Sweep Sampling Comparison of Terrestrial Insect Communities Associated with Herbaceous Stratum in the Riparian Zone of the Miho River, Korea
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
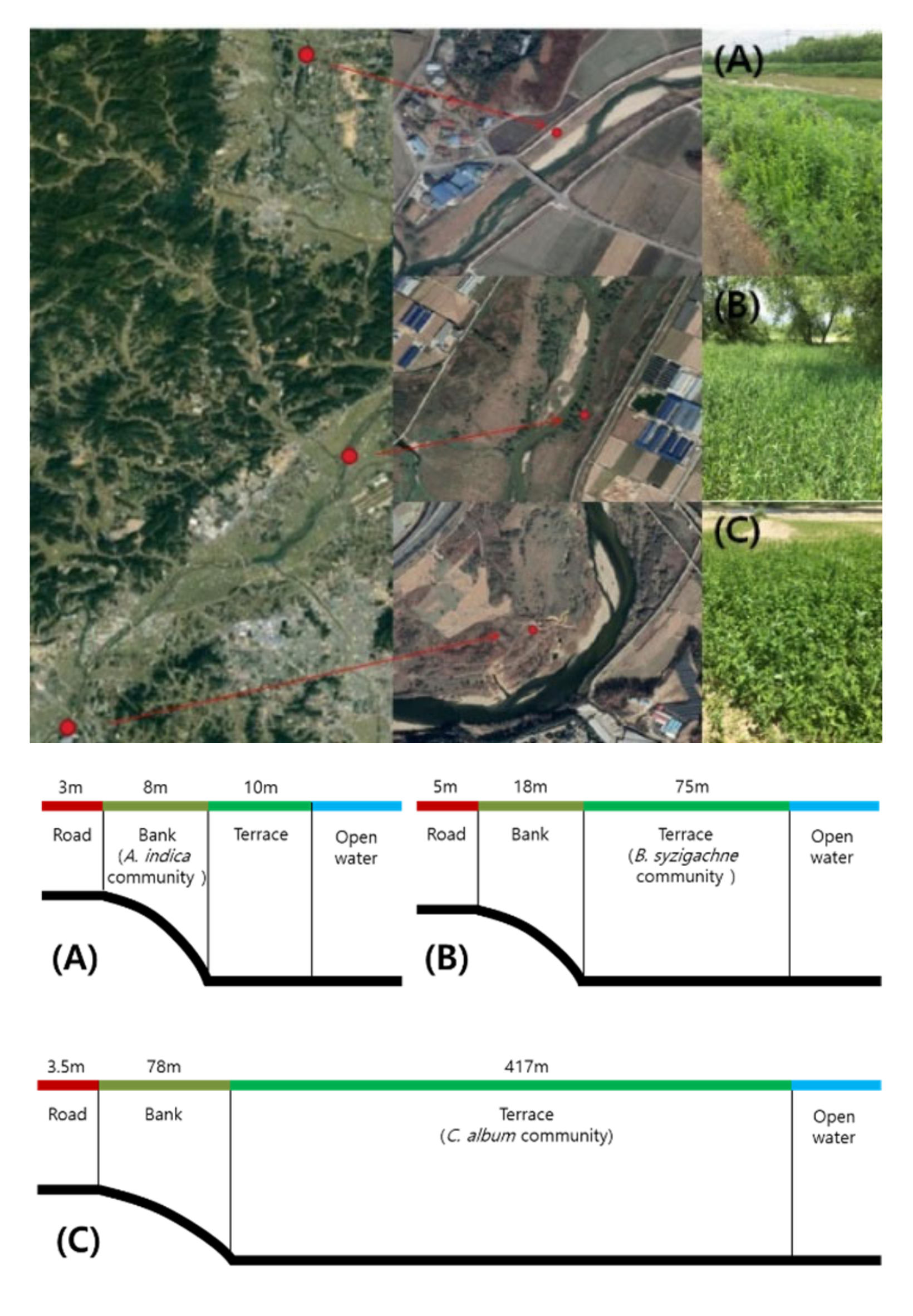
2.1. Survey Site Characteristics
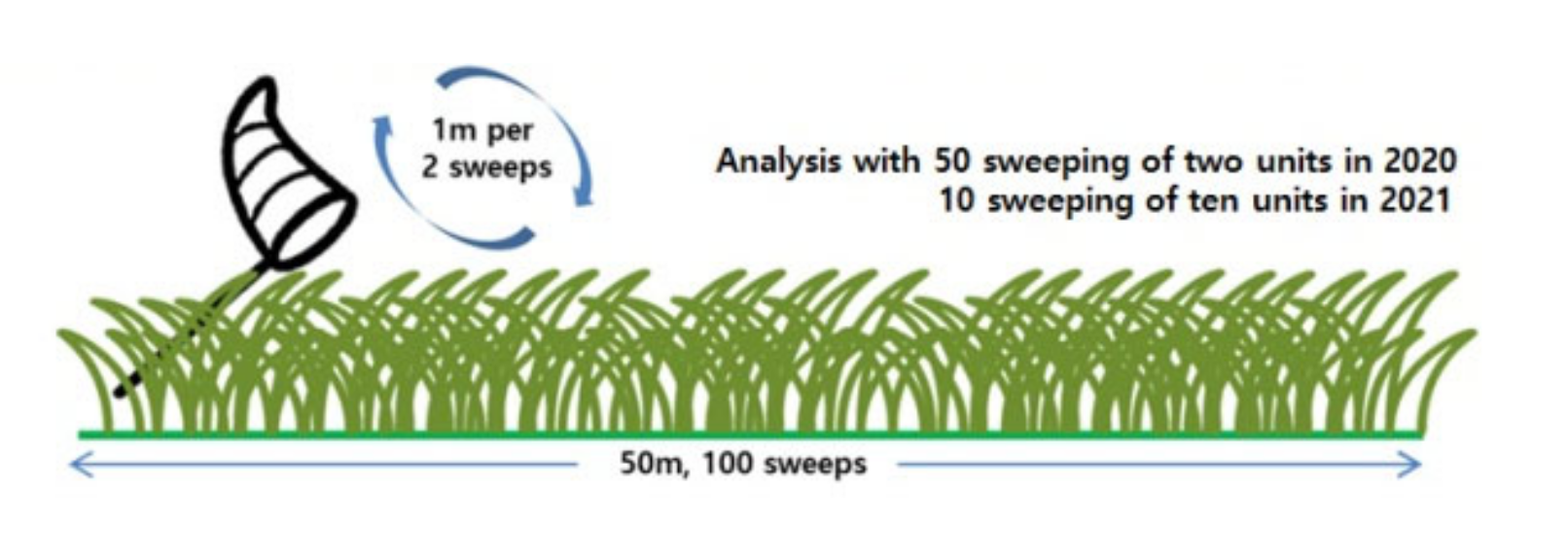
2.2. Survey Methods
2.3. Identification of Plant and Insect Species
2.4. Community Structure Analysis
3. Results
3.1. Analysis by Site and Year
3.2. Comparison of the Number of Species and Individuals by Order in Each Site and Season
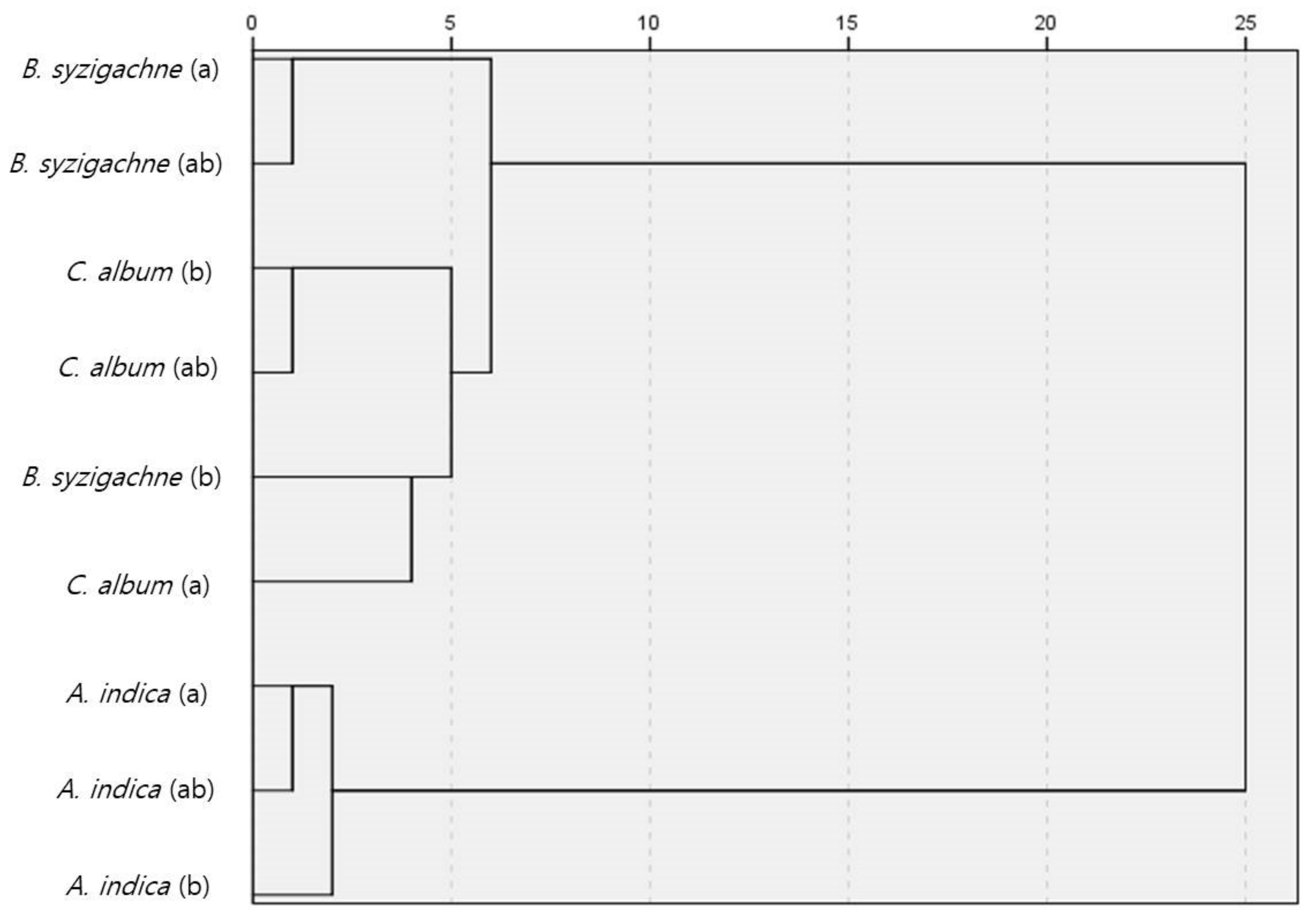
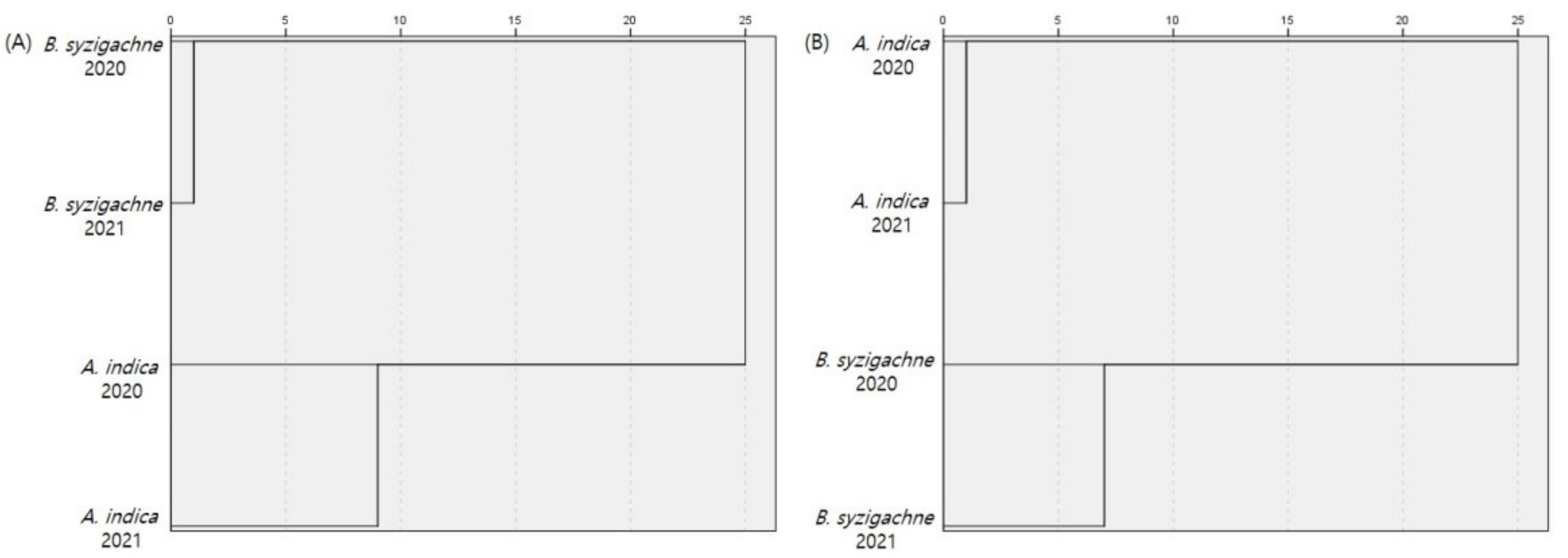
3.3. Similarity Analysis
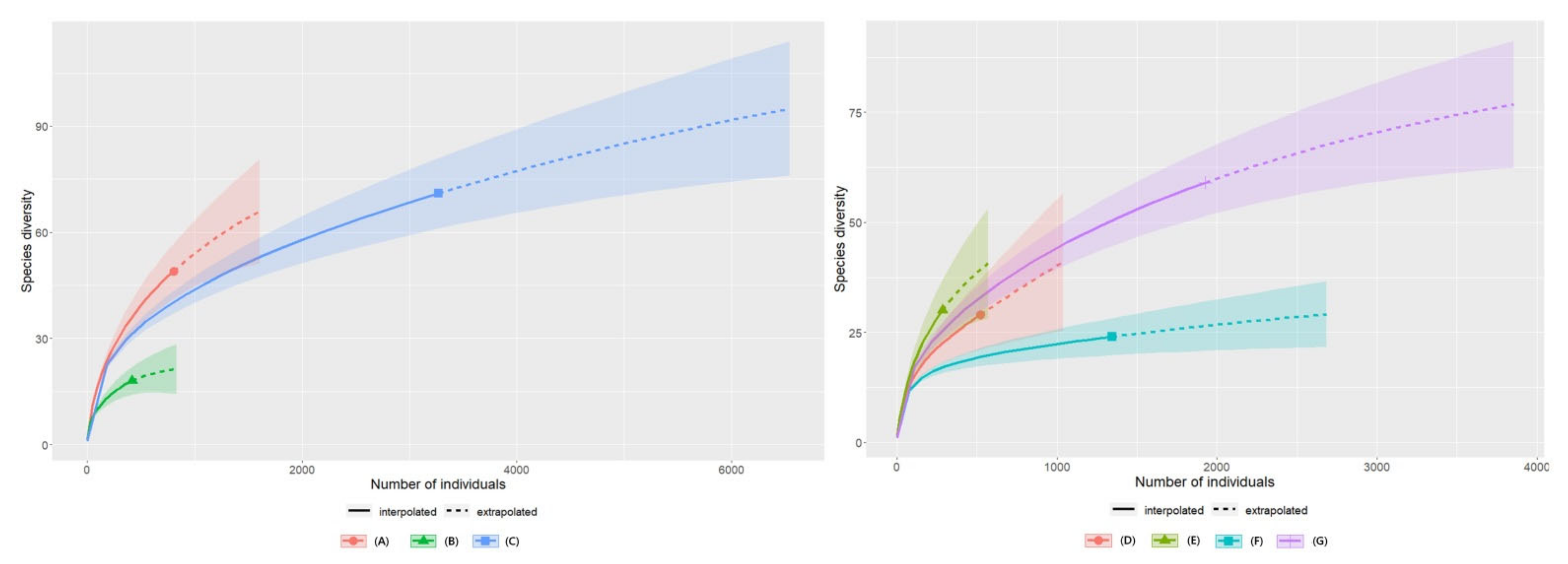
3.4. Species Diversity Abundance Curves of the Insects According to Plant Communities in 2020 and 2021
3.5. Sampling Method Impact on Species Diversity and Insect Sample Coverage Curves by Plant Communities in 2021
3.6. The Coefficient of Variation of the Insect Samples in 2021
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Granados-Sánchez, D.; Hernández-García, M.Á.; López-Ríos, G.F. Ecología de las Zonas Ribereñas; Universidad Autonoma Chapingo: Texcoco, Mexico, 2006; pp. 55–69. ISSN 2007-3828. [Google Scholar]
- Naiman, R.J.; Decamps, H.; Pollock, M. The role of riparian corridors in maintaining regional biodiversity. Ecol. Appl. 1993, 3, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.H.; Kim, S.Y.; Kim, E.; Yoon, J.H.; Yim, M.Y.; An, S.L. Terrestrial insect herbivore communities of riparian zone in urban ecological river, Korea. J. Asia Pac. Biodivers. 2021, 14, 313–320. [Google Scholar] [CrossRef]
- García-Martínez, M.Á.; Valenzuela-González, J.E.; Escobar-Sarria, F.; López-Barrera, F.; Castaño-Meneses, G. The surrounding landscape influences the diversity of leaf-litter ants in riparian cloud forest remnants. PLoS ONE 2017, 12, e0172464. [Google Scholar] [CrossRef] [PubMed]
- García-Martínez, M.Á.; Escobar-Sarria, F.; López-Barrera, F.; Castaño-Meneses, G.; Valenzuela-González, J.E. Value of riparian vegetation remnants for leaf-litter ants (Hymenoptera: Formicidae) in a human-dominated landscape in Central Veracruz, Mexico. Environ. Entomol. 2015, 44, 1488–1497. [Google Scholar] [CrossRef]
