Mitochondrial Genomes of Two Asexual Trichogramma (Hymenoptera: Trichogrammatidae) Strains and Comparison with Their Sexual Relatives
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation and DNA Extraction
2.2. Mitochondrial Genome Sequencing
2.3. Mitochondrial Genome Assembly
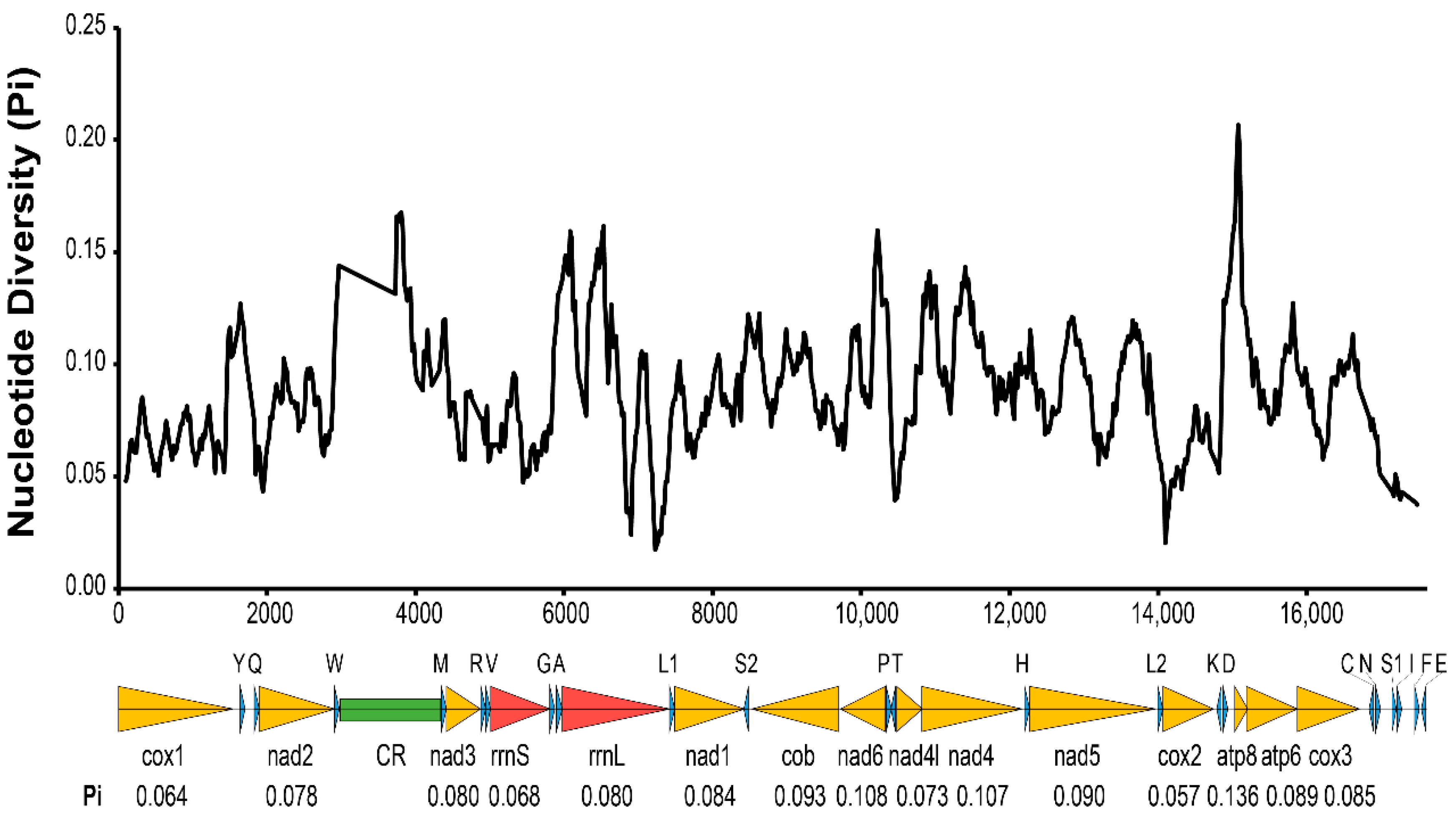
2.4. Gene Annotation and Nucleotide Diversity Analyses
2.5. Phylogenetic Analyses
2.6. Relaxed Selection Test
3. Results
3.1. Features of Two Asexual Mitochondrial Genomes
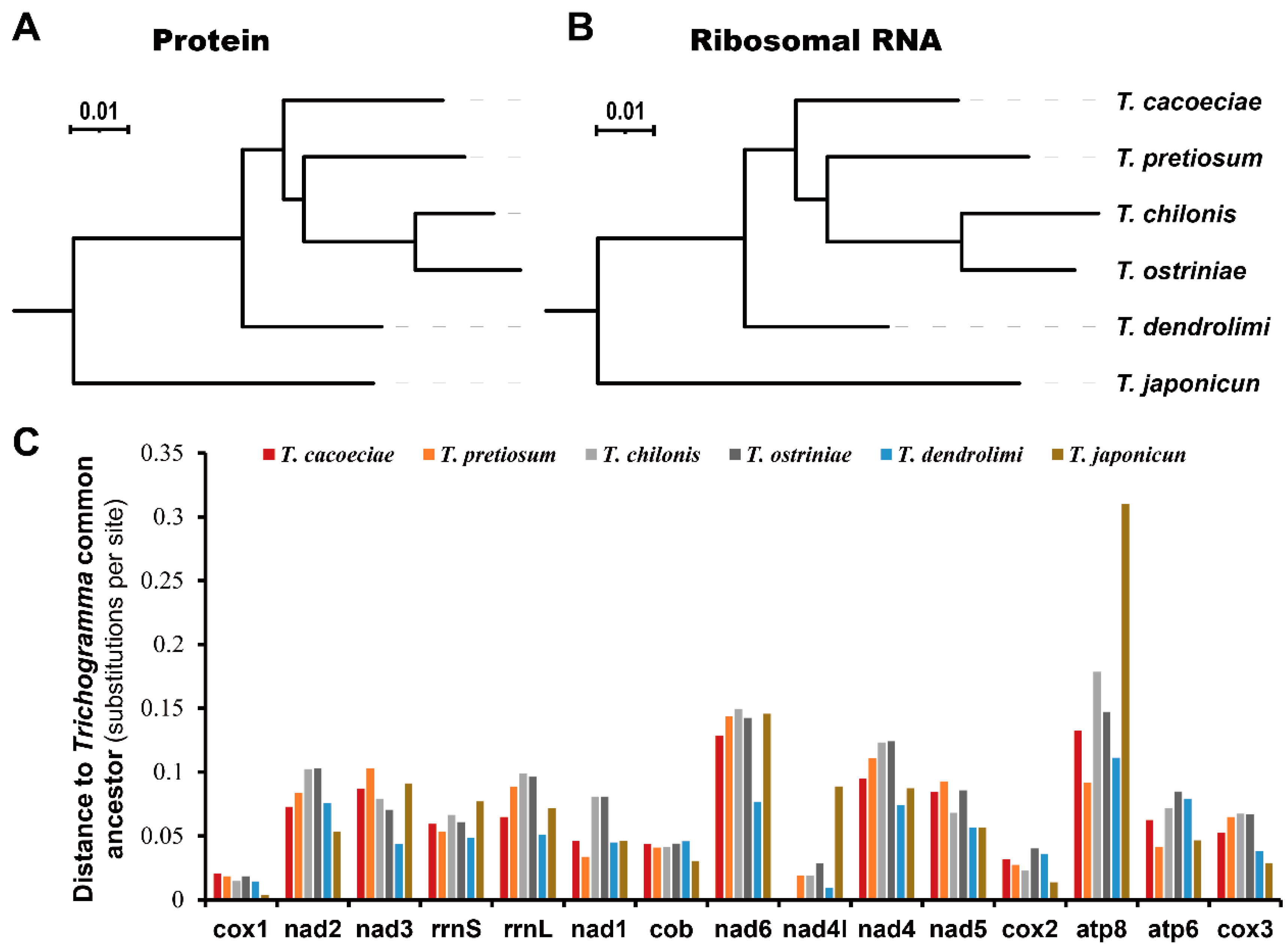
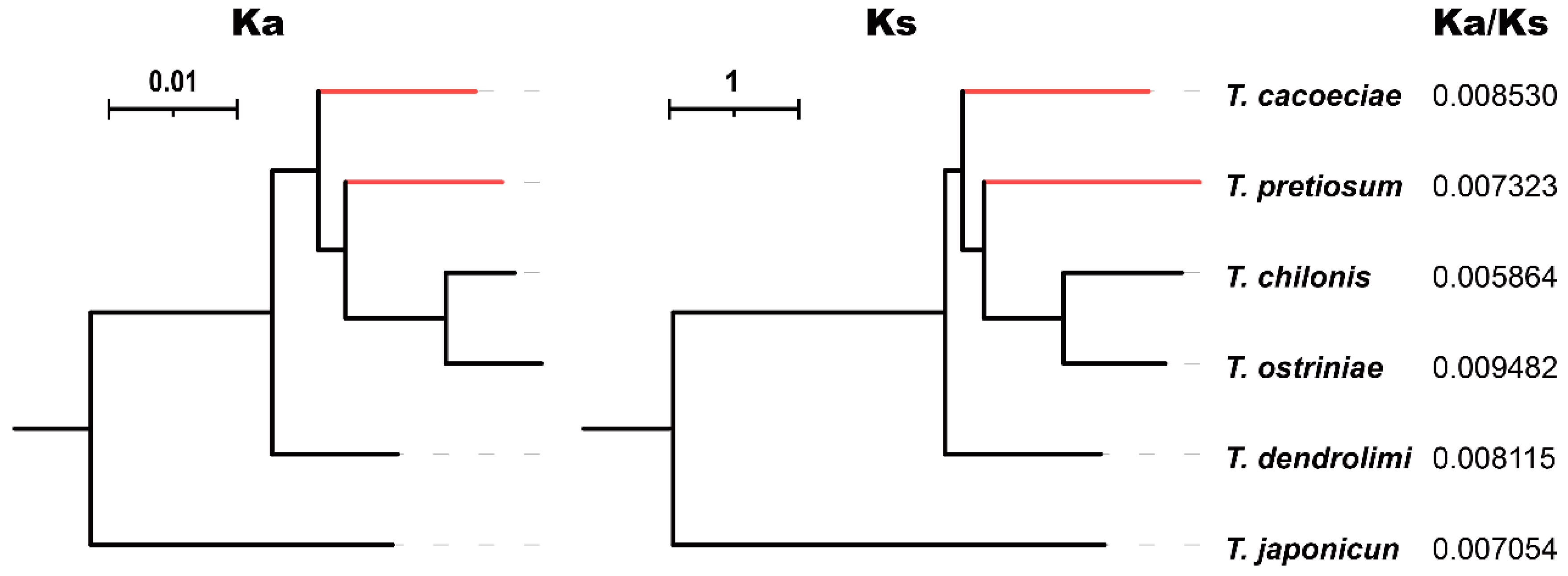
3.2. Comparison of Substitution Rates between Asexual and Sexual Mitogenomes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Otto, S.P.; Lenormand, T. Resolving the paradox of sex and recombination. Nat. Rev. Genet. 2002, 3, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Tvedte, E.S.; Logsdon, J.M., Jr.; Forbes, A.A. Sex loss in insects: Causes of asexuality and consequences for genomes. Curr. Opin. Insect Sci. 2019, 31, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Meirmans, S.; Meirmans, P.G.; Kirkendall, L.R. The costs of sex: Facing real-world complexities. Q. Rev. Biol. 2012, 87, 19–40. [Google Scholar] [CrossRef] [PubMed]
- Schwander, T.; Crespi, B.J. Twigs on the tree of life? Neutral and selective models for integrating macroevolutionary patterns with microevolutionary processes in the analysis of asexuality. Mol. Ecol. 2009, 18, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Kondrashov, A.S. Deleterious mutations and the evolution of sexual reproduction. Nature 1988, 336, 435–440. [Google Scholar] [CrossRef]
- Wertheim, J.O.; Murrell, B.; Smith, M.D.; Kosakovsky Pond, S.L.; Scheffler, K. RELAX: Detecting relaxed selection in a phylogenetic framework. Mol. Biol. Evol. 2015, 32, 820–832. [Google Scholar] [CrossRef]
