Comparison of Alternative Application Methods for Anti-Varroa Lithium Chloride Treatments
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Experimental Set-Up
2.2. Statistical Analysis
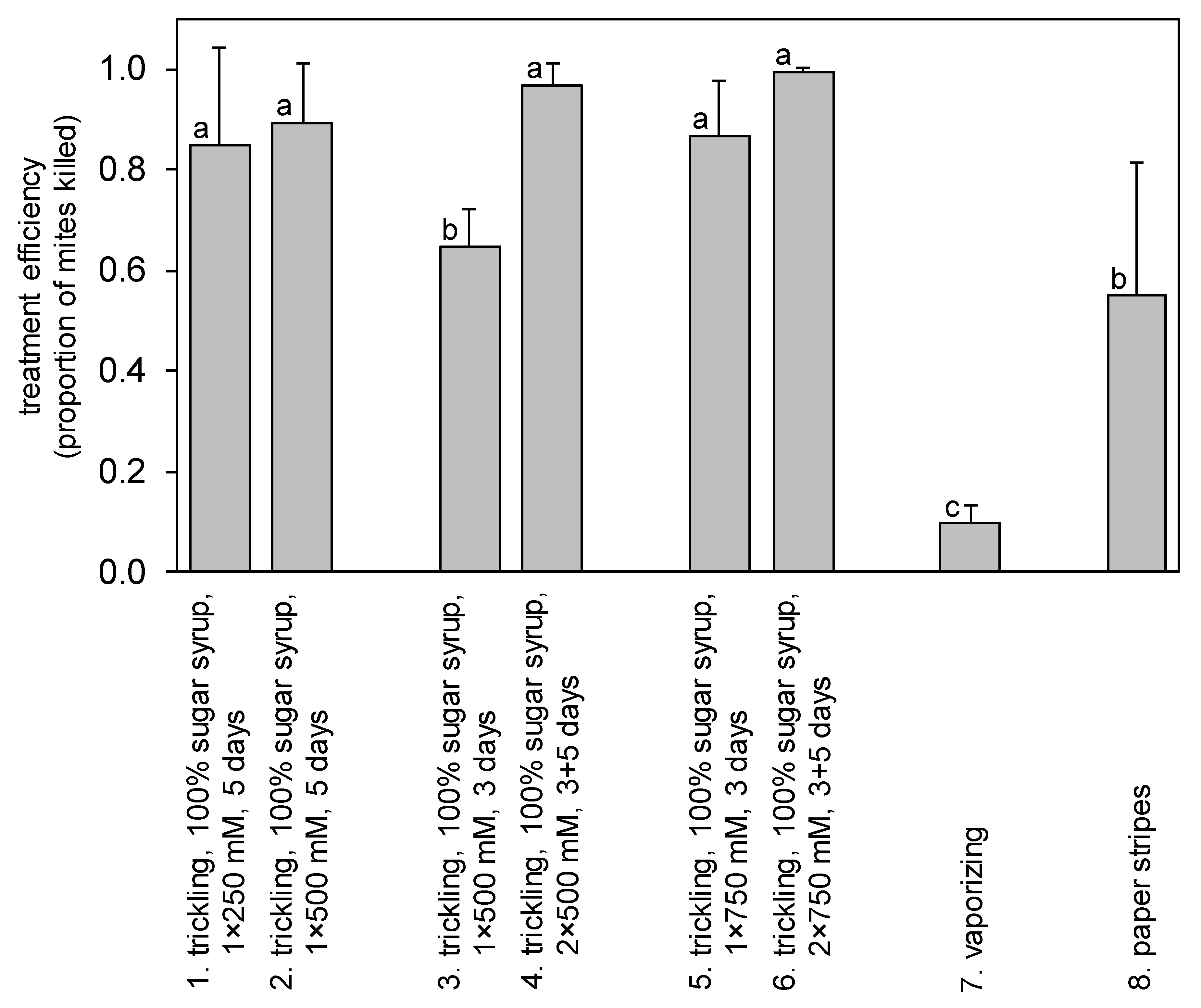
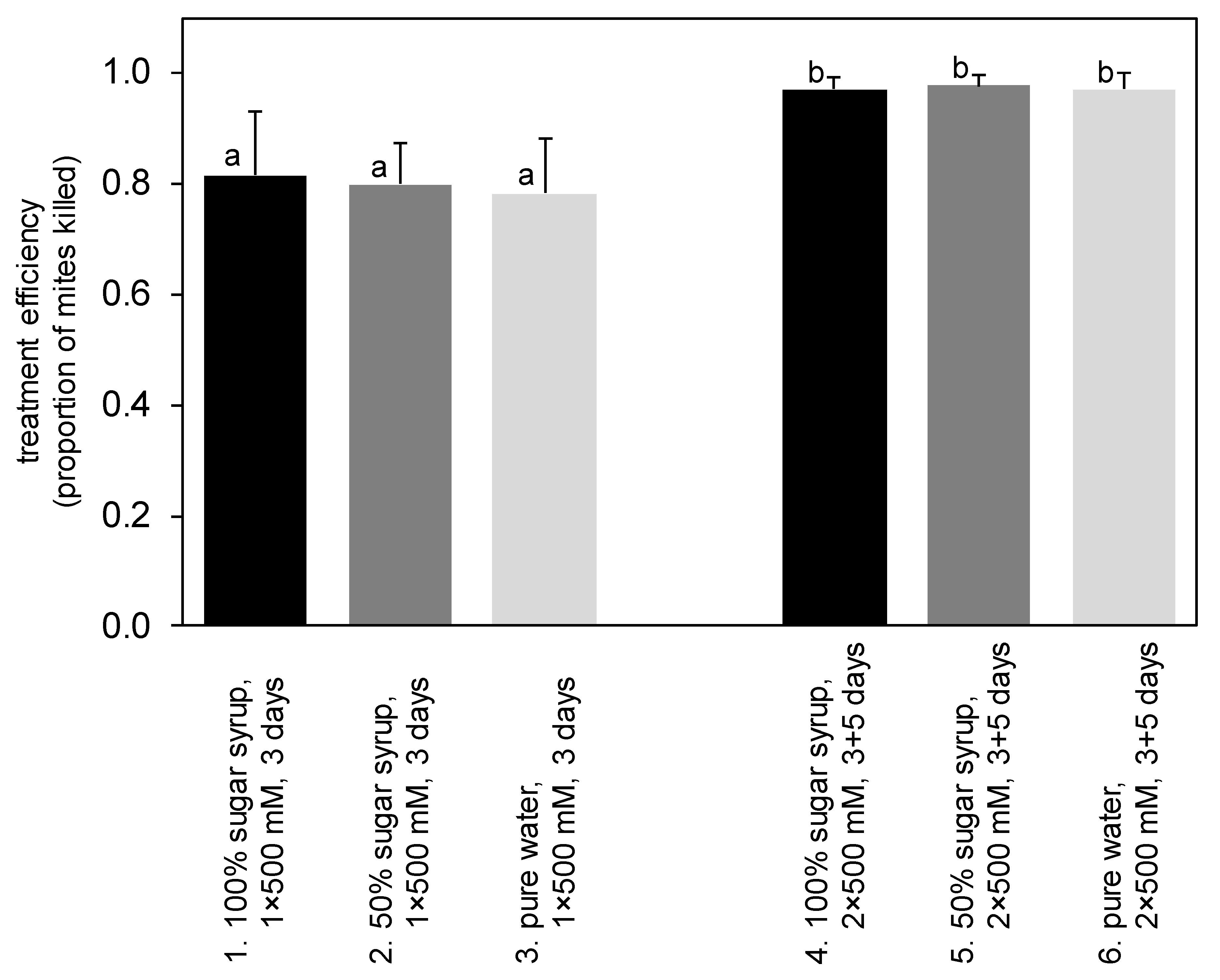
3. Results
4. Discussion
4.1. Fumigation and Cold Vaporisation Methods
4.2. Paper Strip Method
4.3. Trickling Method
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, D.; Trueman, J. Varroa jacobsoni (Acari: Varroidae) is more than one species. Exp. Appl. Acarol. 2000, 24, 165–189. [Google Scholar] [CrossRef] [PubMed]
- Le Conte, Y.; Ellis, M.; Ritter, W. Varroa mites and honey bee health: Can Varroa explain part of the colony losses? Apidologie 2010, 41, 353–363. [Google Scholar] [CrossRef] [Green Version]
- Spivak, M.; Reuter, G. A sustainable approach to controlling honey bee diseases and Varroa mites. Sustain. Agric. Res. Educ. 2005. Available online: https://conservancy.umn.edu/bitstream/handle/11299/182082/Spivak%20and%20Reuter%202005.pdf?sequence=1 (accessed on 19 May 2021).
