A Simple CO2 Generating System Incorporated with CDC Light Trap for Sampling Mosquito Vectors
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
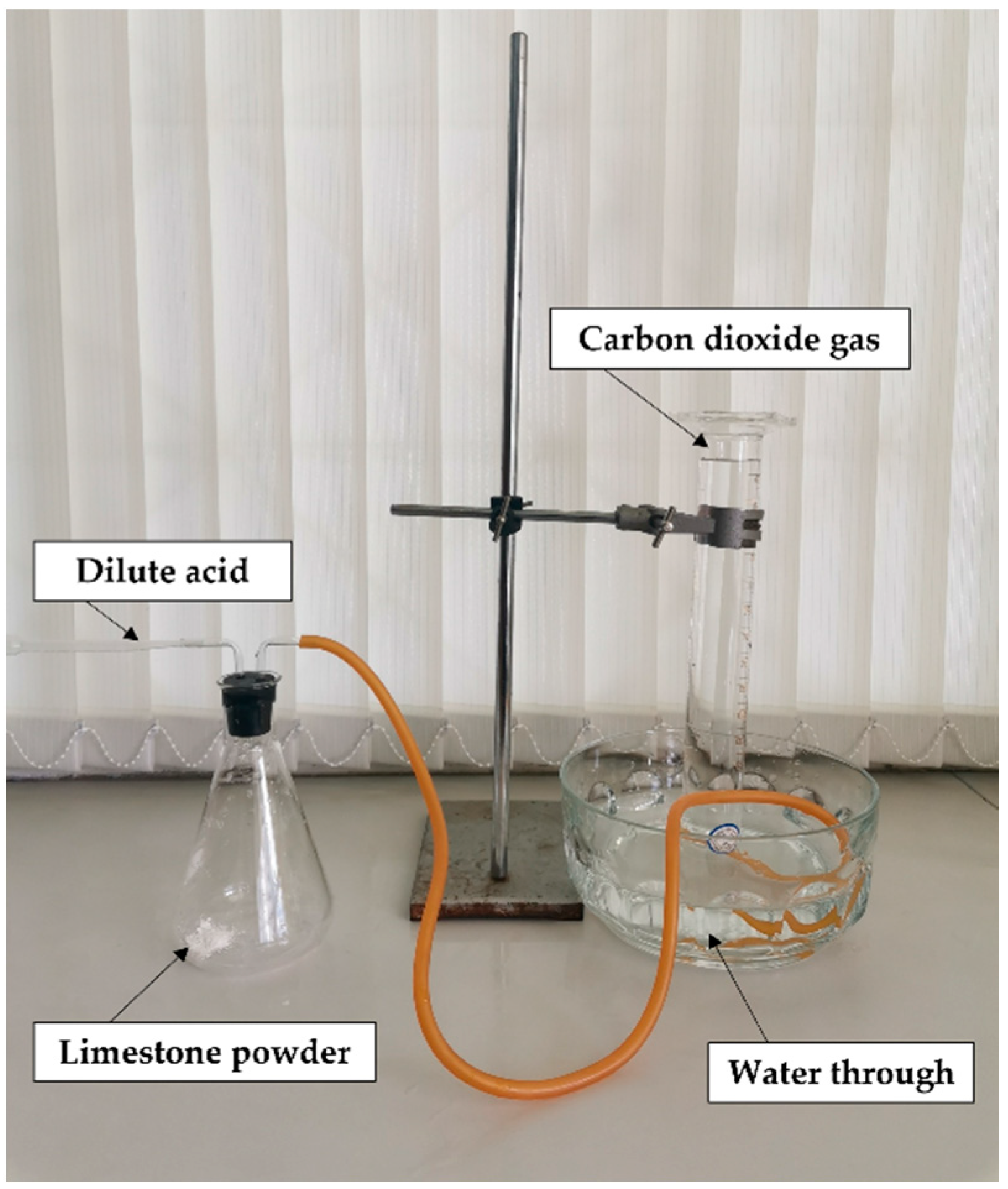
2.1. CO2 Production from Limestone
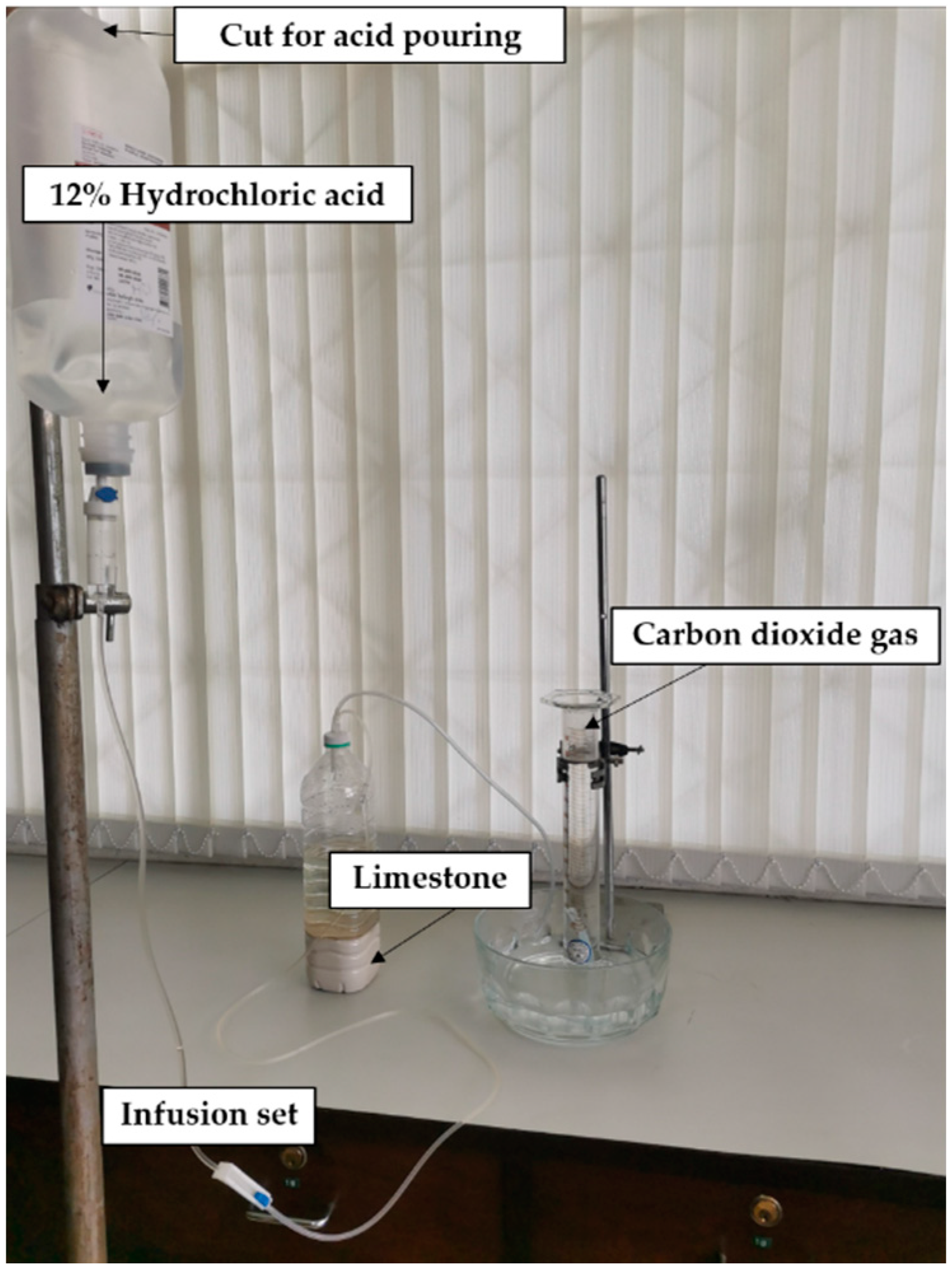
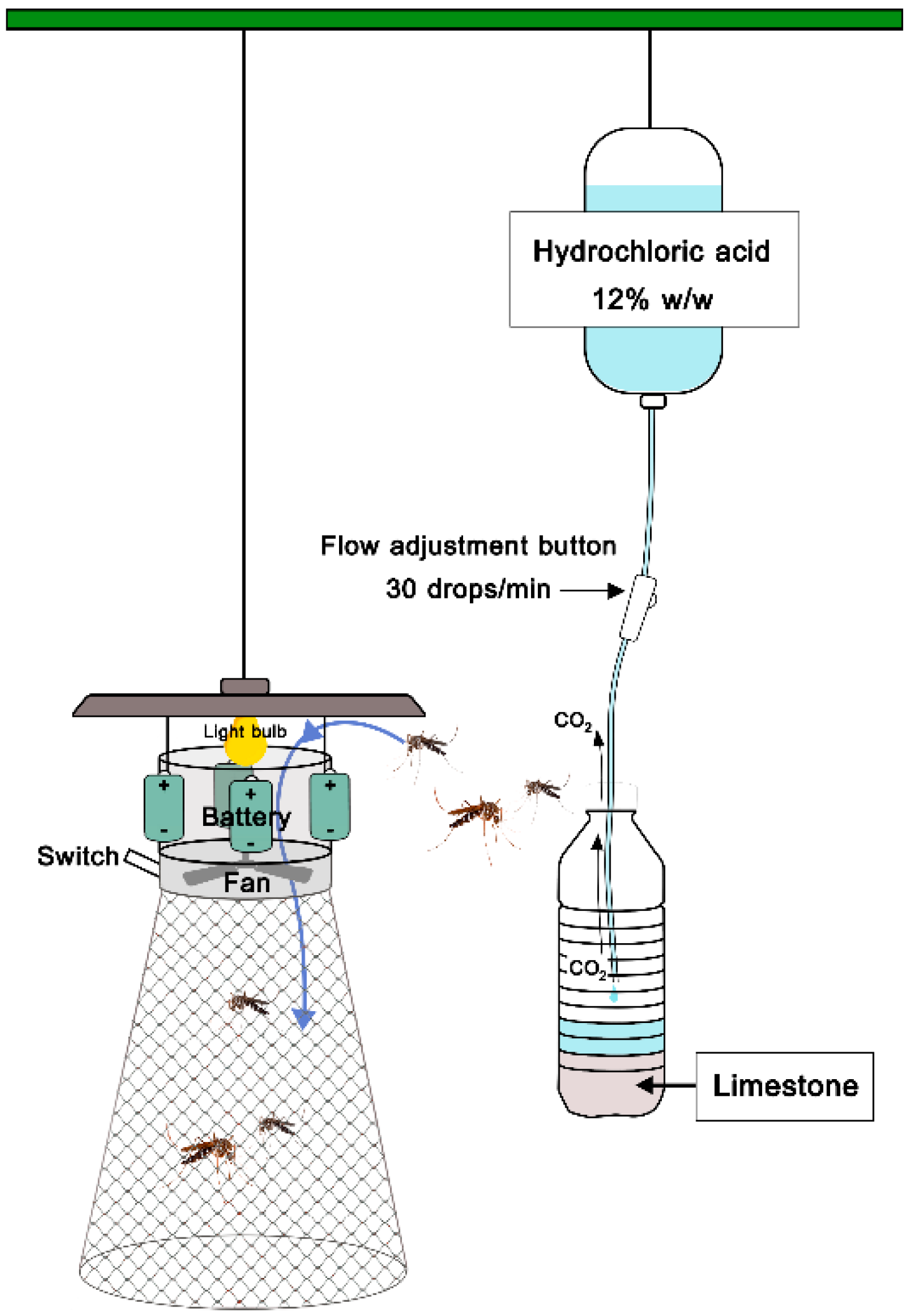
2.2. CO2 Generating System for Overnight Collection
2.3. Field Evaluation
2.3.1. Experimental Design
2.3.2. Mosquito Identification and Dissection
2.4. Statistical Analysis
3. Results
3.1. CO2 Production from Limestone
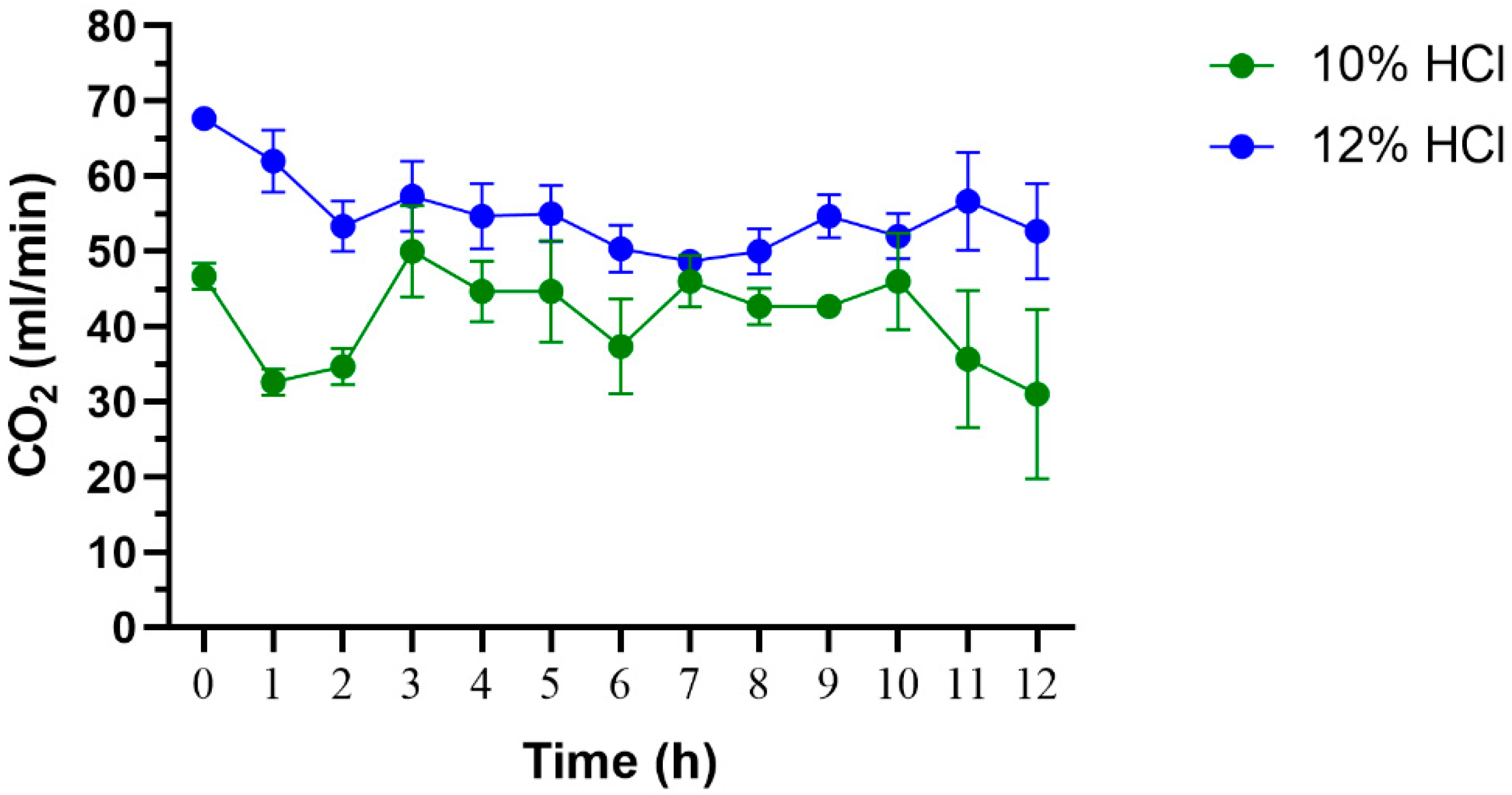
3.2. CO2 Generating System for Overnight Collection
