Seasonal and Year-Round Distributions of Bactrocera dorsalis (Hendel) and Its Risk to Temperate Fruits under Climate Change
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Databases Used in Modeling
2.1.1. Species Occurrence Data of B. dorsalis
2.1.2. Environmental Data
2.2. Protocol of MaxEnt Modeling
2.2.1. Background Selection
2.2.2. Parameter Set and Model Evaluation
2.2.3. Model Projection to Predict the Potential Distribution
2.3. Spatial Analyses for Quantifying the Area at Risk of Damage
3. Results
3.1. Model Evaluation
3.2. Current Potential Distributions
3.3. Future Changes in Suitable Areas
3.4. Risks to Global Fruit Production
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hulme, P.E.; Pyšek, P.; Nentwig, W.; Vilà, M. Will Threat of Biological Invasions Unite the European Union? Science 2009, 324, 40–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Blackburn, T.M.; Song, T.; Li, X.; Huang, C.; Li, Y. Risks of Biological Invasion on the Belt and Road. Curr. Biol. 2019, 29, 499–505.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bacon, S.J.; Aebi, A.; Calanca, P.; Bacher, S. Quarantine arthropod invasions in Europe: The role of climate, hosts and propagule pressure. Divers. Distrib. 2014, 20, 84–94. [Google Scholar] [CrossRef]
- Paini, D.R.; Sheppard, A.W.; Cook, D.C.; De Barro, P.J.; Worner, S.P.; Thomas, M.B. Global threat to agriculture from invasive species. Proc. Natl. Acad. Sci. USA 2016, 113, 7575–7579. [Google Scholar] [CrossRef] [Green Version]
- Ekesi, S.; Meyer, M.D.; Mohamed, S.A.; Virgilio, M.; Borgemeister, C. Taxonomy, Ecology, and Management of Native and Exotic Fruit Fly Species in Africa. Annu. Rev. Entomol. 2016, 61, 219–238. [Google Scholar] [CrossRef]