- Graziano, M.P.; Deguire, A.K.; Surasinghe, T.D. Riparian buffers as a critical landscape feature: Insights for riverscape conservation and policy renovations. Diversity 2022, 14, 172. [Google Scholar] [CrossRef]
- Farrell, B.D.; Mitter, C.; Futuyma, D.J. Diversification at the insect-plant interface. BioScience 1992, 42, 34–42. [Google Scholar] [CrossRef]
- Frenzel, M.; Brandl, R. Diversity and abundance patterns of phytophagous insect communities on alien and native host plants in the Brassicaceae. Ecography 2003, 26, 723–730. [Google Scholar] [CrossRef]
- Wenninger, E.J.; Inouye, R.S. Insect community response to plant diversity and productivity in a sagebrush–steppe ecosystem. J. Arid Environ. 2008, 72, 24–33. [Google Scholar] [CrossRef]
- Zhang, K.; Lin, S.; Ji, Y.; Yang, C.; Wang, X.; Yang, C.; Wang, H.; Jiang, H.; Harrison, R.D.; Yu, D.W. Plant diversity accurately predicts insect diversity in two tropical landscapes. Mol. Ecol. 2016, 25, 4407–4419. [Google Scholar] [CrossRef]
- Klein, J.T.; Redaelli, L.R.; Barcellos, A. Andropogon bicornis (Poales, Poaceae): A hibernation site for Pentatomoidea (Hemiptera: Heteroptera) in a rice-growing region of southern Brazil. Neotrop. Entomol. 2013, 42, 240–245. [Google Scholar] [CrossRef]
- Denys, C.; Tscharntke, T. Plant-insect communities and predator-prey ratios in field margin strips, adjacent crop fields, and fallows. Oecologia 2002, 130, 315–324. [Google Scholar] [CrossRef]
- Choo, H.Y.; Woo, K.S.; Shea, P.J. Phytophagous insect fauna of Dicotyledoneae (Tracheophyta: Angiospermae) weeds. Korean J. Appl. Entomol. 1992, 31, 496–508. [Google Scholar]
- Paik, C.H.; Lee, G.H.; Kang, J.G.; Jeon, Y.K.; Choi, M.Y.; Seo, H.Y. Plant flora and insect fauna in the fallow paddy fields of Jeonnam and Jeonbuk province. Korean J. Appl. Entomol. 2009, 48, 285–294. [Google Scholar] [CrossRef]