- Normark, B.B.; Moran, N.A. Testing for the accumulation of deleterious mutations in asexual eukaryote genomes using molecular sequences. J. Nat. Hist. 2000, 34, 1719–1729. [Google Scholar] [CrossRef]
- Hollister, J.D.; Greiner, S.; Wang, W.; Wang, J.; Zhang, Y.; Wong, G.K.; Wright, S.I.; Johnson, M.T.J. Recurrent loss of sex is associated with accumulation of deleterious mutations in Oenothera. Mol. Biol. Evol. 2015, 32, 896–905. [Google Scholar] [CrossRef]
- Henry, L.; Schwander, T.; Crespi, B.J. Deleterious mutation accumulation in asexual Timema stick insects. Mol. Biol. Evol. 2012, 29, 401–408. [Google Scholar] [CrossRef]
- Sharbrough, J.; Luse, M.; Boore, J.L.; Logsdon, J.M., Jr.; Neiman, M. Radical amino acid mutations persist longer in the absence of sex. Evolution 2018, 72, 808–824. [Google Scholar] [CrossRef]
- Paland, S.; Lynch, M. Transitions to asexuality result in excess amino acid substitutions. Science 2006, 311, 990–992. [Google Scholar] [CrossRef] [PubMed]
- Neiman, M.; Hehman, G.; Miller, J.T.; Logsdon, J.M., Jr.; Taylor, D.R. Accelerated mutation accumulation in asexual lineages of a freshwater snail. Mol. Biol. Evol. 2010, 27, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Warren, W.C.; García-Pérez, R.; Xu, S.; Lampert, K.P.; Chalopin, D.; Stöck, M.; Loewe, L.; Lu, Y.; Kuderna, L.; Minx, P.; et al. Clonal polymorphism and high heterozygosity in the celibate genome of the Amazon molly. Nat. Ecol. Evol. 2018, 2, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Blanc-Mathieu, R.; Perfus-Barbeoch, L.; Aury, J.-M.; Rocha, M.D.; Gouzy, J.; Sallet, E.; Martin-Jimenez, C.; Bailly-Bechet, M.; Castagnone-Sereno, P.; Flot, J.-F.; et al. Hybridization and polyploidy enable genomic plasticity without sex in the most devastating plant-parasitic nematodes. PLoS Genet. 2017, 13, e1006777. [Google Scholar] [CrossRef]
