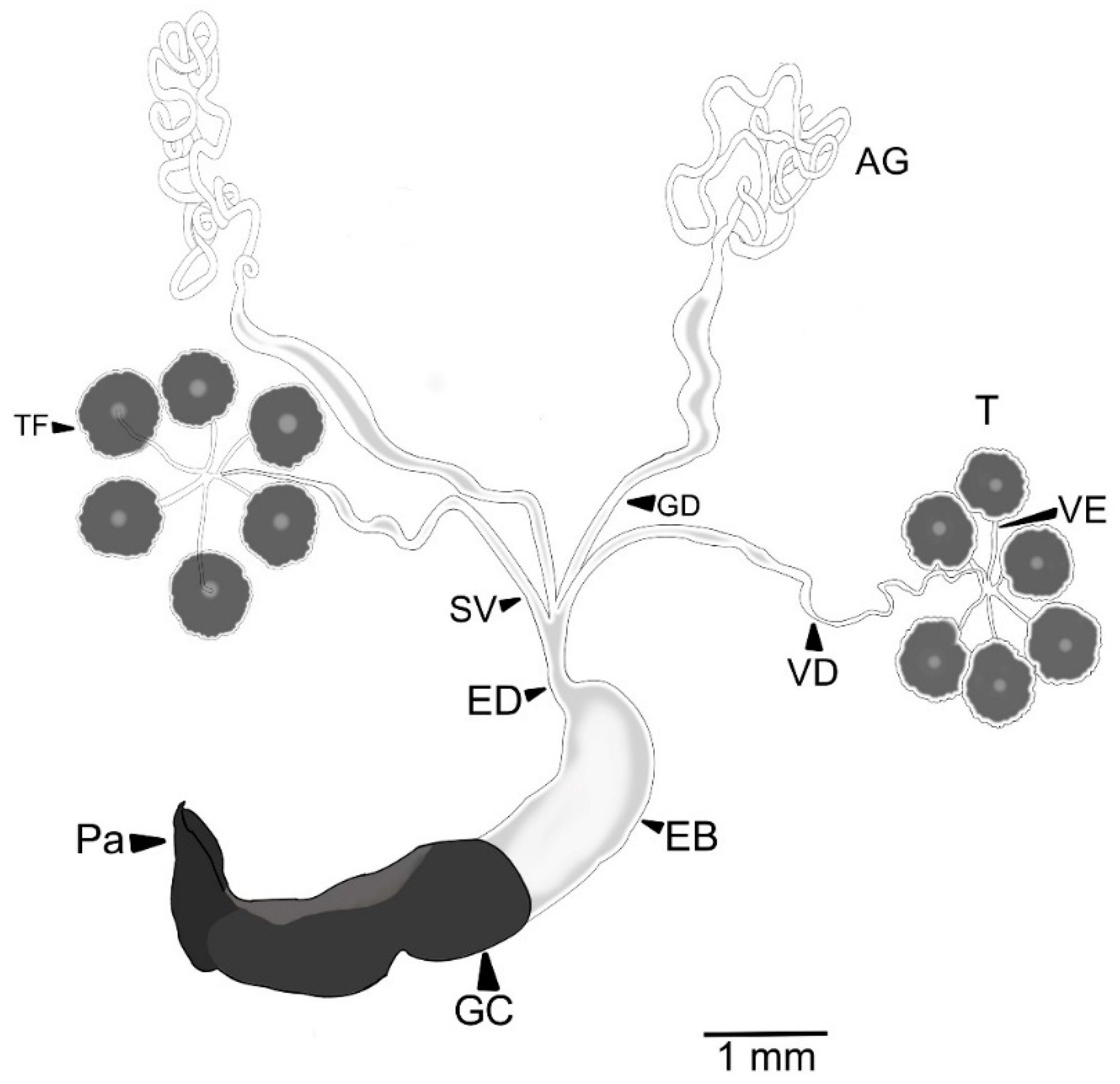
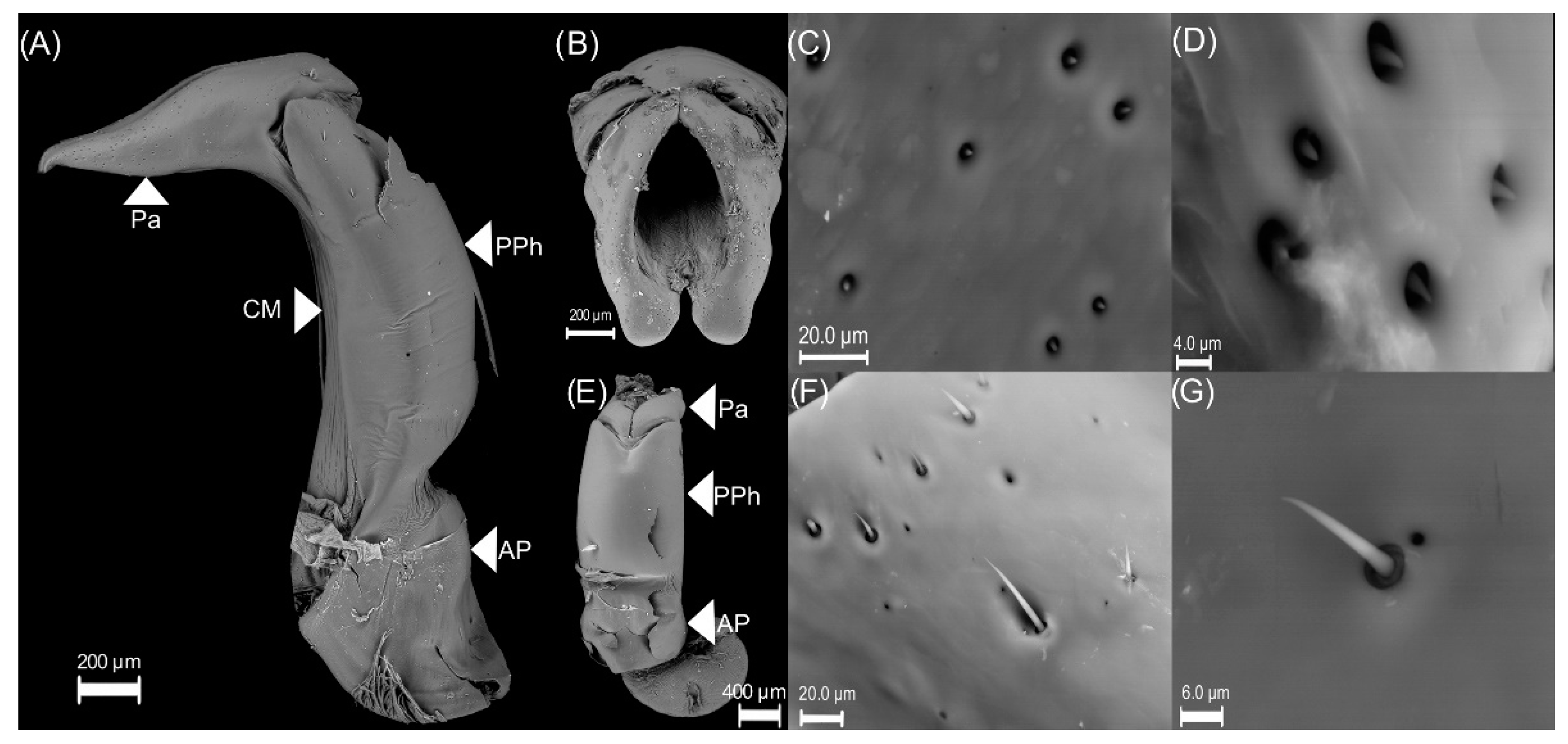
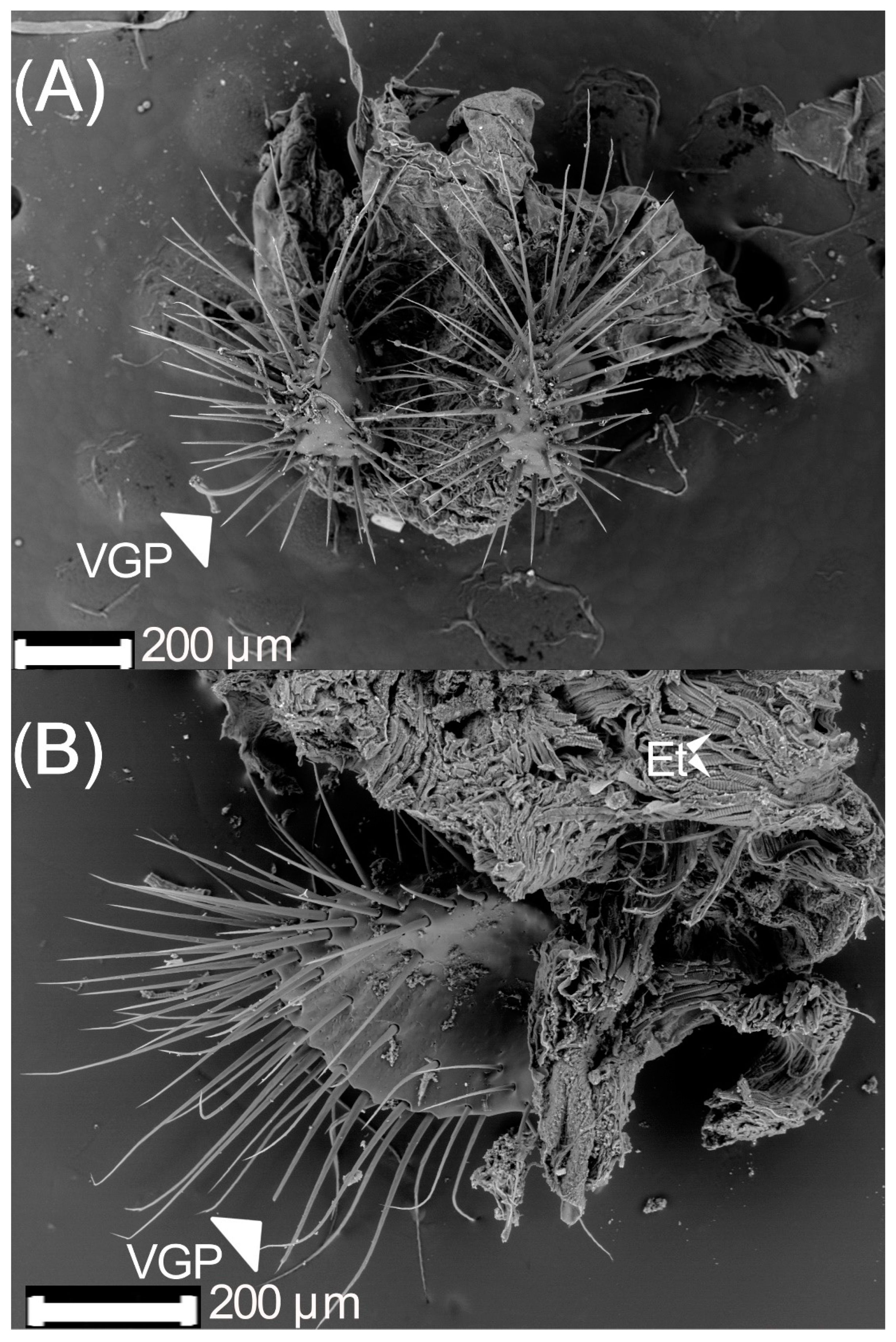
3.1. Male Reproductive Apparatus
- (1)
- The connecting membrane (Figure 3A) is in the ventral zone, connecting the phallobase to parameres.
- (2)
- (3)
- Posterior phallobase and anterior phallobase (Figure 3E). The posterior phallobase is a sub-cylindric structure that involves the aedeagus and connects to the parameres. It is made up of one piece and presents a high diversity of ultrastructure (Figure 3F). The posterior phallobase has four types of ultrastructure: (1) large needle, (2) small needle, (3) pits (Figure 3F), and (4) small seta with a circular base (Figure 3G).