- Park, C.H.; Choi, M.Y.; Seo, H.Y.; Lee, G.H.; Kim, J.D. Stink bug species and host plants occurred in fallow lands for rice product regulation. Korean J. Appl. Entomol. 2007, 46, 221–227. [Google Scholar] [CrossRef]
- Spafford, R.D.; Lortie, C.J. Sweeping beauty: Is grassland arthropod community composition effectively estimated by sweep netting? Ecol. Evol. 2013, 3, 3347–3358. [Google Scholar] [CrossRef]
- Yi, Z.; Jinchao, F.; Dayuan, X.; Weiguo, S.; Axmacher, J.C. A comparison of terrestrial arthropod sampling methods. J. Resour. Ecol. 2012, 3, 174–182. [Google Scholar] [CrossRef]
- Doxon, E.D.; Davis, C.A.; Fuhlendorf, S.D. Comparison of two methods for sampling invertebrates: Vacuum and sweep-net sampling. J. Field Ornithol. 2011, 82, 60–67. [Google Scholar] [CrossRef]
- Swart, R.C.; Pryke, J.S.; Roets, F. Optimising the sampling of foliage arthropods from scrubland vegetation for biodiversity studies. Afr. Entomol. 2017, 25, 164–174. [Google Scholar] [CrossRef]
- Shweta, M.; Rajmohana, K. A comparison of efficiencies of sweep net, yellow pan trap and Malaise trap in sampling Platygastridae (Hymenoptera: Insecta). J. Exp. Zool. India 2016, 19, 393–396. [Google Scholar]
- Musser, F.; Stewart, S.; Bagwell, R.; Lorenz, G.; Catchot, A.; Burris, E.; Cook, D.; Robbins, J.; Greene, J.; Studebaker, G.; et al. Comparison of direct and indirect sampling methods for tarnished plant bug (Hemiptera: Miridae) in flowering cotton. J. Econ. Entomol. 2007, 100, 1916–1923. [Google Scholar] [CrossRef]
- Janzen, D.H.; Pond, C.M. A comparison, by sweep sampling, of the arthropod fauna of secondary vegetation in Michigan, England and Costa Rica. Trans. R. Entomol. Soc. Lond. 1975, 127, 33–50. [Google Scholar] [CrossRef]
- Lowman, M.D. Seasonal variation in insect abundance among three Australian rain forests, with particular reference to phytophagous types. Aust. J. Ecol. 1982, 7, 353–361. [Google Scholar] [CrossRef]
- Liao, Y.C.; Lin, A.C.; Tsai, H.N.; Yen, Y.T.; Tzeng, C.S.; Yang, M.M.; Lin, H.J. The significance of riparian communities in the energy flow of subtropical stream ecosystems. Aquat. Sci. 2022, 84, 20. [Google Scholar] [CrossRef]