- Ament-Velásquez, S.L.; Figuet, E.; Ballenghien, M.; Zattara, E.E.; Norenburg, J.L.; Fernández-Álvarez, F.A.; Bierne, J.; Bierne, N.; Galtier, N. Population genomics of sexual and asexual lineages in fissiparous ribbon worms (Lineus, Nemertea): Hybridization, polyploidy and the Meselson effect. Mol. Ecol. 2016, 25, 3356–3369. [Google Scholar] [CrossRef]
- Tucker, A.E.; Ackerman, M.S.; Eads, B.D.; Xu, S.; Lynch, M. Population-genomic insights into the evolutionary origin and fate of obligately asexual Daphnia pulex. Proc. Natl. Acad. Sci. USA 2013, 110, 15740–15745. [Google Scholar] [CrossRef]
- Kočí, J.; Röslein, J.; Pačes, J.; Kotusz, J.; Halačka, K.; Koščo, J.; Fedorčák, J.; Iakovenko, N.; Janko, K. No evidence for accumulation of deleterious mutations and fitness degradation in clonal fish hybrids: Abandoning sex without regrets. Mol. Ecol. 2020, 29, 3038–3055. [Google Scholar] [CrossRef]
- Lindsey, A.R.I.; Kelkar, Y.D.; Wu, X.; Sun, D.; Martinson, E.O.; Yan, Z.; Rugman-Jones, P.F.; Hughes, D.S.T.; Murali, S.C.; Qu, J.; et al. Comparative genomics of the miniature wasp and pest control agent Trichogramma pretiosum. BMC Biol. 2018, 16, 54. [Google Scholar] [CrossRef]
- Brandt, A.; Schaefer, I.; Glanz, J.; Schwander, T.; Maraun, M.; Scheu, S.; Bast, J. Effective purifying selection in ancient asexual oribatid mites. Nat. Commun. 2017, 8, 873. [Google Scholar] [CrossRef]
- Gokhman, V.E.; Kuznetsova, V.G. Parthenogenesis in Hexapoda: Holometabolous insects. J. Zool. Syst. Evol. Res. 2018, 56, 23–34. [Google Scholar] [CrossRef]
- Vershinina, A.O.; Kuznetsova, V.G. Parthenogenesis in Hexapoda: Entognatha and non-holometabolous insects. J. Zool. Syst. Evol. Res. 2016, 54, 257–268. [Google Scholar] [CrossRef]