3.3. Field Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leake, C.J. Mosquito Ecology Field Sampling Methods, 2nd ed.; M. W. Service. Barking; Elsevier Science Publishers: Essex, UK, 1993; pp. xiii + 988. [Google Scholar]
- Hoy, J.B. Trapping the stable fly by using CO2 or CO as attractants. J. Econ. Entomol. 1970, 63, 792–795. [Google Scholar] [CrossRef]
- Burkett, D.A.; Lee, W.J.; Lee, K.W.; Kim, H.C.; Lee, H.I.; Lee, J.S.; Shin, E.H.; Wirtz, R.A.; Cho, H.W.; Claborn, D.M.; et al. Light, carbon dioxide, and octenol-baited mosquito trap and host-seeking activity evaluations for mosquitoes in a malarious area of the Republic of Korea. J. Am. Mosq. Control Assoc. 2001, 17, 196–205. [Google Scholar] [PubMed]
- Saitoh, Y.; Hattori, J.; Chinone, S.; Nihei, N.; Tsuda, Y.; Kurahashi, H.; Kobayashi, M. Yeast-generated CO2 as a convenient source of carbon dioxide for adult mosquito sampling. J. Am. Mosq. Control Assoc. 2004, 20, 261–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steiger, D.B.; Ritchie, S.A.; Laurance, S.G. Overcoming the challenges of mosquito (Diptera: Culicidae) sampling in remote localities: A comparison of CO2 attractants on mosquito communities in three tropical forest habitats. J. Med. Entomol. 2014, 51, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Holscher, K.; Gearhart, H.L.; Barker, R.W. Electrophysiological responses of three tick species to carbon