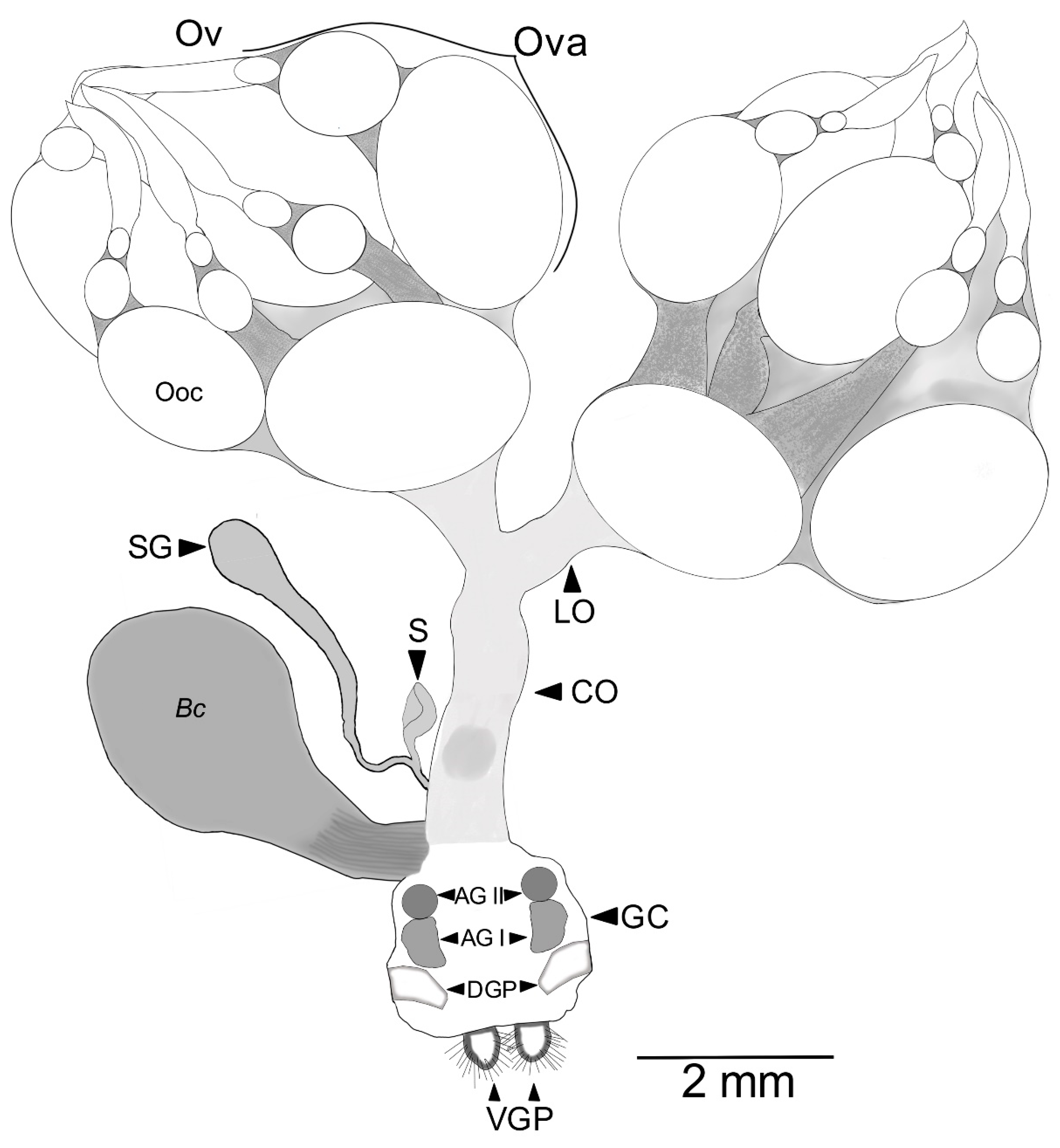
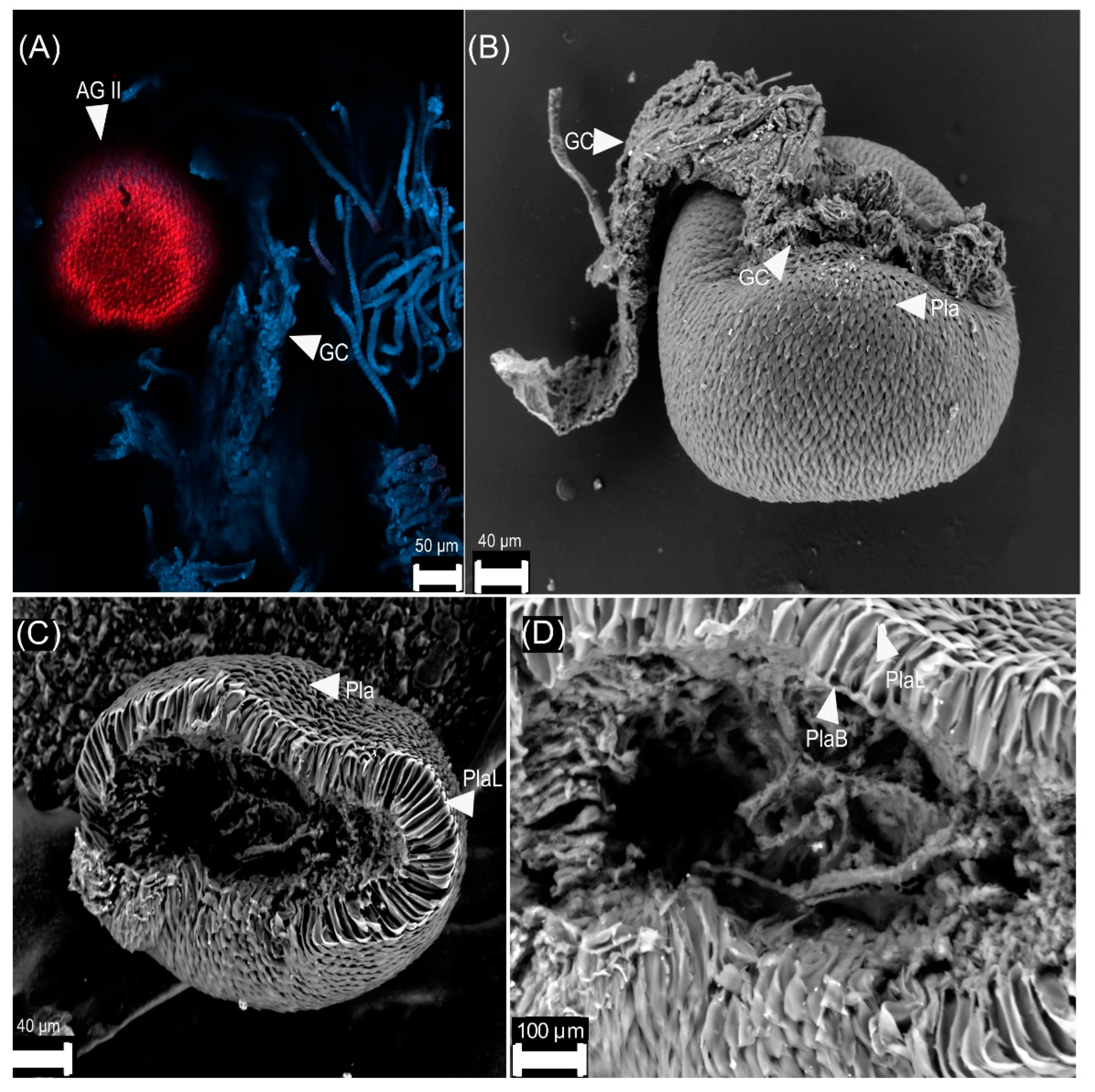
3.2. Female Reproductive Apparatus
- (1)
- Dorsal plates are sclerotized rectangular shape structures. They insert near the accessory glands, around the genital chamber.
- (2)
- (1)
- Type I is oval-shaped, and it inserts in the genital chamber (Figure 5).