- Zang, L.-S.; Wang, S.; Zhang, F.; Desneux, N. Biological control with Trichogramma in China: History, present status, and perspectives. Annu. Rev. Entomol. 2021, 66, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.; Ras, E.; Ferguson, K.B.; Ariëns, S.; Babendreier, D.; Bijma, P.; Bourtzis, K.; Brodeur, J.; Bruins, M.A.; Centurión, A.; et al. Next-generation biological control: The need for integrating genetics and genomics. Biol. Rev. Camb. Philos. Soc. 2020, 95, 1838–1854. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.E.; Stouthamer, R. Sex ratio modulators of egg parasitoids. In Egg Parasitoids in Agroecosystems with Emphasis on Trichogramma; Consoli, F.L., Parra, J.R.P., Zucchi, R.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 167–190. [Google Scholar]
- Stouthamer, R.; Pinto, J.D.; Platner, G.R.; Luck, R.F. Taxonomic status of thelytokous forms of Trichogramma (Hymenoptera: Trichogrammatidae). Ann. Entomol. Soc. Am. 1990, 83, 475–481. [Google Scholar] [CrossRef]
- Stouthamer, R.; Luck, R.F.; Hamilton, W.D. Antibiotics cause parthenogenetic Trichogramma (Hymenoptera, Trichogrammatidae) to revert to sex. Proc. Natl. Acad. Sci. USA 1990, 87, 2424–2427. [Google Scholar] [CrossRef]
- Stouthamer, R.; Breeuwer, J.A.J.; Luck, R.F.; Werren, J.H. Molecular-identification of microorganisms associated with parthenogenesis. Nature 1993, 361, 66–68. [Google Scholar] [CrossRef]
- Vavre, F.; de Jong, J.H.; Stouthamer, R. Cytogenetic mechanism and genetic consequences of thelytoky in the wasp Trichogramma cacoeciae. Heredity 2004, 93, 592–596. [Google Scholar] [CrossRef]
- Stouthamer, R.; Kazmer, D.J. Cytogenetics of microbe-associated parthenogenesis and its consequences for gene flow in Trichogramma wasps. Heredity 1994, 73, 317–327. [Google Scholar] [CrossRef]
- Hua, H.; Zhao, Z.; Zhang, Y.; Hu, J.; Zhang, F.; Li, Y. Inter- and intra-specific differentiation of Trichogramma (Hymenoptera: Trichogrammatidae) species using PCR–RFLP targeting COI. J. Econ. Entomol. 2018, 111, 1860–1867. [Google Scholar] [CrossRef]
- Simon, C.; Frati, F.; Beckenbach, A.; Crespi, B.; Liu, H.; Flook, P. Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann. Entomol. Soc. Am. 1994, 87, 651–701. [Google Scholar] [CrossRef]