dioxide in the laboratory and field. Ann. Entomol. Soc. Am. 1980, 73, 288–292. [Google Scholar] [CrossRef]
- Butler, J.; Holscher, K.; Adeyeye, O.; Gibbs, E. Sampling techniques for burrow dwelling ticks in reference to potential African swine fever virus vectors. Acarol. VI/Ed. DA Griffiths CE Bowman 1984, 2, 1065–1074. [Google Scholar]
- Cançado, P.H.; Piranda, E.M.; Mourão, G.M.; Faccini, J.L. Spatial distribution and impact of cattle-raising on ticks in the Pantanal region of Brazil by using the CO2 tick trap. Parasitol. Res. 2008, 103, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Benante, J.P.; Fox, J.; Lawrence, K.; Fansiri, T.; Pongsiri, A.; Ponlawat, A.; Chaskopoulou, A. A comparative study of mosquito and sand fly (Diptera: Psychodidae: Phlebotominae) sampling using dry ice and chemically generated carbon dioxide from three different prototype CO2 generators. J. Econ. Entomol. 2019, 112, 494–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burkett-Cadena, N.D.; Blosser, E.M.; Young, R.M.; Toe, L.D.; Unnasch, T.R. Carbon dioxide generated from carbonates and acids for sampling blood-feeding arthropods. Acta Trop. 2015, 149, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Limestone, What Is Limestone and How Is It Used? Available online: https://geology.com/rocks/limestone.shtml (accessed on 7 June 2022).