- Barlow, V.M.; Fell, R.D. Sampling Methods for Varroa Mites on The Domesticated Honeybee; Virginia Cooperative Extension; Virginia Polytechnic Institute and State University: Blacksburg, VA, USA, 2006; pp. 103–444. [Google Scholar]
- Mozes-Koch, R.; Slabezki, Y.; Efrat, H.; Kalev, H.; Kamer, Y.; Yakobson, B.; Dag, A. First detection in Israel of fluvalinate resistance in the varroa mite using bioassay and biochemical methods. J. Exp. Appl. Acarol. 2000, 24, 35–43. [Google Scholar] [CrossRef]
- Spreafico, M.; Eördegh, F.R.; Bernardinelli, I.; Colombo, M. First detection of strains of Varroa destructor resistant to coumaphos. Results of laboratory tests and field trials. Apidologie 2001, 32, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Bogdanov, S. Contaminants of bee products. Apidologie 2006, 37, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Bogdanov, S.; Martin, P. Honey authenticity: A review. Mitt. Lebensm. Hyg. 2002, 93, 232–254. [Google Scholar]
- Floris, I.; Satta, A.; Cabras, P.; Garau, V.L.; Angioni, A. Comparison between two thymol formulations in the control of Varroa destructor: Effectiveness, persistence, and residues. J. Econ. Entomol. 2004, 97, 187–191. [Google Scholar] [CrossRef]
- Higes, M.; Meana, A.; Suárez, M.; Llorente, J. Negative long-term effects on bee colonies treated with oxalic acid against Varroa jacobsoni Oud. Apidologie 1999, 30, 289–292. [Google Scholar] [CrossRef] [Green Version]
- Imdorf, A.; Bogdanov, S.; Ochoa, R.I.; Calderone, N.W. Use of essential oils for the control of Varroa jacobsoni Oud. in honey bee colonies. Apidologie 1999, 30, 209–228. [Google Scholar]
- Calderone, N.W.; Nasr, M.E. Evaluation of a formic acid formulation for the fall control of Varroa jacobsoni (Acari: Varroidae) in colonies of the honey bee Apis mellifera (Hymenoptera: Apidae) in a temperate climate. J. Econ. Entomol. 1999, 92, 526–533. [Google Scholar] [CrossRef]
- Charriére, J.-D.; Imdorf, A. Oxalic acid treatment by trickling against Varroa destructor: Recommendations for use in central Europe and under temperate climate conditions. Bee World 2002, 83, 51–60. [Google Scholar] [CrossRef]
- Fries, I. Short-interval treatments with formic acid for control of Varroa jacobsoni in honey bee (Apis mellifera) colonies in cold climates. Swed. J. Agric. Res. 1989, 19, 213–316. [Google Scholar]
- Kraus, B.; Berg, S. Effect of a lactic acid treatment during winter in temperate climate upon Varroa jacobsoni Oud. and the bee (Apis mellifera L.) colony. Exp. Appl. Acarol. 1994, 18, 459–468. [Google Scholar] [CrossRef]
- Milani, N. Activity of oxalic and citric acids on the mite Varroa destructor in laboratory assays. Apidologie 2001, 32, 127–138. [Google Scholar] [CrossRef] [Green Version]
- Nanetti, A.; Bartolomei, P.; Bellato, S.; De Salvio, M.; Gattavecchia, E.; Ghini, S. Pharmacodynamics of oxalic acid in the honey bee colony. In Proceedings of the 38th Apimondia International Congress, Ljubljana, Slovenia, 24–29 August 2003. [Google Scholar]