- Clarke, A.R.; Armstrong, K.F.; Carmichael, A.E.; Milne, J.R.; Raghu, S.; Roderick, G.K.; Yeates, D.K. Invasive phytophagous pests arising through a recent tropical evolutionary radiation: The Bactrocera dorsalis complex of fruit flies. Annu. Rev. Entomol. 2005, 50, 293–319. [Google Scholar] [CrossRef] [Green Version]
- Aketarawong, N.; Bonizzoni, M.; Thanaphum, S.; Gomulski, L.M.; Gasperi, G.; Malacrida, A.R.; Gugliemino, C.R. Inferences on the population structure and colonization process of the invasive oriental fruit fly, Bactrocera dorsalis (Hendel). Mol. Ecol. 2007, 16, 3522–3532. [Google Scholar] [CrossRef]
- EPPO. Bactrocera dorsalis. In Data Sheets on Quarantine Pests; CAB International: Wallingford, UK, 2009. [Google Scholar]
- Ye, H.; Chen, P. Expansion tendency of Bactrocera dorsalis in China. In Research on Biological Invasions in China; Wang, F.H., Guo, J.Y., Zhang, F., Eds.; Science Press: Beijing, China, 2009; pp. 90–92. [Google Scholar]
- De Villiers, M.; Hattingh, V.; Kriticos, D.J.; Brunel, S.; Vayssières, J.F.; Sinzogan, A.; Billah, M.K.; Mohamed, S.A.; Mwatawala, M.; Abdelgader, H.; et al. The potential distribution of Bactrocera dorsalis: Considering phenology and irrigation patterns. Bull. Entomol. Res. 2016, 106, 19–33. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Hui, C.; Plant, R.E.; Su, M.; Papadopoulos, N.T.; Carpenter, T.E.; Li, Z.; Carey, J.R. The failure of success: Cyclic recurrences of a globally invasive pest. Ecol. Appl. 2019, 29, e01991. [Google Scholar] [CrossRef]