- Charabidze, D.; Bourel, B.; Hedouin, V.; Gosset, D. Repellent effect of some household products on fly attraction to cadavers. Forensic Sci. Int. 2009, 189, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Boullis, A.; Mulatier, M.; Delannay, C.; Héry, L.; Verheggen, F.; Vega-Rúa, A. Behavioural and antennal responses of Aedes aegypti (L.) (Diptera: Culicidae) gravid females to chemical cues from conspecific larvae. PLoS ONE 2021, 16, e0247657. [Google Scholar] [CrossRef] [PubMed]
- Rattanarithikul, R.; Harbach, R.; Harrison, B.; Panthusiri, P.; Jones, J.; Coleman, R. Illustrated keys to the mosquitoes of Thailand. II. Genera Culex and Lutzia. Southeast Asian J. Trop. Med. Public Health 2005, 36 (Suppl. 2), 1–97. [Google Scholar] [PubMed]
- Rattanarithikul, R.; Harrison, B.A.; Harbach, R.E.; Panthusiri, P.; Coleman, R.E.; Panthusiri, P. Illustrated keys to the mosquitoes of Thailand. IV. Anopheles. Southeast Asian J. Trop. Med. Public Health 2006, 37 (Suppl. 2), 1–128. [Google Scholar] [PubMed]
- Rattanarithikul, R.; Harrison, B.; Panthusiri, P.; Peyton, E.; Coleman, R. Illustrated keys to the mosquitoes of Thailand III. Genera Aedeomyia, Ficalbia, Mimomyia, Hodgesia, Coquillettidia, Mansonia, and Uranotaenia. Southeast Asian J. Trop. Med. Public Health 2006, 37 (Suppl. 1), 1–85. [Google Scholar] [PubMed]
- Rattanarithikul, R.; Harbach, R.; Harrison, B.; Panthusiri, P.; Coleman, R.; Richardson, J. Illustrated keys to the mosquitoes of Thailand VI. Tribe Aedini. Southeast Asian J. Trop. Med. Public Health 2010, 41 (Suppl. 1), 1–225. [Google Scholar] [PubMed]
- Detinova, T.S. Age-Grouping Methods in Diptera of Medical Importance, with Special Reference to Some Vectors of Malaria; World Health Organization: Geneva, Switzerland, 1962. [Google Scholar]
- WHO. Manual on Practical Entomology in Malaria. Part II. Methods and Techniques; WHO Offset Publication 13; WHO: Geneva, Switzerland, 1975. [Google Scholar]
- Rattanarithikul, R.; Panthusiri, P. Illustrated keys to the medically important mosquitos of Thailand. Southeast Asian J. Trop. Med. Public Health 1994, 25 (Suppl. 1), 1–66. [Google Scholar] [PubMed]
- Sirivanakarn, S. The systematics of Culex vishnui complex in Southeast Asia with the diagnosis of three common species (Diptera: Culicidae). Mosq. Syst. 1975, 7, 69–86. [Google Scholar]
- McPhatter, L.; Gerry, A.C. Effect of CO2 concentration on mosquito collection rate using odor-baited suction traps. J. Vector Ecol. 2017, 42, 44–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Substrate | Excess H2SO4 Solution | Excess HCl Solution | CO2 Gas (mL) | Average ± S.D. (mL) |
|---|---|---|---|---|
| CaCO3 1 g | 15% | 238 | 236 ± 2.8 | |
| 234 | ||||
| 10% | 282 | 281 ± 1.4 | ||
| 280 | ||||
| 12% | 265 | 260 ± 7.1 | ||
| 255 | ||||
| Limestone 1 g | 15% | 190 | 184 ± 3.0 | |
| 183 | ||||