- Rademacher, E.; Harz, M. Oxalic acid for the control of varroosis in honey bee colonies—A review. Apidologie 2006, 37, 98–120. [Google Scholar] [CrossRef] [Green Version]
- Skinner, J.; Parkman, J.; Studer, M. Evaluation of honey bee miticides, including temporal and thermal effects on formic acid gel vapours, in the central south-eastern USA. J. Apic. Res. 2001, 40, 81–89. [Google Scholar] [CrossRef]
- Van Engelsdorp, D.; Underwood, R.M.; Cox-Foster, D.L. Short-term fumigation of honey bee (Hymenoptera: Apidae) colonies with formic and acetic acids for the control of Varroa destructor (Acari: Varroidae). J. Econ. Entomol. 2008, 101, 256–264. [Google Scholar] [CrossRef]
- Rosenkranz, P.; Aumeier, P.; Ziegelmann, B. Biology and control of Varroa destructor. J. Invertebr. Pathol. 2010, 103, S96–S119. [Google Scholar] [CrossRef]
- Ziegelmann, B.; Abele, E.; Hannus, S.; Beitzinger, M.; Berg, S.; Rosenkranz, P. Lithium chloride effectively kills the honey bee parasite Varroa destructor by a systemic mode of action. Sci. Rep. 2018, 8, 683. [Google Scholar] [CrossRef] [Green Version]
- Ziegelmann, B.; Blumenschein, M.; Rein, C.; Lang, V.; Hannus, S.; Rosenkranz, P. Varroa treatment of brood-free honey bee colonies with lithium chloride. In Proceedings of the 46th APIMONDIA—International Apicultural Congress, Montréal, QC, Canada, 8–12 September 2019; p. 56. [Google Scholar]
- Stanimirovic, Z.; Glavinic, U.; Ristanic, M.; Aleksic, N.; Jovanovic, N.; Vejnovic, B.; Stevanovic, J. Looking for the causes of and solutions to the issue of honey bee colony losses. Acta Vet.-Beogr. 2019, 69, 1–31. [Google Scholar]
- Prešern, J.; Kur, U.; Bubnič, J.; Šala, M. Lithium contamination of honeybee products and its accumulation in brood as a consequence of anti-varroa treatment. Food Chem. 2020, 330, 127334. [Google Scholar] [CrossRef]
- Tutun, H.; Kahraman, H.A.; Aluc, Y.; Avci, T.; Ekici, H. Investigation of some metals in honey samples from West Mediterranean region of Turkey. Vet. Res. Forum 2019, 10, 181–186. [Google Scholar]
- Bogdanov, S.; Jurendic, T.; Sieber, R.; Gallmann, P. Honey for nutrition and health: A review. J. Am. Coll. Nutr. 2008, 27, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Kolics, É.; Sajtos, Z.; Mátyás, K.; Szepesi, K.; Solti, I.; Németh, G.; Taller, J.; Baranyai, E.; Specziár, A.; Kolics, B. Changes in Lithium Levels in Bees and Their Products Following Anti-Varroa Treatment. Insects 2021, 12, 579. [Google Scholar] [CrossRef] [PubMed]
- Stanimirovic, Z.; Glavinic, U.; Jovanovic, N.M.; Ristanic, M.; Milojković-Opsenica, D.; Mutic, J.; Stevanovic, J. Preliminary trials on effects of lithium salts on Varroa destructor, honey and wax matrices. J. Apic. Res. 2022, 61, 375–391. [Google Scholar]
- Kolics, B.; Sajtos, Z.; Matyas, K.; Kolics, É.; Taller, J.; E, B. Lithium chloride—hazard or possibility? In Proceedings of the 46th APIMONDIA—International Apicultural Congress, Montréal, QC, Canada, 8–12 September 2019; p. 285. [Google Scholar]