- (2)
3.3. Allometry in Cyclocephala barrerai
3.3.1. Relationships between Body Parts and Weight
3.3.2. Sexual Dimorphism in Lab-Reared Adults
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thornhill, R.; Alcock, J. The Evolution of Insect Mating Systems; Harvard University Press: London, UK, 2013; pp. 28–50. [Google Scholar]
- Chen, S. Biochemistry of insect male accessory glands. Annu. Rev. Entomol. 1984, 29, 233–255. [Google Scholar] [CrossRef]
- Honěk, A. Intraspecific variation in body size and fecundity in insects: A general relationship. Oikos 1993, 66, 492. [Google Scholar] [CrossRef]
- Roff, D.A. Life History Evolution; Sinauer Associates: Sunderland, MA, USA, 2002. [Google Scholar]
- Wilson, D.S.; Knollenberg, W.G. Food discrimination and ovarian development in burying beetles (Coleoptera: Silphidae: Nicrophorus). Ann. Entomol. Soc. Am. 1984, 77, 165–170. [Google Scholar] [CrossRef]
- Gillott, C. Male accessory gland secretions: Modulators of female reproductive physiology and behavior. Annu. Rev. Entomol. 2003, 48, 163–184. [Google Scholar] [CrossRef]
- Zhao, M.-T.; Wang, Y.; Zhou, Z.-S.; Wang, R.; Guo, J.-Y.; Wan, F.-H. Effects of periodically repeated heat events on reproduction and ovary development of Agasicles hygrophila (Coleoptera: Chrysomelidae). J. Econ. Entomol. 2016, 109, 1586–1594. [Google Scholar] [CrossRef]
- Benítez-Herrera, L.N.; Martínez-Morales, I.; Romero-López, A.A. Anatomy of the reproductive system of Macrodactylus mexicanus (Coleoptera: Scarabaeoidea: Melolonthidae) and its possible relationship with sexual chemical communication. Southwest. Entomol. 2015, 40, 189–198. [Google Scholar] [CrossRef]
- Martínez-Morales, I.; Rivera-Gasperín, S.L.; Carrillo-Ruiz, H.; Morón, M.A. Comparative aspects of the internal reproductive system of males in species of Melolonthinae, Dynastinae, and Rutelinae (Coleoptera: Scarabaeoidea) from Mexico. Acta Zool. Mex. 2021, 37, 1–17. [Google Scholar] [CrossRef]
- Cristóvão, J.P.; Vaz-de-Mello, F.Z. The terminalia of the superfamily Scarabaeoidea (Coleoptera): Specific glossary, dissecting methodology, techniques and previously unrecorded sexual dimorphism in some difficult groups. Zool. J. Linn. Soc. 2021, 191, 1001–1043. [Google Scholar] [CrossRef]
- Sanmartín, I.; Martín-Piera, F. First phylogenetic analysis of the subfamily Pachydeminae (Coleoptera, Scarabaeoidea, Melolonthidae): The Palearctic Pachydeminae. J. Zool. Syst. Evol. Res. 2003, 41, 2–46. [Google Scholar] [CrossRef]
- Richmond, M.P.; Park, J.; Henry, C.S. The function and evolution of male and female genitalia in Phyllophaga Harris scarab beetles (Coleoptera: Scarabaeidae). J. Evol. Biol. 2016, 29, 2276–2288. [Google Scholar] [CrossRef]
- Carrillo-Ruiz, H.; Martínez, M.I.; Morón, M.-A. Comparative study of the reproductive system of two species of Hoplia (Coleoptera: Scarabaeidae: Hopliinae). Proc. Entomol. Soc. Wash. 2008, 110, 778–788. [Google Scholar] [CrossRef]
- Romero-López, A.; Arzuffi, R.; Valdez, J.; Sánchez-Espíndola, E.; Morón, M.A. Tissues involved in sex pheromone production in Phyllophaga obsoleta (Coleoptera: Scarabaeoidea: Melolonthidae). Ann. Entomol. Soc. Am. 2011, 104, 960–965. [Google Scholar] [CrossRef]
- Yi-Zhen, L.; Lian-Xin, L.; Yan-Jie, S. Variation in the reproduction of some phytophagous chafers and its bearing on pest control. Acta Entomol. Sin. 1987, 30, 152. [Google Scholar]