- Hill, M.P.; Terblanche, J.S. Niche overlap of congeneric invaders supports a single-species hypothesis and provides insight into future invasion risk: Implications for global management of the Bactrocera dorsalis complex. PLoS ONE 2014, 9, e90121. [Google Scholar] [CrossRef] [Green Version]
- Bale, J.S.; Masters, G.J.; Hodkinson, I.D.; Awmack, C.; Bezemer, T.M.; Brown, V.K.; Butterfield, J.; Buse, A.; Coulson, J.C.; Farrar, J.; et al. Herbivory in global climate change research: Direct effects of rising temperature on insect herbivores. Glob. Chang. Biol. 2002, 8, 1–16. [Google Scholar] [CrossRef]
- IPCC. Summary for Policymakers. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., Eds.; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Peterson, A.T. Townsend Predicting the Geography of Species’ Invasions via Ecological Niche Modeling. Q. Rev. Biol. 2003, 78, 419–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeschke, J.M.; Strayer, D.L. Usefulness of Bioclimatic Models for Studying Climate Change and Invasive Species. Ann. N. Y. Acad. Sci. 2008, 1134, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Veloz, S.D. Spatially autocorrelated sampling falsely inflates measures of accuracy for presence-only niche models. J. Biogeogr. 2009, 36, 2290–2299. [Google Scholar] [CrossRef]
- Boria, R.A.; Olson, L.E.; Goodman, S.M.; Anderson, R.P. Spatial filtering to reduce sampling bias can improve the performance of ecological niche models. Ecol. Model. 2014, 275, 73–77. [Google Scholar] [CrossRef]