- Lalitha, S. Primer Premier 5. Biotech Softw. Internet Rep. 2000, 1, 270–272. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.; Li, Y.; Yang, C.; Liu, S. MitoZ: A toolkit for animal mitochondrial genome assembly, annotation and visualization. Nucleic Acids Res. 2019, 47, e63. [Google Scholar] [CrossRef]
- Bernt, M.; Donath, A.; Jühling, F.; Externbrink, F.; Florentz, C.; Fritzsch, G.; Pütz, J.; Middendorf, M.; Stadler, P.F. MITOS: Improved de novo metazoan mitochondrial genome annotation. Mol. Phylogenetics Evol. 2013, 69, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Laslett, D.; Canbäck, B. ARWEN: A program to detect tRNA genes in metazoan mitochondrial nucleotide sequences. Bioinformatics 2008, 24, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Capella-Gutiérrez, S.; Silla-Martínez, J.M.; Gabaldón, T. trimAl: A tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 2009, 25, 1972–1973. [Google Scholar] [CrossRef]
- Moretti, S.; Wilm, A.; Higgins, D.G.; Xenarios, I.; Notredame, C. R-Coffee: A web server for accurately aligning noncoding RNA sequences. Nucleic Acids Res. 2008, 36, W10–W13. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z. PAML 4: Phylogenetic Analysis by Maximum Likelihood. Mol. Biol. Evol. 2007, 24, 1586–1591. [Google Scholar] [CrossRef] [PubMed]
- Cameron, S.L. Insect mitochondrial genomics: Implications for evolution and phylogeny. Annu. Rev. Entomol. 2014, 59, 95–117. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, P.; Xue, X.; Hua, H.; Li, Y.; Zhang, F.; Wei, S. Extensive gene rearrangements in the mitochondrial genomes of two egg parasitoids, Trichogramma japonicum and Trichogramma ostriniae (Hymenoptera: Chalcidoidea: Trichogrammatidae). Sci. Rep. 2018, 8, 7034. [Google Scholar] [CrossRef]