- Stringer, I.A.N. The female reproductive system of Costelytra zealandica (White) (Coleoptera: Scarabaeidae: Melolonthinae). N. Z. J. Zool. 1988, 15, 513–533. [Google Scholar] [CrossRef]
- Gayon, J. History of the concept of allometry. Am. Zool. 2000, 40, 748–758. [Google Scholar] [CrossRef]
- Simmons, L.W.; Tomkins, J.L.; Hunt, J. Sperm competition games played by dimorphic male beetles. Proc. R. Soc. B Biol. Sci. 1999, 266, 145–150. [Google Scholar] [CrossRef]
- Okada, K.; Miyatake, T. Sexual dimorphism in mandibles and male aggressive behavior in the presence and absence of females in the beetle Librodor japonicus (Coleoptera: Nitidulidae). Ann. Entomol. Soc. Am. 2004, 97, 1342–1346. [Google Scholar] [CrossRef]
- Okada, K.; Miyatake, T.; Nomura, Y.; Kuroda, K. Fighting, dispersing, and sneaking: Body-size dependent mating tactics by male Librodor japonicus beetles. Ecol. Entomol. 2008, 33, 269–275. [Google Scholar] [CrossRef]
- Cayetano, L.; Maklakov, A.A.; Brooks, R.C.; Bonduriansky, R. Evolution of male and female genitalia following release from sexual selection. Evolution 2011, 65, 2171–2183. [Google Scholar] [CrossRef]
- Rowland, J.M.; Qualls, C.R.; Beaudoin-Ollivier, L. Discrimination of alternative male phenotypes in Scapanes australis (Boisduval) (Coleoptera: Scarabaeidae: Dynastinae). Aust. J. Entomol. 2005, 44, 22–28. [Google Scholar] [CrossRef]
- McCullough, E.L.; Ledger, K.J.; O’Brien, D.M.; Emlen, D.J. Variation in the allometry of exaggerated rhinoceros beetle horns. Anim. Behav. 2015, 109, 133–140. [Google Scholar] [CrossRef]
- Álvarez, H.; Carrillo-Ruiz, H.; Morón, M. Horns positive allometry in a Mexican population of Strategus aloeus (L.) (Coleoptera: Scarabaeoidea: Dynastinae). Entomotropica 2013, 28, 87–94. [Google Scholar]
- Johnson, J.P. Cyclocephala (ochrosidia) borealis in Connecticut 1. J. Agric. Res. 1941, 62, 79–86. [Google Scholar]
- Maia, A.C.D.; Schlindwein, C. Caladium bicolor (Araceae) and Cyclocephala celata (Coleoptera, Dynastinae): A well-established pollination system in the Northern Atlantic rainforest of Pernambuco, Brazil. Plant Biol. 2006, 8, 529–534. [Google Scholar] [CrossRef]
- Duchini, P.G.; Echeverria, J.R.; Américo, L.F.; Guzatti, G.C.; Cherman, M.A.; Sbrissia, A.F. White grubs (Cyclocephala flavipennis) damaging perennial winter pastures in the south region of Brazil. Ciência Rural 2017, 47, e20160662. [Google Scholar] [CrossRef][Green Version]
- Parizotto, D.R.; Grossi, P.C. Revisiting pollinating Cyclocephala scarab beetles (Coleoptera: Melolonthidae: Dynastinae) associated with the soursop (Annona muricata, Annonaceae). Neotrop. Entomol. 2019, 48, 415–421. [Google Scholar] [CrossRef]
- Martínez-Morales, I.; Morón, M.A. Female reproductive systems in Melolonthinae, Rutelinae, and Dynastinae (Coleoptera: Scarabaeoidea, Melolonthidae). Southwest. Entomol. 2015, 40, 369–386. [Google Scholar] [CrossRef]
- Sanchez-Cruz, A.; Robledo, N.; Rosete-Enríquez, M.; Romero-López, A.A. Attraction of adults of Cyclocephala lunulata and Cyclocephala barrerai (Coleoptera: Scarabaeoidea: Melolonthidae) towards Bacteria Volatiles Isolated from Their Genital Chambers. Molecules 2020, 25, 4430. [Google Scholar] [CrossRef]