- Brown, J.L.; Bennett, J.R.; French, C.M. SDMtoolbox 2.0: The next generation Python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. PeerJ 2017, 5, e4095. [Google Scholar] [CrossRef] [Green Version]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Marchioro, C.A. Global Potential Distribution of Bactrocera carambolae and the Risks for Fruit Production in Brazil. PLoS ONE 2016, 11, e0166142. [Google Scholar] [CrossRef] [Green Version]
- Phillips, S.J.; Dudík, M.; Elith, J.; Graham, C.H.; Lehmann, A.; Leathwick, J.; Ferrier, S. Sample selection bias and presence-only distribution models: Implications for background and pseudo-absence data. Ecol. Appl. 2009, 19, 181–197. [Google Scholar] [CrossRef] [Green Version]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Kriticos, D.J.; Webber, B.L.; Leriche, A.; Ota, N.; Macadam, I.; Bathols, J.; Scott, J.K. CliMond: Global high-resolution historical and future scenario climate surfaces for bioclimatic modelling. Methods Ecol. Evol. 2012, 3, 53–64. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A., Jr. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Morales, N.S.; Fernández, I.C.; Baca-González, V. MaxEnt’s parameter configuration and small samples: Are we paying attention to recommendations? A systematic review. PeerJ 2017, 5, e3093. [Google Scholar] [CrossRef] [PubMed]
- Radosavljevic, A.; Anderson, R.P. Making better Maxent models of species distributions: Complexity, overfitting and evaluation. J. Biogeogr. 2014, 41, 629–643. [Google Scholar] [CrossRef]
- Cobos, M.E.; Peterson, A.T.; Barve, N.; Osorio-Olvera, L. kuenm: An R package for detailed development of ecological niche models using Maxent. PeerJ 2019, 7, e6281. [Google Scholar] [CrossRef] [Green Version]
- Peterson, A.T.; Papeş, M.; Soberón, J. Rethinking receiver operating characteristic analysis applications in ecological niche modeling. Ecol. Model. 2008, 213, 63–72. [Google Scholar] [CrossRef]