- Halligan, D.L.; Keightley, P.D. Spontaneous mutation accumulation studies in evolutionary genetics. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 151–172. [Google Scholar] [CrossRef]
- Tvedte, E.S. Genome Evolution in Parasitic Wasps: Comparisons of Sexual and Asexual Species. Ph.D. Thesis, The University of Iowa, Iowa City, IA, USA, 2018. [Google Scholar]
- Meiklejohn, C.D.; Montooth, K.L.; Rand, D.M. Positive and negative selection on the mitochondrial genome. Trends Genet. 2007, 23, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Ye, G.; Werren, J.H. Evolutionary rate correlation between mitochondrial-encoded and mitochondria-associated nuclear-encoded proteins in insects. Mol. Biol. Evol. 2019, 36, 1022–1036. [Google Scholar] [CrossRef]
- Kraaijeveld, K.; Anvar, S.Y.; Frank, J.; Schmitz, A.; Bast, J.; Wilbrandt, J.; Petersen, M.; Ziesmann, T.; Niehuis, O.; de Knijff, P.; et al. Decay of sexual trait genes in an asexual parasitoid wasp. Genome Biol. Evol. 2016, 8, 3685–3695. [Google Scholar] [CrossRef][Green Version]
- Wang, X.; Qi, L.; Jiang, R.; Du, Y.; Li, Y. Incomplete removal of Wolbachia with tetracycline has two-edged reproductive effects in the thelytokous wasp Encarsia formosa (Hymenoptera: Aphelinidae). Sci. Rep. 2017, 7, 44014. [Google Scholar] [CrossRef]
- Hughes, J.F.; Skaletsky, H.; Brown, L.G.; Pyntikova, T.; Graves, T.; Fulton, R.S.; Dugan, S.; Ding, Y.; Buhay, C.J.; Kremitzki, C.; et al. Strict evolutionary conservation followed rapid gene loss on human and rhesus Y chromosomes. Nature 2012, 483, 82–86. [Google Scholar] [CrossRef]
- Kang, D.; Hamasaki, N. Maintenance of mitochondrial DNA integrity: Repair and degradation. Curr. Genet. 2002, 41, 311–322. [Google Scholar] [CrossRef] [PubMed]
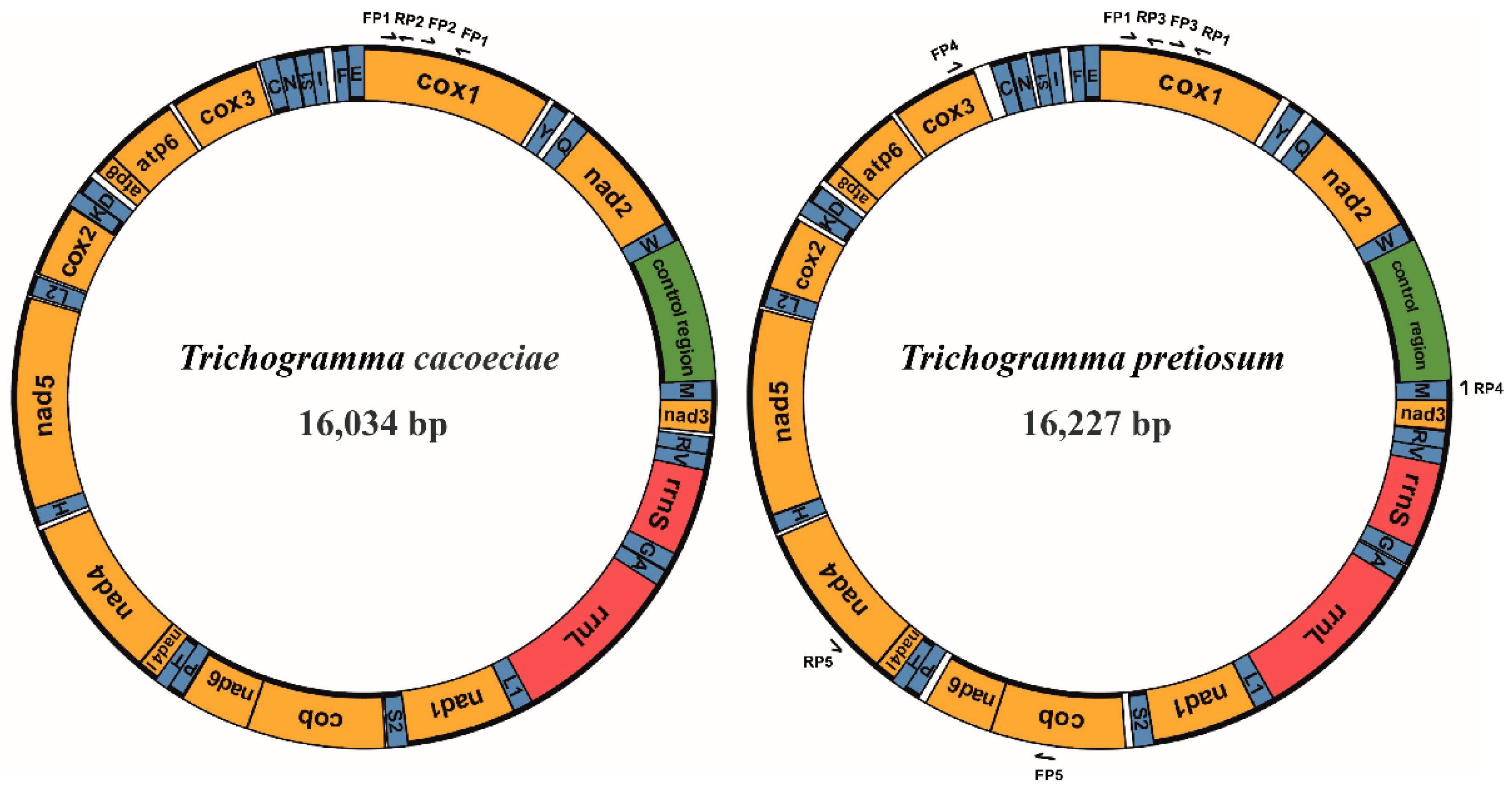
| Gene | Strand | T. cacoeciae | T. pretiosum | Tchi | Tden | Tjap | Tost | ||
|---|---|---|---|---|---|---|---|---|---|