- Aragón, A.; Morón, M.A.; Tapia-Rojas, A.M.; Rojas-García, R. Fauna de Coleoptera Melolonthidae en el rancho “La Joya”, Atlixco, Puebla, México. Acta Zool. Mex. 2001, 164, 143–164. [Google Scholar] [CrossRef]
- Morón, M.A.; Lugo-García, G.A.; Aragón-García, A. Description of the third instar larvae of five species of Cyclocephala (Coleoptera, Melolonthidae, Dynastinae) from Mexico. Rev. Bras. Entomol. 2014, 58, 219–228. [Google Scholar] [CrossRef]
- Moore, M.R.; Cave, R.D.; Branham, M.A. Annotated catalog and bibliography of the cyclocephaline scarab beetles (Coleoptera, Scarabaeidae, Dynastinae, Cyclocephalini). Zookeys 2018, 745, 101–378. [Google Scholar] [CrossRef] [PubMed]
- Tai, S.S.; Tang, X.M. Manipulating biological samples for environmental scanning electron microscopy observation. Scanning 2001, 23, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Kirk, S.E.; Skepper, J.N.; Donald, A.M. Application of environmental scanning electron microscopy to determine biological surface structure. J. Microsc. 2009, 233, 205–224. [Google Scholar] [CrossRef]
- Carson, F.L.; Martin, J.H.; Lynn, J.A. Formalin fixation for electron microscopy: A re-evaluation. Am. J. Clin. Pathol. 1973, 59, 365–373. [Google Scholar] [CrossRef]
- García-Hernández, C.; Rojo-Rubio, R.; Olmedo-Juárez, A.; Zamilpa, A.; Mendoza de Gives, P.; Antonio-Romo, I.A.; Aguilar-Marcelino, L.; Arece-García, J.; Tapia-Maruri, D.; González-Cortazar, M. Galloyl derivatives from Caesalpinia coriaria exhibit in vitro ovicidal activity against cattle gastrointestinal parasitic nematodes. Exp. Parasitol. 2019, 200, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Núñez, E.J.; Zamilpa, A.; González-Cortazar, M.; Olmedo-Juárez, A.; Cardoso-Taketa, A.; Sánchez-Mendoza, E.; Tapia-Maruri, D.; Salinas-Sánchez, D.O.; Mendoza-De Gives, P. Isorhamnetin: A nematocidal flavonoid from Prosopis laevigata leaves against Haemonchus contortus eggs and larvae. Biomolecules 2020, 10, 773. [Google Scholar] [CrossRef]
- Grodowitz, M.J.; Brewer, F.D. Ovarian anatomy and physiological age-grading of the female boll weevil, Anthonomus grandis grandis Boheman (Coleoptera: Curculionidae). Ann. Entomol. Soc. Am. 1987, 80, 642–651. [Google Scholar] [CrossRef]
- Rasband, W.S. ImageJ Version 1.53s, Rasband, IL, USA; US National Institutes of Health: Bethesda, MD, USA, 1997–2009.
- Wang, Q.; Zeng, W.-Y.; Li, J.-S. Reproductive behavior of Paraglenea fortunei (Coleoptera: Cerambycidae). Ann. Entomol. Soc. Am. 1990, 83, 860–866. [Google Scholar] [CrossRef]
- Hoffman, G.D.; Raffa, K.F. Maturation of the male pales weevil (Coleoptera: Curculionidae) reproductive system and its effect on male response to females. Ann. Entomol. Soc. Am. 1992, 85, 571–577. [Google Scholar] [CrossRef]
- Wang, Q.; Shi, G.; Davis, L.K. Reproductive potential and daily reproductive rhythms of Oemona hirta (Coleoptera: Cerambycidae). J. Econ. Entomol. 1998, 91, 1360–1365. [Google Scholar] [CrossRef]
- Breeschoten, T.; Clark, D.R.; Schilthuizen, M. Evolutionary patterns of asymmetric genitalia in the beetle tribe cyclocephalini (Coleoptera: Scarabaeidae: Dynastinae). Contrib. Zool. 2013, 82, 95–106. [Google Scholar] [CrossRef]
- Kim, J.Y.; Leal, W.S. Ultrastructure of pheromone-detecting sensillum placodeum of the Japanese beetle, Popillia japonica Newmann (Coleoptera: Scarabaeidae). Arthropod Struct. Dev. 2000, 29, 121–128. [Google Scholar] [CrossRef]