- Monfreda, C.; Ramankutty, N.; Foley, J.A. Farming the planet: 2. Geographic distribution of crop areas, yields, physiological types, and net primary production in the year 2000. Glob. Biogeochem. Cycles 2008, 22. [Google Scholar] [CrossRef]
- Clarke, A.R.; Li, Z.; Qin, Y.; Zhao, Z.H.; Liu, L.; Schutze, M.K. Bactrocera dorsalis (Hendel) (Diptera: Tephritidae) is not invasive through Asia: It’s been there all along. J. Appl. Entomol. 2019, 143, 797–801. [Google Scholar] [CrossRef]
- Stephens, A.E.A.; Kriticos, D.J.; Leriche, A. The current and future potential geographical distribution of the oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae). Bull. Entomol. Res. 2007, 97, 369–378. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, C.; Zhao, Z.; Pan, X.; Li, Z. Climate change impacts on the global potential geographical distribution of the agricultural invasive pest, Bactrocera dorsalis (Hendel) (Diptera: Tephritidae). Clim. Chang. 2019, 155, 145–156. [Google Scholar] [CrossRef]
- Biosecurity Australia. Report of the Assessment of Northern China’s Fruit Fly Pest Free Areas: Hebei, Shandong and Xinjiang (Technical Report); Biosecurity: Canberra, Australia, 2009. [Google Scholar]
- Han, P.; Wang, X.; Niu, C.-Y.; Dong, Y.-C.; Zhu, J.-Q.; Desneux, N. Population dynamics, phenology, and overwintering of Bactrocera dorsalis (Diptera: Tephritidae) in Hubei Province, China. J. Pest. Sci. 2011, 84, 289–295. [Google Scholar] [CrossRef]
- European Food Safety Authority; Loomans, A.; Diakaki, M.; Kinkar, M.; Schenk, M.; Vos, S. Pest survey card on Bactrocera dorsalis. EFSA Support. Publ. 2019, 16, 1714E. [Google Scholar] [CrossRef]