- Kolics, É.; Specziár, A.; Taller, J.; Mátyás, K.K.; Kolics, B. Lithium chloride outperformed oxalic acid sublimation in a preliminary experiment for Varroa mite control in pre-wintering honey bee colonies. Acta Vet. Hung. 2021, 68, 370–373. [Google Scholar] [CrossRef]
- Kolics, É.; Mátyás, K.; Taller, J.; Specziár, A.; Kolics, B. Contact Effect Contribution to the High Efficiency of Lithium Chloride Against the Mite Parasite of the Honey Bee. Insects 2020, 11, 333. [Google Scholar] [CrossRef]
- Pohorecka, K.; Skubida, P.; Semkiw, P. Varroacidal efficiency of treatment with amitraz in honey bee colonies with brood. J. Apic. Sci. 2018, 62, 285–292. [Google Scholar] [CrossRef] [Green Version]
- Semkiw, P.; Skubida, P.; Pohorecka, K. The amitraz strips efficacy in control of Varroa destructor after many years application of amitraz in apiaries. J. Apic. Sci. 2013, 57, 107–121. [Google Scholar]
| Treatment 1 | Treatment 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment Type | Method | Concentration LiCl × 1H2O | Li+ Dosage (mg) | Observation Period | Method | Concentration LiCl × 1H2O | Li+ Dosage (mg) | Observation Period | Number of Hives Treated |
| Experiment I | |||||||||
| 1. | trickling, 100% sugar syrup | 250 mM | 69.4 | 5 days | - | - | - | 10 | |
| 2. | trickling, 100% sugar syrup | 500 mM | 138.8 | 5 days | - | - | - | 10 | |
| 3. | trickling, 100% sugar syrup | 500 mM | 138.8 | 3 days | - | - | - | 10 | |
| 4. | trickling, 100% sugar syrup | 500 mM | 138.8 | 3 days | trickling | 500 mM | 138.8 | 5 days | 10 |
| 5. | trickling, 100% sugar syrup | 750 mM | 208.2 | 3 days | - | - | - | 10 | |
| 6. | trickling, 100% sugar syrup | 750 mM | 208.2 | 3 days | trickling | 750 mM | 208.2 | 5 days | 10 |
| 7. | vaporising | 5.5 M | 69.4 | 8 days | - | - | - | 5 | |
| 8. | paper strips | 5.5 M | 138.8 | 8 days | - | - | - | 10 | |
| Experiment II | |||||||||
| 1. | trickling, 100% sugar syrup | 500 mM | 138.8 | 3 days | - | - | - | 9 | |
| 2. | trickling, 50% sugar syrup | 500 mM | 138.8 | 3 days | - | - | - | 10 | |
| 3. | trickling, pure water | 500 mM | 138.8 | 3 days | - | - | - | 10 | |
| 4. | trickling, 100% sugar syrup | 500 mM | 138.8 | 3 days | trickling | 500 mM | 138.8 | 5 days | 9 |
| 5. | trickling, 50% sugar syrup | 500 mM | 138.8 | 3 days | trickling | 500 mM | 138.8 | 5 days | 10 |
| 6. | trickling, pure water | 500 mM | 138.8 | 3 days | trickling | 500 mM | 138.8 | 5 days | 10 |
| (a) | ||||||
|---|---|---|---|---|---|---|
| Preparation of trickling solution directly from different compounds | 250 mM solution | 500 mM solution | 750 mM solution | |||
| Lithium chloride monohydrate (LiCl × H2O) | 15.10 g L−1 | 30.20 g L−1 | 45.29 g L−1 | |||
| Lithium chloride anhydrate (LiCl) | 10.60 g L−1 | 21.20 g L−1 | 31.79 g L−1 | |||
| (b) | ||||||
| Preparation of stock solution | Preparation of trickling solution from the stock | |||||
| agent used (g) | final volume (mL) | final concentration (M) | stock solution (mL) | final volume (mL) | final concentration (mM) | |
| LiCl anhydrate | 500 | 2137 | 5.5 | 45.3 | 1000 | 250 |
| LiCl monohydrate | 500 | 1500 | 5.5 | 45.3 | 1000 | 250 |
| (c) | ||||||