- Romero-López, A.; Morón, M.; Valdez, J. Sexual dimorphism in antennal receptors of Phyllophaga ravida Blanchard (Coleoptera: Scarabaeoidea: Melolonthidae). Neotrop. Entomol. 2010, 39, 957–966. [Google Scholar] [CrossRef] [PubMed]
- Bal, N.; Özkdikmen, H. Aedeagus structure of Cassida prasina Illiger, 1798 (Coleoptera: Chrysomelidae: Cassidinae) in Scanning Electrone Microscope (SEM). KSU J. Agric. Nat. 2020, 23, 748–753. [Google Scholar] [CrossRef]
- Özdikmen, Ü.; Bal, N.; Amutkan Mutlu, D.; Suludere, Z. A SEM study of the aedeagus and spermatheca of Cassida viridis Linnaeus, 1758 (Coleoptera: Chrysomelidae: Cassidinae) from Turkey. Turk. Entomoloji Derg. 2020, 44, 355–364. [Google Scholar] [CrossRef]
- Özyurt Koçakoğlu, N.; Candan, S.; Güllü, M. Anatomy and histology of reproductive system of adult male mint leaf beetle Chrysolina herbacea (Duftschmid, 1825) (Coleoptera: Chrysomelidae). Microsc. Res. Tech. 2021, 84, 512–520. [Google Scholar] [CrossRef]
- Acebes, A.; Cobb, M.; Ferveur, J.F. Species-specific effects of single sensillum ablation on mating position in Drosophila. J. Exp. Biol. 2003, 206, 3095–3100. [Google Scholar] [CrossRef]
- Schubert, L.F.; Krüger, S.; Moritz, G.B.; Schubert, V. Male reproductive system and spermatogenesis of Limodromus assimilis (Paykull 1790). PLoS ONE 2017, 12, e0180492. [Google Scholar] [CrossRef]
- Pascini, T.V.; Martins, G.F. The insect spermatheca: An overview. Zoology 2017, 121, 56–71. [Google Scholar] [CrossRef]
- Eberhard, W.G. Rapid divergent evolution of sexual morphology: Comparative tests of antagonistic coevolution and traditional female choice. Evolution 2004, 58, 1947–1970. [Google Scholar] [CrossRef]
- Shapiro, A. The lock-and-key hypothesis: Evolutionary and biosystematic interpretation of insect genitalia. Annu. Rev. Entomol. 1989, 34, 231–245. [Google Scholar] [CrossRef]
- Fernadez, M.; Salgado, J.; Pajares, J. The seasonal development of the gonads and fat content of Tomicus minor (Coleoptera Scolytidae). Belg. J. Entomol. 1999, 1, 311–324. [Google Scholar]
- Vega-Petlacalco, M.; Arzuffi, R.; Valdez, J.; Rodríguez-Monroy, M.; Jiménez-Pérez, A.; Robledo, N. Food quality influences ovarian development in Scyphophorus acupunctatus (Coleoptera: Dryophthoridae). Fla. Entomol. 2018, 101, 447–452. [Google Scholar] [CrossRef]
- Sasakawa, K. Diet affects male gonad maturation, female fecundity, and larval development in the granivorous ground beetle Anisodactylus punctatipennis. Ecol. Entomol. 2009, 34, 406–411. [Google Scholar] [CrossRef]
- Ghoneim, K.K.; Abdel-Khaliq, A.A.; Bream, A.S.; Emam, D.M. Effects of food type on the adult performace of black blister beetle Meloe proscaeabaeus (Coleoptera: Meloidae). Int. J. Biol. Sci. 2012, 1, 5–17. [Google Scholar]
- Zhou, P.; Yang, H.; Jin, D.C.; He, X.Z.; Wang, Q. Sex-specific allometry of morphometric and reproductive traits in oriental fruit flies (Diptera: Tephritidae). J. Econ. Entomol. 2016, 109, 1249–1253. [Google Scholar] [CrossRef]
- Carne, P.B. Cyclocephala signaticollis Burmeister, an introduced pasture scarab (Coleoptera). Proc. Linn. Soc. N. S. W. 1957, 81, 217–221. [Google Scholar]
- Stern, D.L.; Emlen, D.J. The developmental basis for allometry in insects. Development 1999, 126, 1091–1101. [Google Scholar] [CrossRef]