- O’Donnell, M.S.; Ignizio, D.A. Bioclimatic predictors for supporting ecological applications in the conterminous United States. U.S. Geol. Surv. Data Ser. 2012, 691, 4–9. [Google Scholar]
- Lodge, D.M.; Williams, S.; MacIsaac, H.J.; Hayes, K.R.; Leung, B.; Reichard, S.; Mack, R.N.; Moyle, P.B.; Smith, M.; Andow, D.A.; et al. Biological invasions: Recommendations for U.S. policy and management. Ecol. Appl. 2006, 16, 2035–2054. [Google Scholar] [CrossRef] [Green Version]
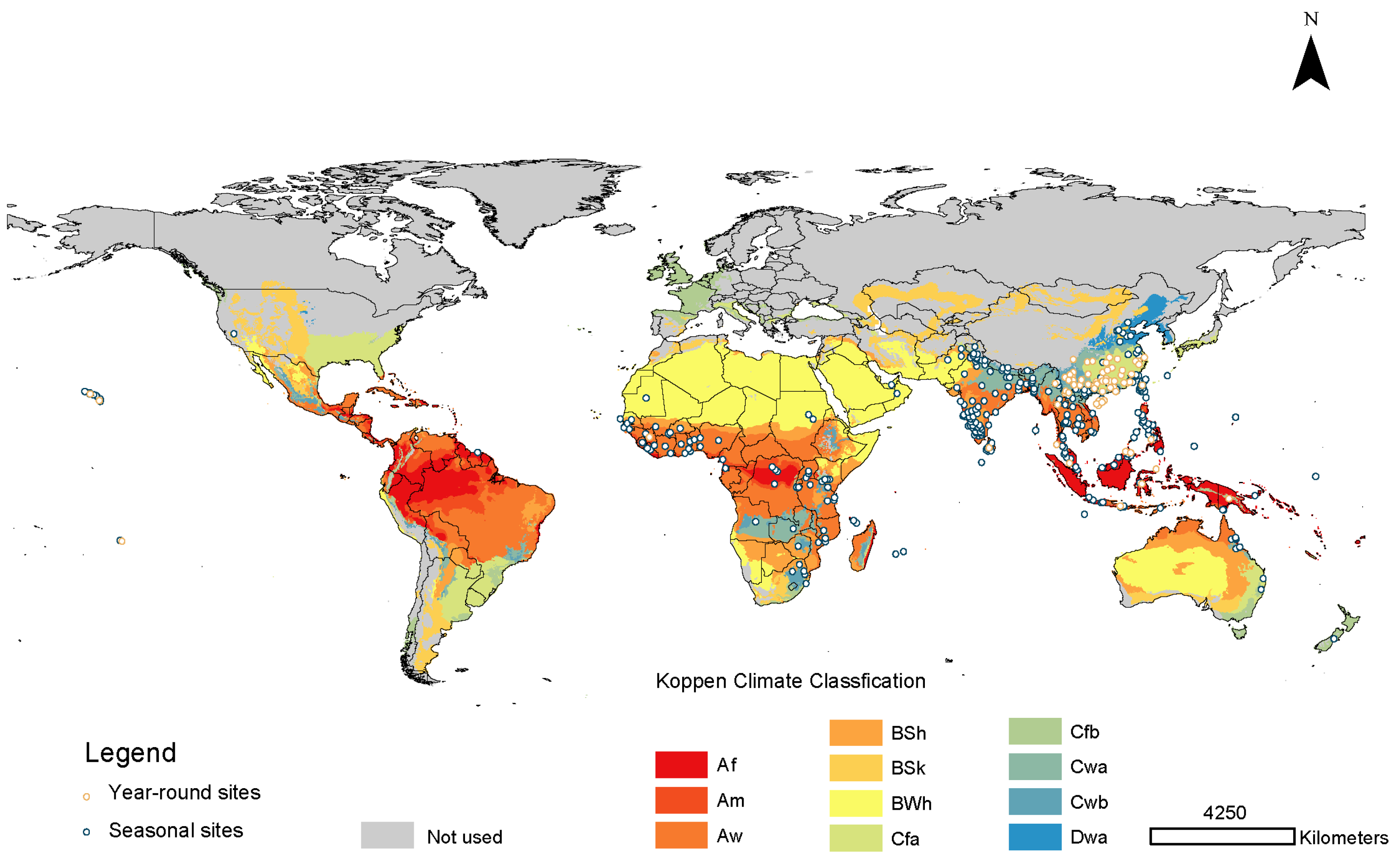
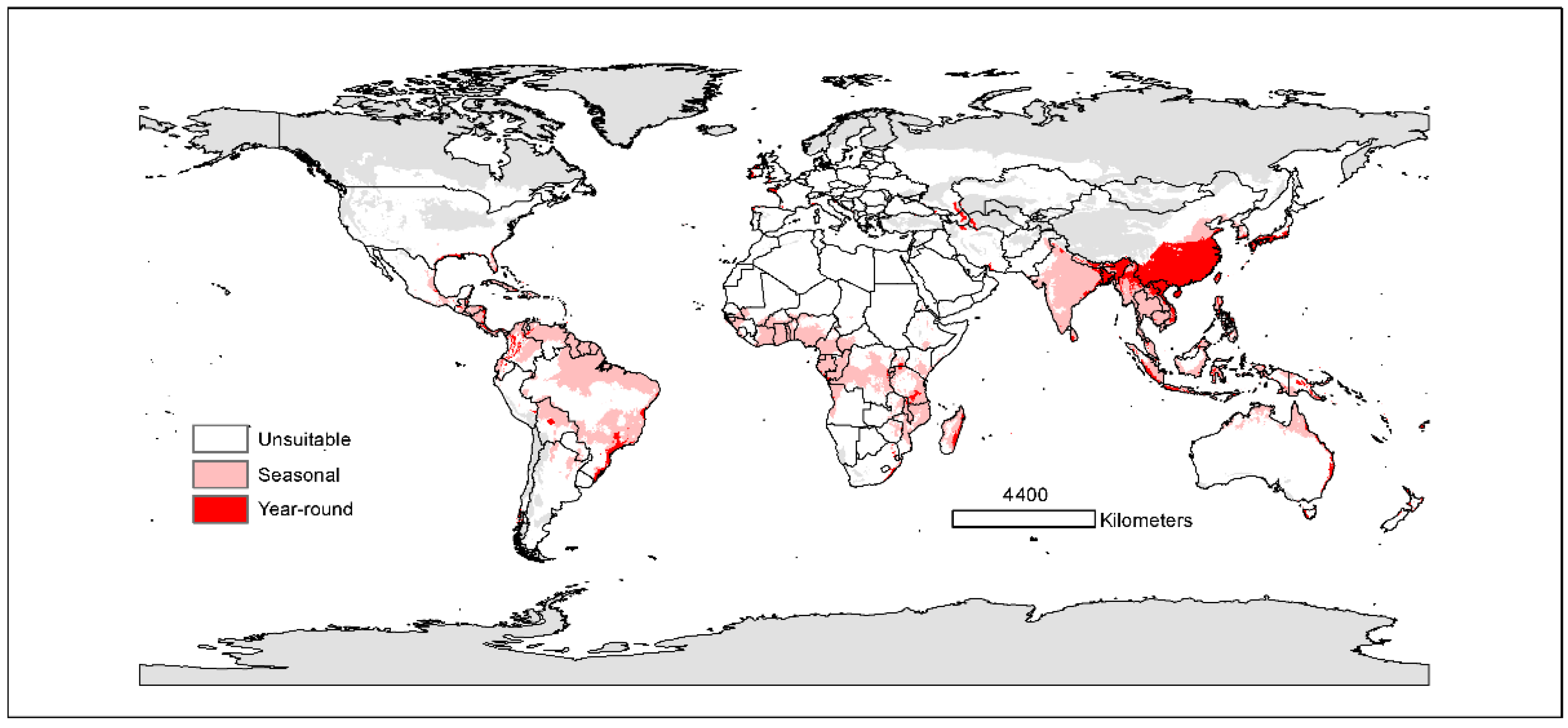

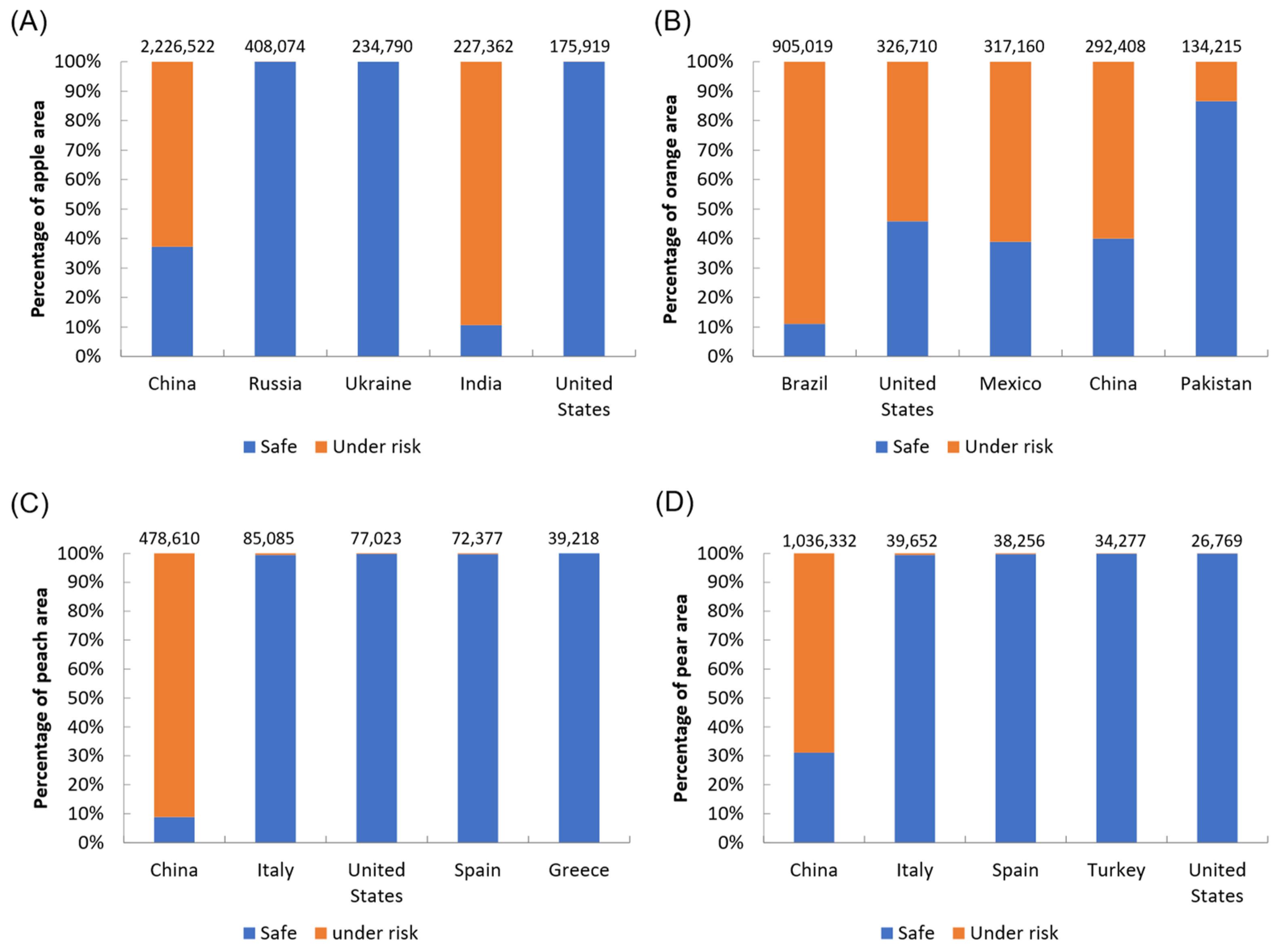
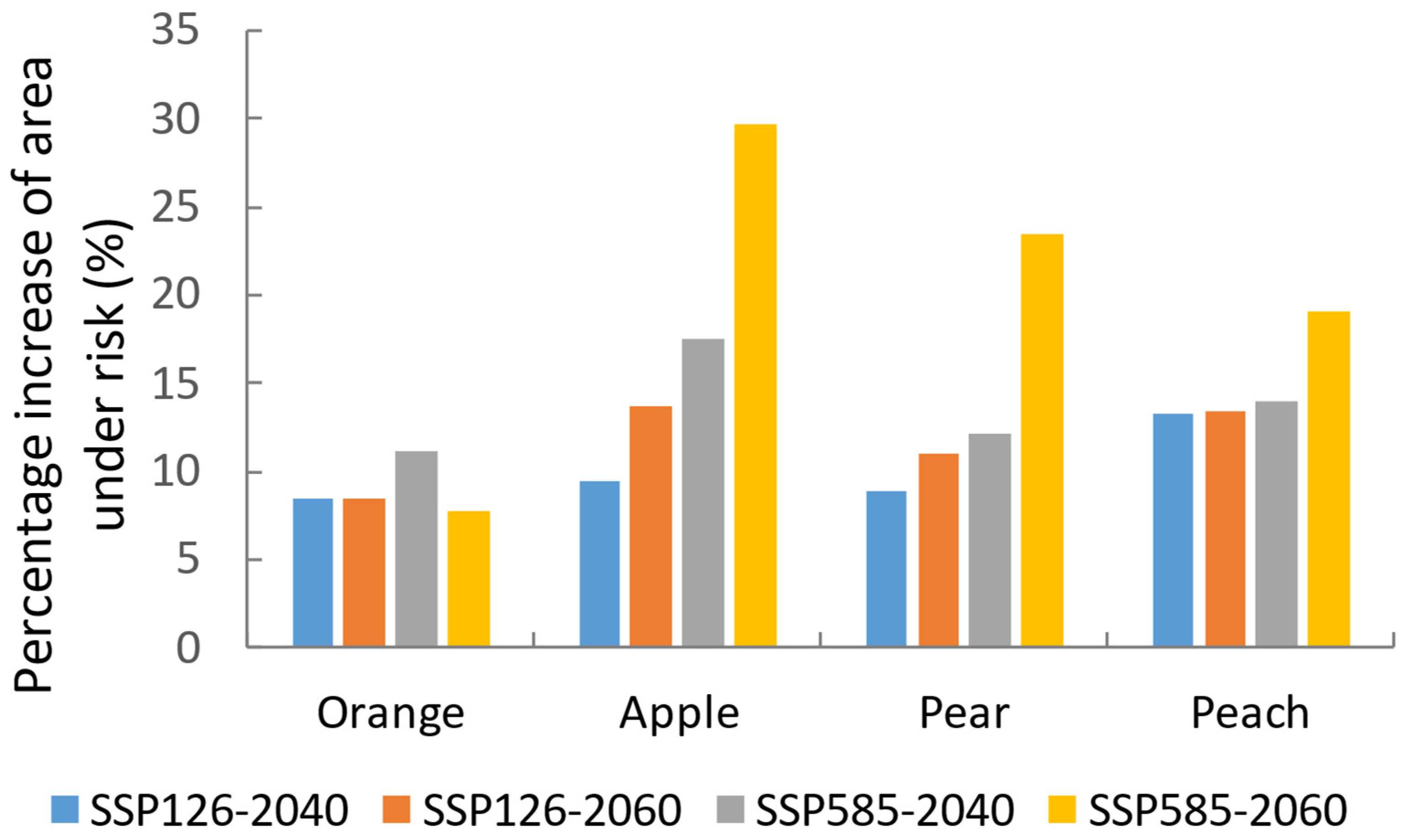
| RM | FC | Pred. Sets | Partial ROC | Omission Rate | AICc | Delta AICc | Weight AICc | |
|---|---|---|---|---|---|---|---|---|
| Seasonal | 3 | PH | Set 1 | 0 | 0.04 | 7422.17 | 0.00 | 0.51 |
| 1 | LQP | Set 2 | 0 | 0.04 | 7423.00 | 0.82 | 0.34 | |
| Year-round | 1 | LQP | Set 1 | 0 | 0.03 | 1385.06 | 0.00 | 0.99 |
| Seasonal | Year-Round | ||||
|---|---|---|---|---|---|
| Variable Name | Variable Description | Contribution (%) | Permutation Importance | Contribution (%) | Permutation Importance |
| Bio1 | Annual mean temperature | 5.3 | 13.7 | 8.0 | 20.5 |
| Bio2 | Mean diurnal temperature range | 27.2 | 32.3 | 57.7 | 26.9 |
| Bio5 | Max temperature of warmest month | 2.6 | 0.8 | 5.0 | 11 |
| Bio6 | Min temperature of coldest month | 1.6 | 8.4 | 19.4 | 30.9 |
| Bio12 | Annual precipitation | 1.3 | 7.3 | 2.7 | 7.3 |
| Bio13 | Precipitation of wettest month | 56.2 | 36.2 | 5.0 | 2 |