| Start/Stop | Length (bp) | Start/Stop | Length (bp) | Length (bp) | Length (bp) | Length (bp) | Length (bp) | ||
| cox1 | + | ATG/TAA | 1536 | ATG/TAA | 1548 | 1536 | 1536 | 1536 | 1536 |
| trnY | + | 68 | 66 | 66 | 67 | 67 | 66 | ||
| trnQ | + | 68 | 70 | 68 | 68 | 68 | 68 | ||
| nad2 | + | ATA/TAA | 1014 | ATA/TAA | 1014 | 1014 | 1014 | 1014 | 1014 |
| trnW | + | 66 | 66 | 67 | 66 | 66 | 67 | ||
| trnM | + | 65 | 65 | 64 | 65 | 68 | 66 | ||
| nad3 | + | ATA/TAA | 360 | ATA/TAA | 360 | 360 | 363 | 363 | 360 |
| trnR | + | 64 | 63 | 63 | 63 | 65 | 63 | ||
| trnV | + | 67 | 67 | 68 | 68 | 66 | 68 | ||
| rrnS | + | 770 | 784 | 777 | 780 | 749 | 774 | ||
| trnG | + | 66 | 67 | 66 | 66 | 65 | 67 | ||
| trnA | + | 63 | 64 | 63 | 65 | 65 | 64 | ||
| rrnL | + | 1388 | 1417 | 1393 | 1393 | 1367 | 1362 | ||
| trnL1 | + | 68 | 70 | 70 | 66 | 65 | 70 | ||
| nad1 | + | ATA/TAA | 939 | ATA/TAA | 939 | 936 | 936 | 939 | 936 |
| trnS2 | − | 64 | 64 | 64 | 65 | 65 | 64 | ||
| cob | − | ATG/TAA | 1140 | ATG/TAA | 1143 | 1140 | 1140 | 1140 | 1140 |
| nad6 | − | ATG/TAA | 573 | ATG/TAA | 573 | 579 | 594 | 573 | 573 |
| trnP | + | 66 | 65 | 66 | 65 | 66 | 65 | ||
| trnT | − | 64 | 64 | 64 | 64 | 65 | 64 | ||
| nad4l | + | ATT/TAG | 288 | ATT/TAG | 288 | 288 | 288 | 288 | 288 |
| nad4 | + | ATG/TAA | 1344 | ATG/TAA | 1344 | 1344 | 1344 | 1344 | 1344 |
| trnH | + | 64 | 67 | 63 | 65 | 67 | 63 | ||
| nad5 | + | ATT/TAA | 1689 | ATT/TAA | 1683 | 1683 | 1689 | 1686 | 1692 |
| trnL2 | + | 66 | 66 | 68 | 66 | 66 | 66 | ||
| cox2 | + | ATT/TAG | 681 | ATT/TAA | 681 | 681 | 681 | 681 | 681 |
| trnK | − | 70 | 72 | 70 | 70 | 70 | 70 | ||
| trnD | + | 66 | 68 | 66 | 67 | 66 | 66 | ||
| atp8 | + | ATT/TAA | 168 | ATA/TAA | 159 | 168 | 168 | 159 | 159 |
| atp6 | + | ATA/TAA | 672 | ATA/TAA | 672 | 681 | 681 | 675 | 675 |
| cox3 | + | ATG/TAA | 792 | ATG/TAA | 792 | 792 | 831 | 792 | 792 |
| trnC | − | 69 | 69 | 69 | 69 | 69 | 68 | ||