- Cook, D.R.; Crow, W.; Gore, J. Performance of selected insecticides against stink bugs infesting soybean, 2018. Arthropod Manag. Tests 2022, 47, tsac048. [Google Scholar] [CrossRef]
- Stoltz, R.L.; Mcneal, C.D., Jr. Assessment of insect emigration from alfalfa hay to bean fields. Environ. Entomol. 1982, 11, 578–580. [Google Scholar] [CrossRef]
- Lee, S.T.; Li, C.; Davis, J.A. Predator-pest dynamics of arthropods residing in louisiana soybean agroecosystems. Insects 2022, 13, 154. [Google Scholar] [CrossRef]
- Supahan, N. Avian assemblage during the development of rice in organic and inorganic rice paddies and its relation to insect pests. Curr. Appl. Sci. Technol. 2022, 22, 34. [Google Scholar] [CrossRef]
- Hur, J.W.; Kim, K.H.; Lee, J.J. Calculation (computation) of habitat suitability index for swimming fish species living in Miho stream in geum river water system. Ecol. Resilient Infrastruct. 2021, 8, 9–21. [Google Scholar] [CrossRef]
- Lee, S.Y.; Jang, R.H.; Han, Y.S.; Jung, Y.H.; Lee, S.I.; Lee, E.P.; You, Y.H. Health condition assessment using the riparian vegetation index and vegetation analysis of Geumgang mainstream and Mihocheon. Korean J. Environ. Ecol. 2018, 32, 105–117. [Google Scholar] [CrossRef]
- Son, Y.M.; Byeon, H.K. The ichthyofauna and dynamics of the fish community in Miho stream, Korea. Korean J. Ichthyol. 2005, 17, 271–278. [Google Scholar]
- Song, H.S.; Seo, J.S.; Nam, Y.G.; Ahn, Y.S.; Park, C.B.; Kim, S.M. Ecological distribution of medicinal plants in Miho stream, Korea. Korean J. Med. Crop. Sci. 2011, 19, 407–413. [Google Scholar] [CrossRef][Green Version]
- AKS (The Academy of Korean Studies). Encyclopedia of Korean Culture. Available online: http://encykorea.aks.ac.kr/Contents/Item/E0020049 (accessed on 10 February 2022).
- KMA (Korean Meteorological Administration). Weather Data Service. Available online: https://data.kma.go.kr/stcs/grnd/grndTaList.do?pgmNo=70 (accessed on 20 October 2021).
- Braun-Blanquet, J. The Study of Plant Communities; Hafner: New York, NY, USA, 1965. [Google Scholar]