| Bio14 | Precipitation of driest month | 5.9 | 1.3 | 2.2 | 1.4 |
| Bio15 | Precipitation seasonality | - | - | - | - |
| Seasonal | Year-Round | |||||||
|---|---|---|---|---|---|---|---|---|
| Unsuitable | Low | Medium | High | Unsuitable | Low | Medium | High | |
| Current | 248.74 | 11.83 | 11.66 | 5.11 | 270.35 | 3.21 | 1.91 | 1.86 |
| SSP126-2040 | 242.24 | 12.58 | 13.83 | 8.69 | 268.46 | 4.01 | 2.77 | 2.10 |
| SSP126-2060 | 241.08 | 12.81 | 14.26 | 9.19 | 268.32 | 4.12 | 3.07 | 1.83 |
| SSP585-2040 | 240.13 | 13.16 | 14.43 | 9.63 | 267.96 | 4.20 | 2.57 | 2.60 |
| SSP585-2060 | 236.79 | 13.30 | 15.03 | 12.21 | 268.07 | 4.13 | 2.72 | 2.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Z.; He, Y.; Ren, Y.; Wang, G.; Chu, D. Seasonal and Year-Round Distributions of Bactrocera dorsalis (Hendel) and Its Risk to Temperate Fruits under Climate Change. Insects 2022, 13, 550. https://doi.org/10.3390/insects13060550
Dong Z, He Y, Ren Y, Wang G, Chu D. Seasonal and Year-Round Distributions of Bactrocera dorsalis (Hendel) and Its Risk to Temperate Fruits under Climate Change. Insects. 2022; 13(6):550. https://doi.org/10.3390/insects13060550
Chicago/Turabian StyleDong, Zhaoke, Yitong He, Yonglin Ren, Guanjin Wang, and Dong Chu. 2022. "Seasonal and Year-Round Distributions of Bactrocera dorsalis (Hendel) and Its Risk to Temperate Fruits under Climate Change" Insects 13, no. 6: 550. https://doi.org/10.3390/insects13060550
APA StyleDong, Z., He, Y., Ren, Y., Wang, G., & Chu, D. (2022). Seasonal and Year-Round Distributions of Bactrocera dorsalis (Hendel) and Its Risk to Temperate Fruits under Climate Change. Insects, 13(6), 550. https://doi.org/10.3390/insects13060550