| 180 | ||||
| 10% | 260 | 259 ± 1.4 | ||
| 258 | ||||
| 12% | 255 | 256 ± 1.4 | ||
| 257 |
| Mosquitoes Species | Set I (Light Trap) | Set II (Light Trap + Dry Ice) | Set III (Light Trap + Limestone + HCl) | Total (%) | |||
|---|---|---|---|---|---|---|---|
| F | M | F | M | F | M | ||
| Aedes aegypti | 0 | 0 | 1 | 0 | 2 | 2 | 5 (0.31) |
| Armigeres subalbatus | 1 | 0 | 14 | 0 | 0 | 0 | 15 (0.93) |
| Anopheles barbirostris s.l. | 0 | 0 | 4 | 0 | 0 | 0 | 4 (0.25) |
| Anopheles hyrcanus group | 0 | 0 | 10 | 0 | 0 | 0 | 10 (0.62) |
| Anopheles vagus | 0 | 0 | 2 | 0 | 0 | 0 | 2 (0.12) |
| Anopheles tessellatus | 0 | 0 | 2 | 0 | 0 | 0 | 2 (0.12) |
| Coquillettidia crassipes | 0 | 0 | 22 | 0 | 2 | 0 | 24 (1.48) |
| Culex bitaeniorhynchus | 4 | 0 | 412 | 15 | 71 | 2 | 504 (31.11) |
| Culex gelidus | 0 | 1 | 8 | 1 | 4 | 2 | 16 (0.99) |
| Culex nigropunctatus | 0 | 0 | 10 | 0 | 3 | 0 | 13 (0.80) |
| Culex quinquefasciatus | 1 | 0 | 24 | 0 | 30 | 2 | 57 (3.52) |
| Culex tritaeniorhynchus | 1 | 0 | 114 | 1 | 41 | 0 | 157 (9.69) |
| Culex vishnui | 4 | 0 | 649 | 5 | 101 | 1 | 760 (46.91) |
| Mansonia uniformis | 0 | 0 | 42 | 5 | 4 | 0 | 51 (3.15) |
| Total | 11 | 1 | 1314 | 27 | 258 | 9 | 1620 |
| Mosquitoes Species | Ovary Status | Set II (Light Trap + Dry Ice) | Set III (Light Trap + Limestone + HCl) |
|---|---|---|---|
| Culex quinquefasciatus | Nulliparous | 7 (29.2%) | 11 (36.7%) |
| Parous | 17 (70.8%) | 19 (63.3%) | |
| Total | 24 | 30 | |
| Pearson’s chi-squared 0.338 (p-value = 0.772) | |||
| Culex vishnui | Nulliparous | 236 (36.4%) | 43 (42.2%) |
| Parous | 413 (63.3%) | 59 (57.8%) | |
| Total | 649 | 102 | |
| Pearson’s chi-squared 1.267 (p-value = 0.272) | |||
| Culex bitaeniorhynchus | Nulliparous | 163 (49.6%) | 29 (42.0%) |
| Parous | 249 (60.4%) | 40 (58.0%) | |
| Total | 412 | 69 | |
| Pearson’s chi-squared 0.150 (p-value = 0.791) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madang, S.; Saingamsook, J.; Saeung, A.; Somboon, P. A Simple CO2 Generating System Incorporated with CDC Light Trap for Sampling Mosquito Vectors. Insects 2022, 13, 637. https://doi.org/10.3390/insects13070637
Madang S, Saingamsook J, Saeung A, Somboon P. A Simple CO2 Generating System Incorporated with CDC Light Trap for Sampling Mosquito Vectors. Insects. 2022; 13(7):637. https://doi.org/10.3390/insects13070637
Chicago/Turabian StyleMadang, Sutasinee, Jassada Saingamsook, Atiporn Saeung, and Pradya Somboon. 2022. "A Simple CO2 Generating System Incorporated with CDC Light Trap for Sampling Mosquito Vectors" Insects 13, no. 7: 637. https://doi.org/10.3390/insects13070637
APA StyleMadang, S., Saingamsook, J., Saeung, A., & Somboon, P. (2022). A Simple CO2 Generating System Incorporated with CDC Light Trap for Sampling Mosquito Vectors. Insects, 13(7), 637. https://doi.org/10.3390/insects13070637