- Vera-Cano, D.A.; Álvarez, H.A.; Morón, M.A. Positive allometry of horns in the rhinoceros beetle Golofa xiximeca does not follow breaking-point patterns. Southwest. Entomol. 2017, 42, 933–940. [Google Scholar] [CrossRef]
- Moczek, A.P. Pupal remodeling and the evolution and development of alternative male morphologies in horned beetles. BMC Evol. Biol. 2006, 7, 711–729. [Google Scholar] [CrossRef]
- Knell, R.J.; Pomfret, J.C.; Tomkins, J.L. The limits of elaboration: Curved allometries reveal the constraints on mandible size in stag beetles. Proc. R. Soc. B Biol. Sci. 2004, 271, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Heddleston, J.M.; Aaron, J.S.; Khuon, S.; Chew, T.L. A guide to accurate reporting in digital image acquisition–can anyone replicate your microscopy data? J. Cell Sci. 2021, 134, jcs254144. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.; McNeill, B.; Irwin, M. Nondestructive Imaging of Pin-Mounted Museum Insect Specimens Using the Field-Emission Environmental Scanning Electron Microscope (ESEM-FEG). Microsc. Microanal. 1999, 5, 338–339. [Google Scholar] [CrossRef]


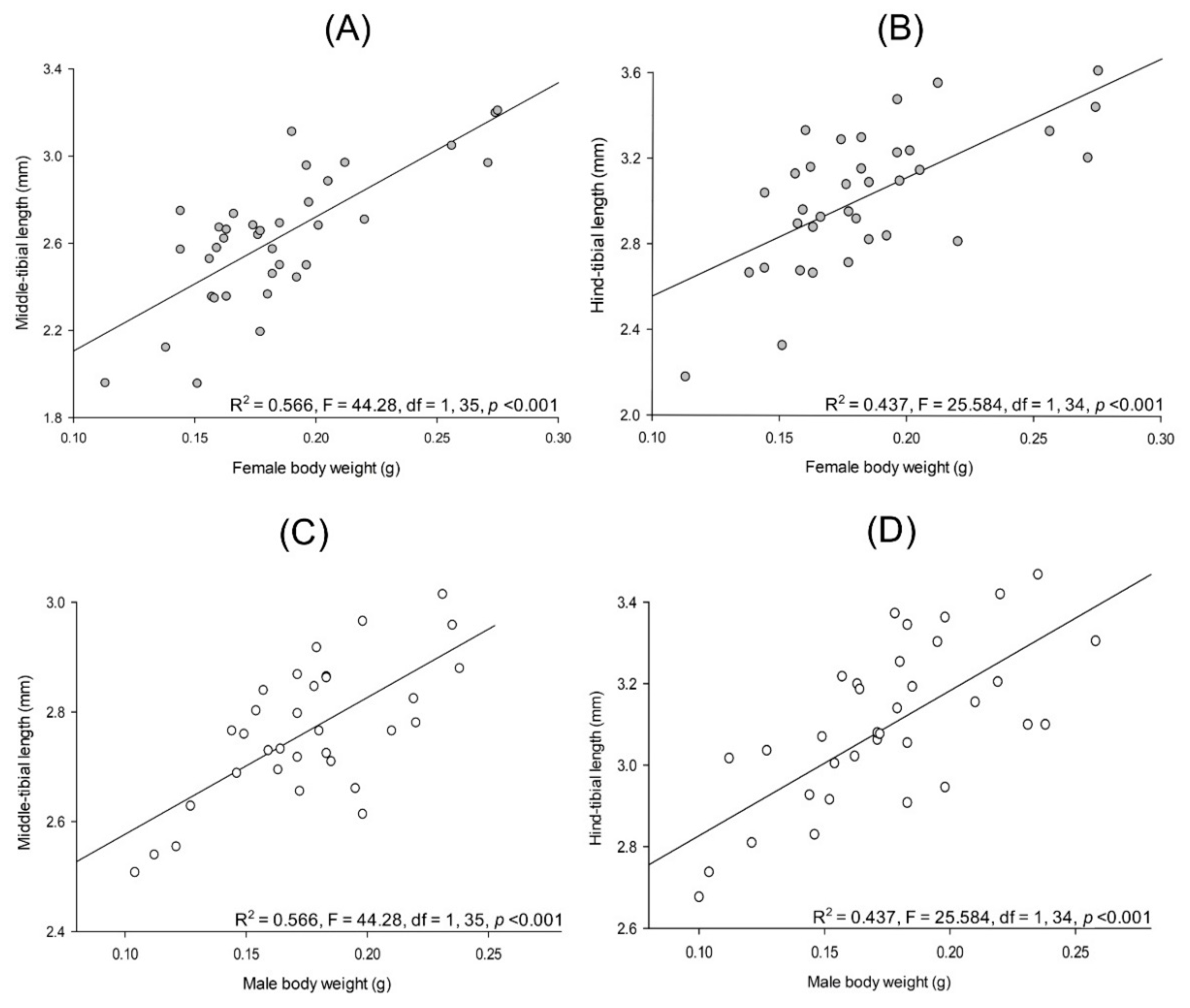
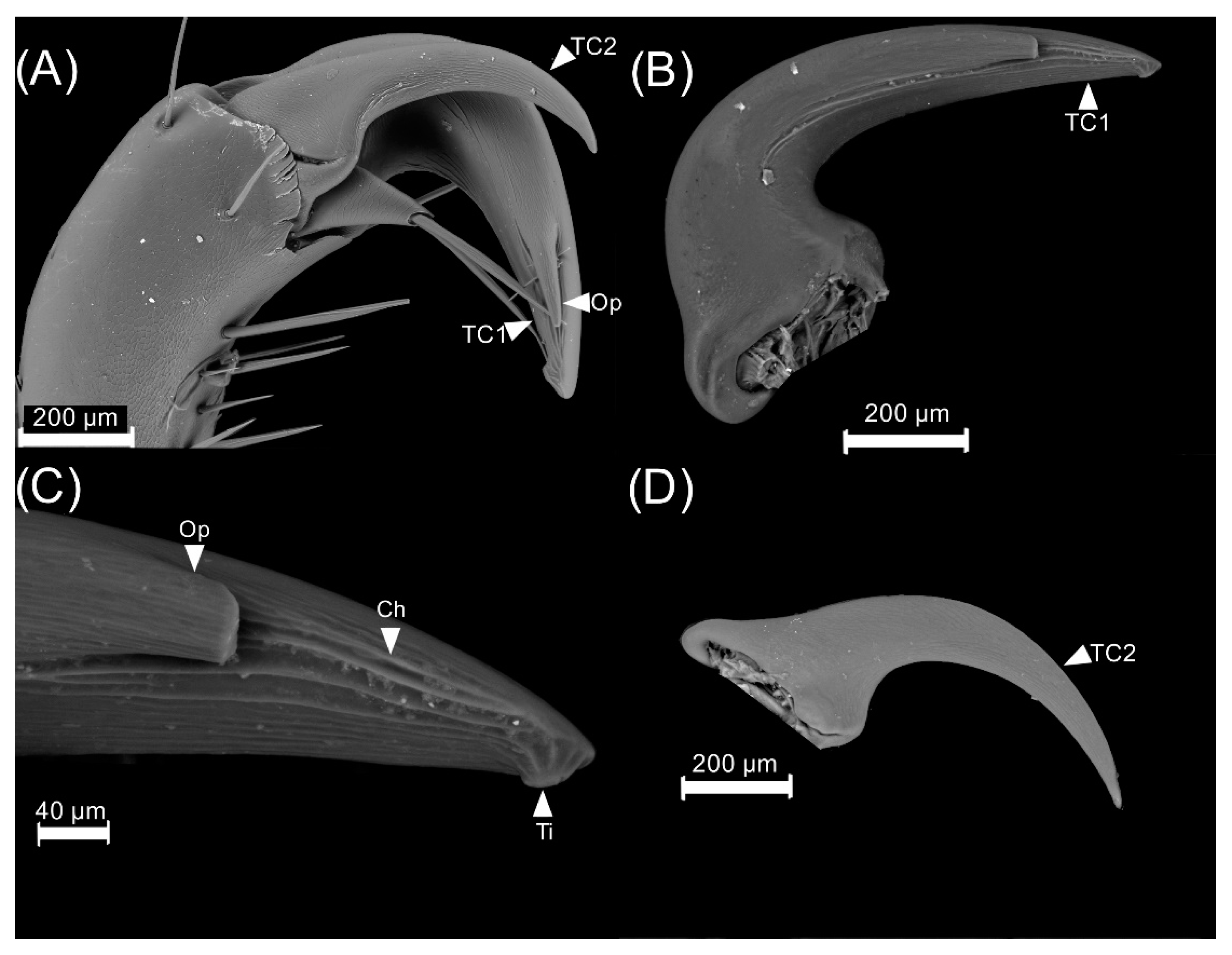
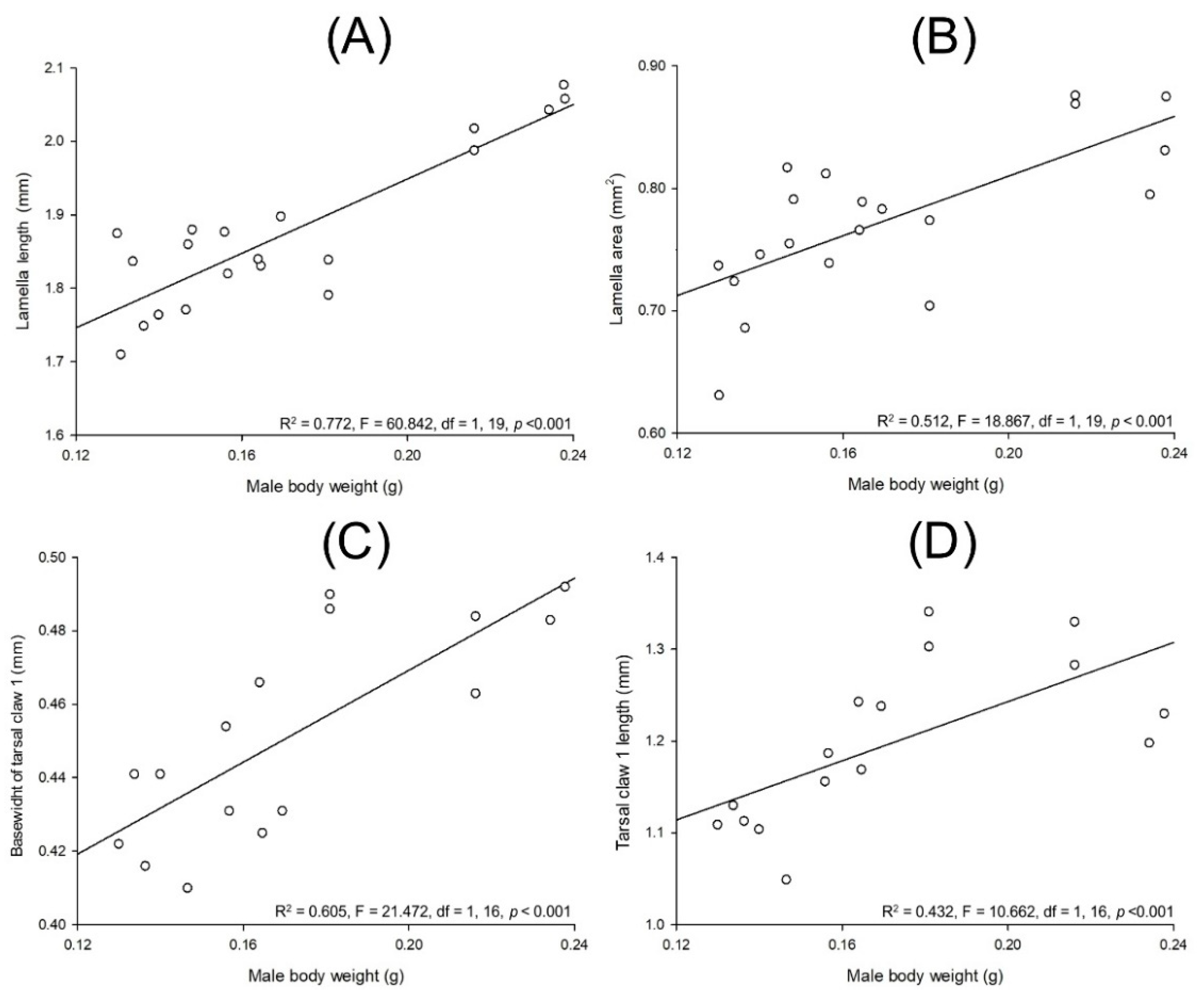

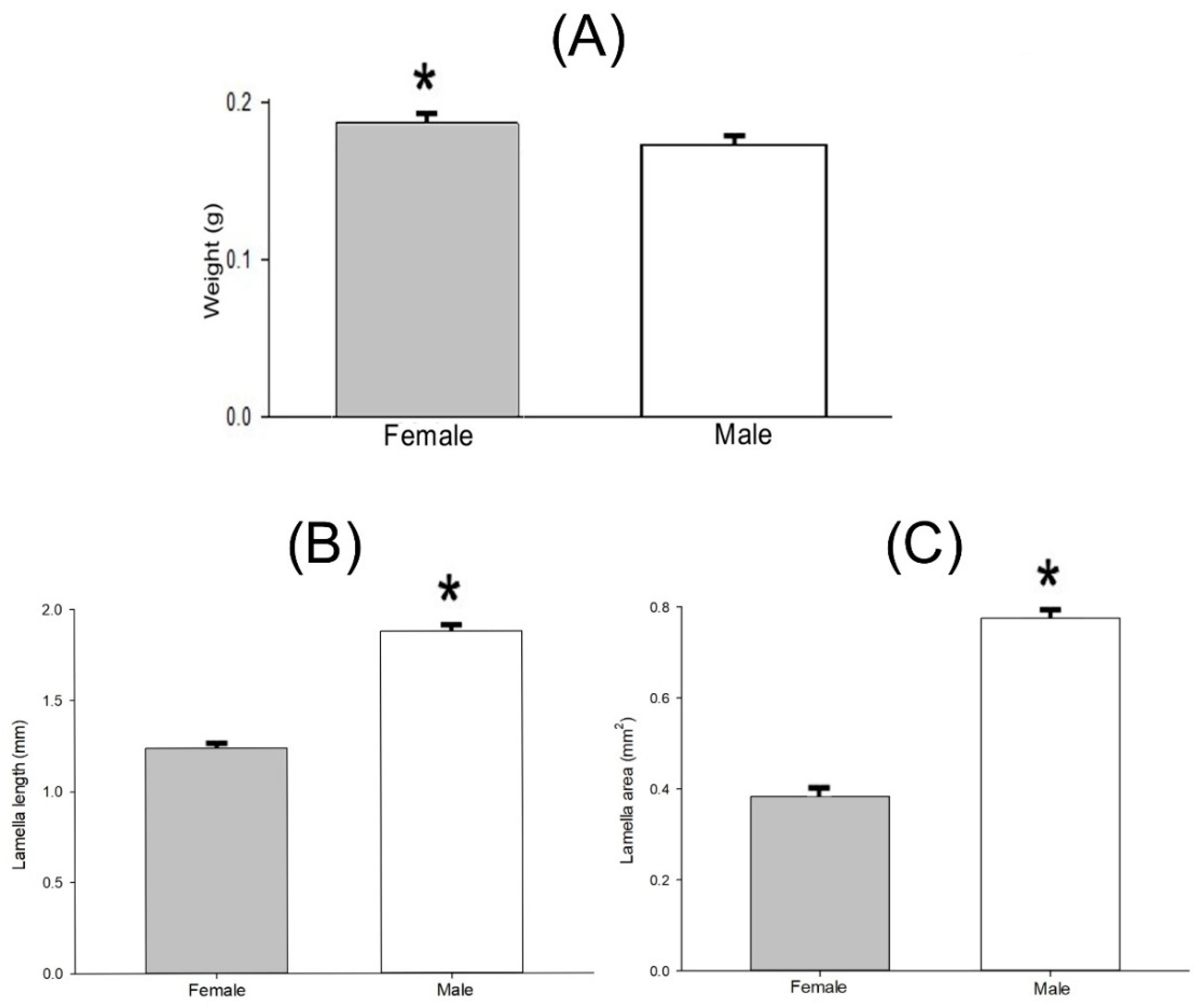
| Structure | Lab-Reared Adults Mean ± SEM (n) | |
|---|---|---|
| Female | Male | |
| Pupa weight | 0.308 ± 0.006 g (58) | 0.291 ± 0.006 g (58) |
| Adult weight | 0.188 ± 0.004 g (58) | 0.175 ± 0.004 g (58) |
| Middle-tibial length | 2.625 ± 0.051 mm (36) | 2.770 ± 0.022 mm (33) |
| Hind-tibial length | 3.025 ± 0.054 mm (35) | 3.077 ± 0.048 mm (36) |
| Lamella length | 1.236 ± 0.089 mm (21) | 1.876 ± 0.024 mm (20) |
| Lamella area | 0.383 ± 0.015 mm2 (16) | 0.775 ± 0.014 mm2 (20) |
| Tarsal claw 1 length | - | 1.199 ± 0.0218 mm (16) |
| Tarsal claw 1 width base | - | 0.452 ± 0.007 mm (16) |
| Tarsal claw 2 length | - | 0.839 ± 0.065 mm (15) |
| Tarsal claw 2 width base | - | 0.0388 ± 0.008 mm (15) |
| Genital chamber length | 1.993 ± 0.109 mm (19) | - |
| Genital chamber area | 3.875 ± 0.291 mm2 (16) | - |
| Bursa copulatrix length | 4.223 ± 0.145 mm (27) | - |
| Bursa copulatrix area | 5.089 ± 0.357 mm2 (26) | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez-Cruz, A.; Tapia-Maruri, D.; Jiménez-Pérez, A. Reproductive Apparatus, Gonadic Maturation, and Allometry of Cyclocephala barrerai Martínez (Coleoptera: Melolonthidae: Dynastinae). Insects 2022, 13, 638. https://doi.org/10.3390/insects13070638
Sanchez-Cruz A, Tapia-Maruri D, Jiménez-Pérez A. Reproductive Apparatus, Gonadic Maturation, and Allometry of Cyclocephala barrerai Martínez (Coleoptera: Melolonthidae: Dynastinae). Insects. 2022; 13(7):638. https://doi.org/10.3390/insects13070638
Chicago/Turabian StyleSanchez-Cruz, Abraham, Daniel Tapia-Maruri, and Alfredo Jiménez-Pérez. 2022. "Reproductive Apparatus, Gonadic Maturation, and Allometry of Cyclocephala barrerai Martínez (Coleoptera: Melolonthidae: Dynastinae)" Insects 13, no. 7: 638. https://doi.org/10.3390/insects13070638
APA StyleSanchez-Cruz, A., Tapia-Maruri, D., & Jiménez-Pérez, A. (2022). Reproductive Apparatus, Gonadic Maturation, and Allometry of Cyclocephala barrerai Martínez (Coleoptera: Melolonthidae: Dynastinae). Insects, 13(7), 638. https://doi.org/10.3390/insects13070638