- National Institute of Ecology (NIE). National Ecosystem Survey Guide; National Institute of Ecology: Seocheon, Korea, 2021; ISBN 979-11-89730-72-7. [Google Scholar]
- Lee, T.B. Coloured Flora of Korea Volume I; Hyangmunsa: Seoul, Korea, 2006; ISBN 978-89-7187-196-6. [Google Scholar]
- Lee, T.B. Coloured Flora of Korea Volume II; Hyangmunsa: Seoul, Korea, 2006; ISBN 978-89-7187-197-3. [Google Scholar]
- Lee, W.T. Standard Illustrations of Korean Plants; Academy Press: Seoul, Korea, 1996; ISBN 978-89-7616-158-1. [Google Scholar]
- KPNIC (Korean Plant Names Index Committee). Checklist of Vascular Plants in Korea; Korea National Arboretum: Pocheon, Korea, 2017; ISBN 979-11-88720-12-5. [Google Scholar]
- National Institute of Biological Resources (NIBR). National Species List of Korea. Available online: http://kbr.go.kr (accessed on 11 June 2021).
- Ahn, S.J.; Kim, W.G.; Kim, S.S.; Park, C.G. The Terrestrial Heteroptera of Korea; Nature & Ecology: Seoul, Korea, 2018; ISBN 978-89-9742-994-3. [Google Scholar]
- An, S.L. Leef beetle of Korea (Coleoptera: Chrysomelidae); The Planning Sinjin: Seoul, Korea, 2011; ISBN 978-89-963385-5-0. [Google Scholar]
- An, S.L.; Kim, E. A Guide Book of Korean Leaf Beetles; Nature & Ecology: Seoul, Korea, 2020; ISBN 979-11-64500-27-7. [Google Scholar]
- Hong, K.J.; Park, S.; Han, K. Insect Fauna of Korea. Volume 12, Number 2. Arthropoda: Insecta: Coleoptera: Curculionidae: Bagonisnae, Baridinae, Ceutorhynchinae, Conoderinae, Cryptorhynchinae, Molytinae, Orobitidinae Weevils I; National Institute of Biological Resources: Incheon, Korea, 2011; ISBN 9788994555461-96470. [Google Scholar]
- Hong, K.J.; Park, S.; Han, K. Insect Fauna of Korea. Volume 12, number 7. Arthropoda: Insecta: Coleoptera: Curculionidae: Curculioninae, Cossoninae, Mesoptiliinae Weevils II; National Institute of Biological Resources: Incheon, Korea, 2012; ISBN 9788997462353-96470. [Google Scholar]
- NARIS (Korean Natural History Research Information System). Species Information. Available online: https://www.naris.go.kr (accessed on 25 May 2021).