| Concentration | Single volume/colony | Way of administration | Single dose/ colony Li+ basis (mg) | Reference | ||
| Lithium chloride | 25 mM | ad libitum | feeding sugar syrup | - | Ziegelmann et al., 2018 [22] Ziegelmann et al., 2019 [23] | |
| Lithium chloride | 50 mM | ad libitum | feeding sugar syrup | - | Ziegelmann et al., 2018 [22] | |
| Lithium chloride | 50 mM | ad libitum | feeding sugar dough | - | Ziegelmann et al., 2019 [23] | |
| Lithium chloride | 25 mM | 1000 mL | feeding sugar syrup | 173.5 | Kolics et al., 2019 [30] | |
| Presern et al., 2020 [25], Kolics et al., 2021a [31] | ||||||
| Lithium chloride | 250 mM | 40 mL | trickling | 69.4 | Kolics et al., 2021b [31], Kolics et al., 2020 [32], present study | |
| Lithium chloride | 500 mM | 40 mL | trickling | 138.8 | present study | |
| Lithium chloride | 750 mM | 40 mL | trickling | 208.2 | present study | |
| Lithium citrate | 5 mM | 1000 mL | feeding sugar syrup | 101 | Stanimirovic et al., 2022 [29] | |
| Lithium citrate | 10 mM | 1000 mL | feeding sugar syrup | 202 | Stanimirovic et al., 2022 [29] | |
| Lithium citrate | 15 mM | 1000 mL | feeding sugar syrup | 302.9 | Stanimirovic et al., 2022 [29] | |
| Lithium citrate | 20 mM | 1000 mL | feeding sugar syrup | 403.9 | Stanimirovic et al., 2022 [29] | |
| Lithium citrate | 25 mM | 1000 mL | feeding sugar syrup | 504.9 | Stanimirovic et al., 2022 [29] | |
| Effect | Overall Model | ||||||
|---|---|---|---|---|---|---|---|
| d.f.error effect | F | p | R2adj. | d.f.model, residual | F | p | |
| Treatment × number of mites | 8, 59 | 7.2 | <0.001 | ||||
| Treatment | 7, 59 | 21.5 | <0.001 | ||||
| Overall model | 0.850 | 15, 59 | 28.9 | <0.001 | |||
| Effect | Overall Model | ||||||
|---|---|---|---|---|---|---|---|
| d.f.error, effect | F | p | R2adj. | d.f.model, residual | F | p | |
| Number of mites | 1, 57 | 5.6 | 0.021 | ||||
| Sugar concentration | 2, 57 | 0.8 | 0.469 | ||||
| Number of treatments | 1, 57 | 96.2 | <0.001 | ||||
| Sugar × number of treatments | 2, 57 | 0.3 | 0.762 | ||||
| Overall model | 0.632 | 6, 51 | 17.3 | <0.001 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolics, B.; Kolics, É.; Mátyás, K.; Taller, J.; Specziár, A. Comparison of Alternative Application Methods for Anti-Varroa Lithium Chloride Treatments. Insects 2022, 13, 633. https://doi.org/10.3390/insects13070633
Kolics B, Kolics É, Mátyás K, Taller J, Specziár A. Comparison of Alternative Application Methods for Anti-Varroa Lithium Chloride Treatments. Insects. 2022; 13(7):633. https://doi.org/10.3390/insects13070633
Chicago/Turabian StyleKolics, Balázs, Éva Kolics, Kinga Mátyás, János Taller, and András Specziár. 2022. "Comparison of Alternative Application Methods for Anti-Varroa Lithium Chloride Treatments" Insects 13, no. 7: 633. https://doi.org/10.3390/insects13070633
APA StyleKolics, B., Kolics, É., Mátyás, K., Taller, J., & Specziár, A. (2022). Comparison of Alternative Application Methods for Anti-Varroa Lithium Chloride Treatments. Insects, 13(7), 633. https://doi.org/10.3390/insects13070633