| trnN | + | 66 | 67 | 66 | 66 | 66 | 62 | ||
| trnS1 | + | 60 | 60 | 60 | 60 | 59 | 60 | ||
| trnI | + | 67 | 67 | 67 | 67 | 67 | 67 | ||
| trnF | + | 66 | 65 | 65 | 65 | 64 | 65 | ||
| trnE | − | 66 | 67 | 66 | 66 | 67 | 66 | ||
| Gene | K | LR | p | q |
|---|---|---|---|---|
| atp6 | 1.13 | 0.15 | 0.700 | 0.899 |
| atp8 | 0.69 | 0.41 | 0.520 | 0.899 |
| cob | 1.00 | 0.00 | 1.000 | 1.000 |
| cox1 | 1.05 | 0.04 | 0.835 | 0.899 |
| cox2 | 1.99 | 2.44 | 0.118 | 0.413 |
| cox3 | 1.07 | 0.06 | 0.799 | 0.899 |
| nad1 | 1.72 | 5.83 | 0.016 | 0.112 |
| nad2 | 1.20 | 0.31 | 0.576 | 0.899 |
| nad3 | 1.55 | 0.64 | 0.425 | 0.899 |
| nad4 | 5.90 | 186.52 | 0 | 0 |
| nad4l | 14.98 | 0.22 | 0.639 | 0.899 |
| nad5 | 0.77 | 3.74 | 0.053 | 0.247 |
| nad6 | 0.93 | 0.08 | 0.771 | 0.899 |
| 13PCGs | 1.11 | 1.17 | 0.280 | 0.784 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Z.-C.; Qi, G.-Y.; Yao, T.-Y.; Li, Y.-X. Mitochondrial Genomes of Two Asexual Trichogramma (Hymenoptera: Trichogrammatidae) Strains and Comparison with Their Sexual Relatives. Insects 2022, 13, 549. https://doi.org/10.3390/insects13060549
Yan Z-C, Qi G-Y, Yao T-Y, Li Y-X. Mitochondrial Genomes of Two Asexual Trichogramma (Hymenoptera: Trichogrammatidae) Strains and Comparison with Their Sexual Relatives. Insects. 2022; 13(6):549. https://doi.org/10.3390/insects13060549
Chicago/Turabian StyleYan, Zhi-Chao, Guang-Yuan Qi, Tian-Yi Yao, and Yuan-Xi Li. 2022. "Mitochondrial Genomes of Two Asexual Trichogramma (Hymenoptera: Trichogrammatidae) Strains and Comparison with Their Sexual Relatives" Insects 13, no. 6: 549. https://doi.org/10.3390/insects13060549
APA StyleYan, Z.-C., Qi, G.-Y., Yao, T.-Y., & Li, Y.-X. (2022). Mitochondrial Genomes of Two Asexual Trichogramma (Hymenoptera: Trichogrammatidae) Strains and Comparison with Their Sexual Relatives. Insects, 13(6), 549. https://doi.org/10.3390/insects13060549