- Park, K.T.; Kwon, Y.J.; Park, J.K.; Bae, Y.S.; Bae, Y.J.; Byun, B.K.; Lee, B.W.; Lee, S.H.; Lee, J.W.; Lee, J.E.; et al. Insect of Korea; Geobook: Seoul, Korea, 2012; ISBN 978-89-94242-11-8. [Google Scholar]
- Takizawa, H. A revision of the genus Psylliodes Latreille in Japan (Chrysomelidae: Alticinae). Insecta Matsumurana. New Ser. J. Fac. Agric. Hokkaido Univ. Ser. Entomol. 2005, 62, 175–185. [Google Scholar]
- Entomological Society of Korean (ESK); Korean Society of Applied Entomology (KSAE). Check List of Insects from Korea; KonKuk University Press: Seoul, Korea, 1994. [Google Scholar]
- Bae, S.; Kim, H.; Mainali, B.P.; Yoon, Y.; Lee, G. Preference of Adult alfalfa Weevil, Hypera postica (Gyllenhal), (Coleoptera: Curculionidae), to Different Seedlings of Upland Crops. Korean J. Appl. Entomol. 2013, 52, 371–377. [Google Scholar] [CrossRef]
- Cristofaro, M.; Lecce, F.; Paolini, A.; Di Cristina, F.; Bon, M.C.; Colonnelli, E.; Smith, L. Host specificity of an Italian population of Cosmobaris scolopacea (Coleoptera: Curculionidae), candidate for the biological control of Salsola tragus (Chenopodiaceae). In Proceeding of the XIII International Symposium on Biological Control of Weeds, Waikoloa, HI, USA, 11–16 September 2011; pp. 20–25. [Google Scholar]
- Heiss, E.; Lee, C.E. On some Piesma from Korea (Heteroptera). Z. Arbeitsgem. Osten Entomol. 1983, 35, 52–54. [Google Scholar]
- Hossain, M.S.; Kwon, J.H. Taxonomic revision of the microleafhopper genus Ziczacella Anufriev 1970 from Korea (Hemiptera: Cicadellidae: Typhlocybinae). Zootaxa 2019, 4571, zootaxa.4571.3.4. [Google Scholar] [CrossRef]
- Kim, J.; Park, H.; Heiss, E.; Jung, S. A new species of the genus Capsus Fabricius (Hemiptera: Heteroptera: Miridae: Mirinae) from the Korean Peninsula, with a key to the Korean Capsus species. Zootaxa 2015, 3905, 585–592. [Google Scholar] [CrossRef][Green Version]
- Kim, D.E.; Kil, J. Geographical distribution and host plants of Corythucha marmorata (Uhler) (Hemiptera: Tingidae) in Korea. Korean J. Appl. Entomol. 2014, 53, 185–191. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lim, G.S. Feeding plants of rice water weevil. Korean J. Appl. Entomol. 1992, 31, 139–143. [Google Scholar]
- Korotyaev, B.A.; Hong, K.J. A revised list of the weevil subfamily Ceutorhynchinae (Coleoptera; Curculionidae) of the Korean fauna, with contribution to the knowledge of the fauna of neighbouring countries. J. Asia Pac. Entomol. 2004, 7, 143–169. [Google Scholar] [CrossRef]
- Lodos, N.; Kalkandelen, A. Preliminary list of Auchenorrhyncha with notes on distribution and importance of species in Turkey. XII. Family Cicadellidae Typhlocybinae: Empoascini. Türkiye Bitki Koruma Derg. 1983, 7, 153–165. [Google Scholar]
- National Institute of Biological Resources (NIBR). Available online: https://species.nibr.go.kr (accessed on 5 January 2022).
- Oh, M.; Yasunaga, T.; Duwal, R.K.; Lee, S. Annotated checklist of the plant bug tribe Mirini (Heteroptera: Miridae: Mirinae) recorded on the Korean Peninsula, with descriptions of three new species. Eur. J. Entomol. 2018, 115, 467–492. [Google Scholar] [CrossRef]
- Pan, H.; Lu, Y.; Wyckhuys, K.A.; Wu, K. Preference of a polyphagous mirid bug, Apolygus lucorum (Meyer-Dür) for flowering host plants. PLoS ONE 2013, 8, e68980. [Google Scholar] [CrossRef]
- Slater, J.A.; Zheng, L.Y. Revision of the genus Stigmatonotum Lindberg (Hemiptera Lygaeidae). Insect Syst. Evol. 1985, 16, 13–25. [Google Scholar] [CrossRef]
- Takeda, M. Genetic basis of photoperiodic control of summer and winter diapause in geographic ecotypes of the rice stem maggot, Chlorops oryzae. Entomol. Exp. Appl. 1998, 86, 59–70. [Google Scholar] [CrossRef]
- Thomas, S.K.; Greeshma, M.; Aswathi, P. Host plant and leaf-age preference of Luprops tristis (Coleoptera: Tenebrionidae: Lagriinae: Lupropini): A home invading nuisance pest in rubber plantation belts. Psyche 2012, 2012, 232735. [Google Scholar] [CrossRef][Green Version]
- Pielou, E.C. Shannon’s formula as a measure of specific diversity, its use and misuse. Am. Nat. 1966, 100, 463–465. [Google Scholar] [CrossRef]
- Margalef, R. Information theory in biology. Gen. Syst. Yearbook 1958, 3, 36–71. [Google Scholar]
- McNaughton, S.J. Relationship among functional properties of California grassland. Nature 1967, 216, 168–169. [Google Scholar] [CrossRef]
- Pielou, E.C. Ecology Diversity; Wiley: New York, NY, USA, 1975; ISBN 978-04-71689-25-6. [Google Scholar]
- Beals, E.W. Bray-Curtis ordination: An effective strategy for analysis of multivariate ecological data. Adv. Ecol. Res. 1984, 14, 1–55. [Google Scholar] [CrossRef]
- Chao, A.; Shen, T.J. User’s Guide for Program SPADE (Species Prediction and Diversity Estimation); National Tsing Hua University: Hsinchu, Taiwan, 2010. [Google Scholar]
- Giovas, C.M. A simple method for quantifying compositional correspondence between Zooarchaeological assemblages using paired similarity indices. J. Archaeol. Method Theor. 2021, 28, 823–844. [Google Scholar] [CrossRef]
- Schroeder, P.J.; Jenkins, D.G. How robust are popular beta diversity indices to sampling error? Ecosphere 2018, 9, e02100. [Google Scholar] [CrossRef]
- Sørensen, T. A method of establishing groups of equal amplitude in plant sociology based on similarity of species content. K. Dan. Vidensk. Selsk. Biol. Skr. 1948, 4, 1–34. [Google Scholar]
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: An R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: http://www.R-project.org/ (accessed on 22 October 2021).
- Zhu, H.; Zou, X.; Wang, D.; Wan, S.; Wang, L.; Guo, J. Responses of community-level plant-insect interactions to climate warming in a meadow steppe. Sci. Rep. 2015, 5, 18654. [Google Scholar] [CrossRef]
- Plum, N. Terrestrial invertebrates in flooded grassland: A literature review. Wetlands 2005, 25, 721–737. [Google Scholar] [CrossRef]
- Fenoglio, M.S.; Rossetti, M.R.; Videla, M. Negative effects of urbanization on terrestrial arthropod communities: A meta-analysis. Glob. Ecol. Biogeogr. 2020, 29, 1412–1429. [Google Scholar] [CrossRef]
- Yim, Y.J.; Jeon, E.S. Distribution of naturalized plants in the Korean Peninsula. Korean J. Bot. 1981, 23, 69–83. [Google Scholar]
- Jones, C.G.; Lawton, J.H. Plant chemistry and insect species richness of British umbellifers. J. Anim. Ecol. 1991, 60, 767–777. [Google Scholar] [CrossRef]
- Dioli, P.; Colamartino, A.D.; Negri, M.; Limonta, L. Hemiptera and Coleoptera on Chenopodium quinoa. Redia 2016, 99, 139–141. [Google Scholar] [CrossRef]
- Huang, D.; Su, Z.; Zhang, R.; Koh, L.P. Degree of urbanization influences the persistence of Dorytomus weevils (Coleoptera: Curculionoidae) in Beijing, China. Landsc. Urban Plan. 2010, 96, 163–171. [Google Scholar] [CrossRef]
- Jo, J.S.; Choe, G.R. Analysis of Insect Community on Mugwort, Artemisia princeps var. orientalis, in Taejon. Korean J. Entomol. 2001, 31, 77–84. [Google Scholar]
- Duwal, R.K.; Jung, S.; Lee, S. Review of Europiella Reuter (Hemiptera: Heteroptera: Miridae: Phylinae: Phylini) from Korea, with a description of a new genus. Zootaxa 2014, 3795, 383–393. [Google Scholar] [CrossRef]
- Crossley, D.A.; Howden, H.F. Insect-vegetation relationships in an area contaminated by radioactive wastes. Ecology 1961, 42, 302–317. [Google Scholar] [CrossRef]
- Hwang, J.H.; Kim, E.; Choi, E.Y.; Choi, J.B.; Park, J.K. Insect diversity in Gonggeom-ji, the first protected paddy field wetland in Korea. Entomol. Res. 2020, 50, 221–228. [Google Scholar] [CrossRef]
- Kang, J.; Park, T.; Park, J.; Kim, J.; Jeong, Y.; Park, J.J. Survey and analysis of insect species in the South Korea habitat (Yeoncheon) of the water spider, Argyroneta aquatica. Korean J. Environ. Biol. 2017, 35, 476–491. [Google Scholar] [CrossRef]
- Son, J.K.; Kong, M.J.; Kang, D.H.; Kang, B.H.; Yun, S.W.; Lee, S.Y. The comparative studies on the terrestrial insect diversity in protected horticulture complex and paddy wetland. J. Wetlands Res. 2016, 18, 386–393. [Google Scholar] [CrossRef]
- Vince, S.W.; Valiela, I.; Teal, J.M. An experimental study of the structure of herbivorous insect communities in a salt marsh. Ecology. 1981, 62, 1662–1678. [Google Scholar] [CrossRef]
- Delong, D.M. 1932. Some problems encountered in the estimation of insect populations by the sweeping method. Ann. Entomol. Soc. Am. 1932, 25, 13–17. [Google Scholar] [CrossRef]
- Dorman, S.J.; Taylor, S.V.; Malone, S.; Roberts, P.M.; Greene, J.K.; Reisig, D.D.; Smith, R.H.; Jacobson, A.L.; Reay-Jones, F.P.F.; Paula-Moraes, S.; et al. Sampling optimization and crop interface effects on Lygus lineolaris populations in southeastern USA cotton. Insects 2022, 13, 88. [Google Scholar] [CrossRef]
- Popescu, C.; Oprina-Pavelescu, M.; Dinu, V.; Cazacu, C.; Burdon, F.J.; Forio, M.A.E.; Kupilas, B.; Friberg, N.; Goethals, P.; McKie, B.G.; et al. Riparian vegetation structure influences terrestrial invertebrate communities in an agricultural landscape. Water 2021, 13, 188. [Google Scholar] [CrossRef]
- Ismay, J.W.; Lee, S. Notes on Korean Chloropidae (Diptera). Korean J. Appl. Entomol. 1998, 37, 1–7. [Google Scholar]
- Lee, K.Y.; Chang, Y.D.; Ahn, K.S.; Kang, H.J.; Park, S.K. Effect of temperature on reproduction and development of rice water weevil, Lissorhoptrus oryzophilus (Coleoptera: Curculionidae). Korean J. Appl. Entomol. 2002, 41, 123–128. [Google Scholar]
- Kogan, M.; Lattin, J.D. Insect conservation and pest management. Biodivers. Conserv. 1993, 2, 242–257. [Google Scholar] [CrossRef]
- Lee, Y.K.; Kim, J.W. Riparian Vegetation of South Korea; Keimyung University Press: Daegu, Korea, 2005; ISBN 978-89-7585-313-5. [Google Scholar]
- Ida, T.Y.; Takanashi, K.; Tamura, M.; Ozawa, R.; Nakashima, Y.; Ohgushi, T. Defensive chemicals of neighboring plants limit visits of herbivorous insects: Associational resistance within a plant population. Ecol. Evol. 2018, 8, 12981–12990. [Google Scholar] [CrossRef]


| Chenopodium album 2020 | Beckmannia syzigachne 2020 | Beckmannia syzigachne 2021 | Artemisia indica 2020 | Artemisia indica 2021 | |
|---|---|---|---|---|---|
| Individuals and species diversity of insects | 2 orders 10 families 18 species 417 individuals | 4 orders 15 families 29 species 520 individuals | 4 orders 18 families 30 species 284 individuals | 4 orders 14 families 24 species 1343 individuals | 4 orders 29 families 59 species 1927 individuals |
| Insect *H’/DI/RI/EI | 1.59/0.68/2.82/0.55 | 1.8/0.7/4.48/0.54 | 1.57/0.76/5.13/0.46 | 1.63/0.73/3.19/0.51 | 2.25/0.54/7.67/0.55 |
| Dominant insect species | P. attenuata | P. attenuata | C. cinctus | A. vittata | A. vittata |
| Subdominant insect species | Orthotylus flavosparsus | C. cinctus | Dorytomus imbecillus | Europiella artemisiae | Europiella artemisiae |
| Diversity of plant taxa | 12 families 19 genera 25 species 1 var.) | 14 families 18 genera 16 species 5 var.) | 10 families 13 genera 15 species 3 var.) | 15 families 21 genera 20 species 2 var.) | 13 families 18 genera 17 species 3 var.) |
| Matching rate of insect host plants with plant flora | 100 | 82.6 | 66.7 | 87.5 | 66.7 |
| Sampling completeness based on individuals | 0.986 | 0.975 | 0.951 | 0.996 | 0.987 |
| C. album 2020 | B. syzigachne 2020 | B. syzigachne 2021 | A. indica 2020 | A. indica 2021 | ||
|---|---|---|---|---|---|---|
| Hemiptera | Species | 7 | 12 | 10 | 13 | 26 |
| Individuals | 128 | 167 | 204 | 1160 | 1358 | |
| Coleoptera | Species | 11 | 14 | 14 | 8 | 21 |
| Individuals | 289 | 340 | 62 | 85 | 117 | |
| Diptera | Species | - | 2 | 4 | 1 | 5 |
| Individuals | - | 11 | 14 | 26 | 267 | |
| Hymenoptera | Species | - | - | 2 | 2 | 7 |
| Individuals | - | - | 4 | 72 | 185 | |
| Odonata | Species | - | 1 | - | - | - |
| Individuals | - | 2 | - | - | - | |
| B. syzigachne | A. indica | |
|---|---|---|
| Hemiptera | 46.1 | 29.18 |
| Coleoptera | 43.16 | 43.08 |
| Diptera | 57.14 | 53.21 |
| Hymenoptera | 165.83 | 150.74 |
| Total | 36.97 | 35.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, J.H.; Yim, M.-Y.; Kim, S.-Y.; Ji, S.J.; Lee, W.-H. Sweep Sampling Comparison of Terrestrial Insect Communities Associated with Herbaceous Stratum in the Riparian Zone of the Miho River, Korea. Insects 2022, 13, 497. https://doi.org/10.3390/insects13060497
Hwang JH, Yim M-Y, Kim S-Y, Ji SJ, Lee W-H. Sweep Sampling Comparison of Terrestrial Insect Communities Associated with Herbaceous Stratum in the Riparian Zone of the Miho River, Korea. Insects. 2022; 13(6):497. https://doi.org/10.3390/insects13060497
Chicago/Turabian StyleHwang, Jeong Ho, Mean-Young Yim, Sung-Yeol Kim, Seong Jin Ji, and Wang-Hee Lee. 2022. "Sweep Sampling Comparison of Terrestrial Insect Communities Associated with Herbaceous Stratum in the Riparian Zone of the Miho River, Korea" Insects 13, no. 6: 497. https://doi.org/10.3390/insects13060497
APA StyleHwang, J. H., Yim, M.-Y., Kim, S.-Y., Ji, S. J., & Lee, W.-H. (2022). Sweep Sampling Comparison of Terrestrial Insect Communities Associated with Herbaceous Stratum in the Riparian Zone of the Miho River, Korea. Insects, 13(6), 497. https://doi.org/10.3390/insects13060497






