A Comparative Analysis of Bombyx mori (Lepidoptera: Bombycidae) β-fructofuranosidase Homologs Reveals Different Post-Translational Regulations in Glyphodes pyloalis Walker (Lepidoptera: Pyralidae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects, Vectors, and Cell Lines
2.2. Enzymatic Determination of β-FFase in Larval Tissues
2.3. Degenerate Polymerase Chain Reaction (Degenerate PCR)
2.4. Reverse Transcription PCR (RT-PCR) and Rapid Amplification of cDNA Ends (RACE)
2.5. Expression of Recombinant Proteins in E. coli and Preparation of GpSUC1a Polyclonal Antibody
2.6. Expression and Purification of Recombinant Proteins through the Bac-to-Bac System
2.7. SDS-PAGE and Western Blotting Analysis
2.8. Immunohistochemistry Assay
2.9. Purification of GpSUC1a and BmSUC1 by Immunoprecipitation (IP) and β-FFase Activity Confirmation
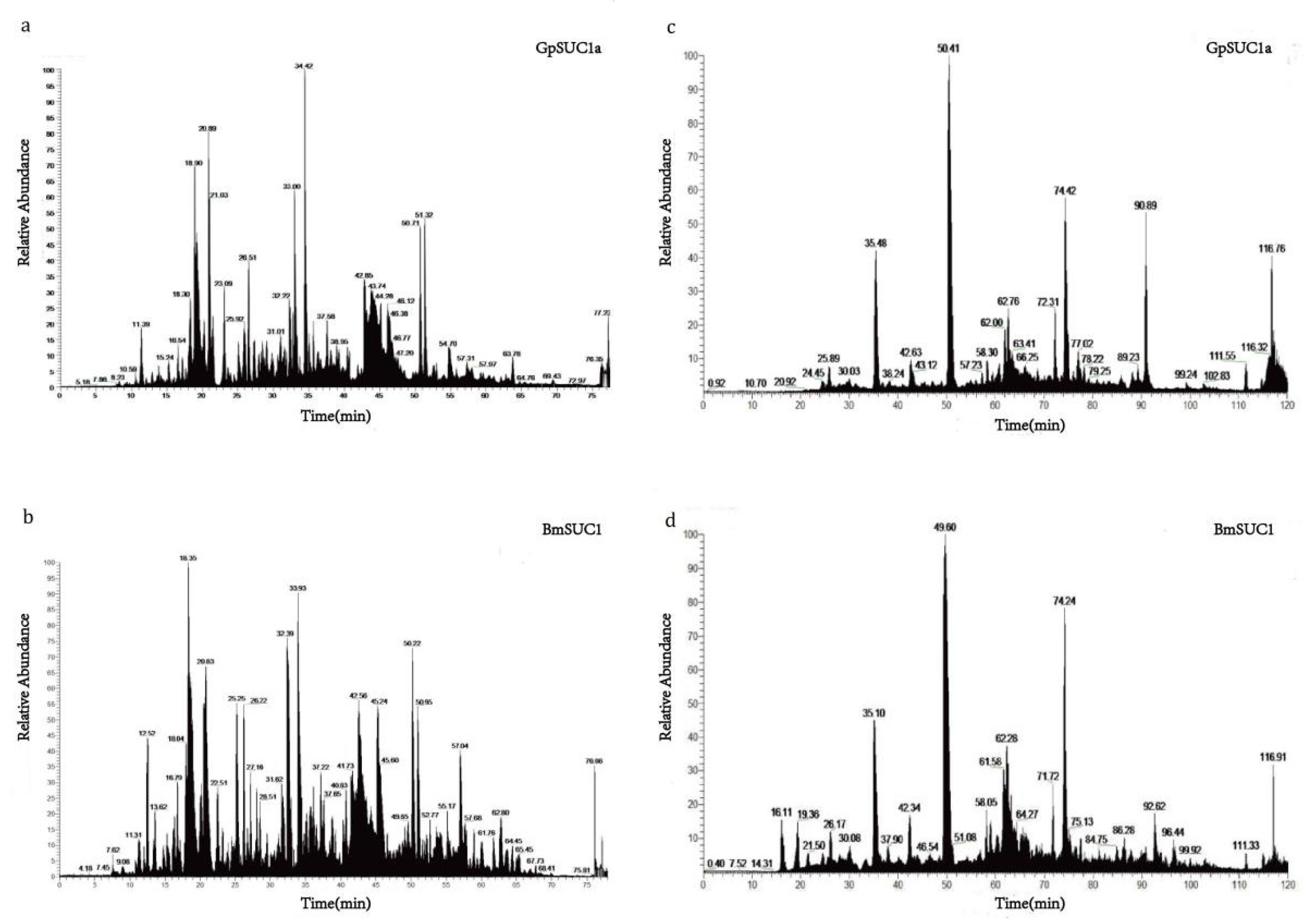
2.10. Liquid Chromatograph-Mass Spectrometer (LC-MS) Analysis
3. Results
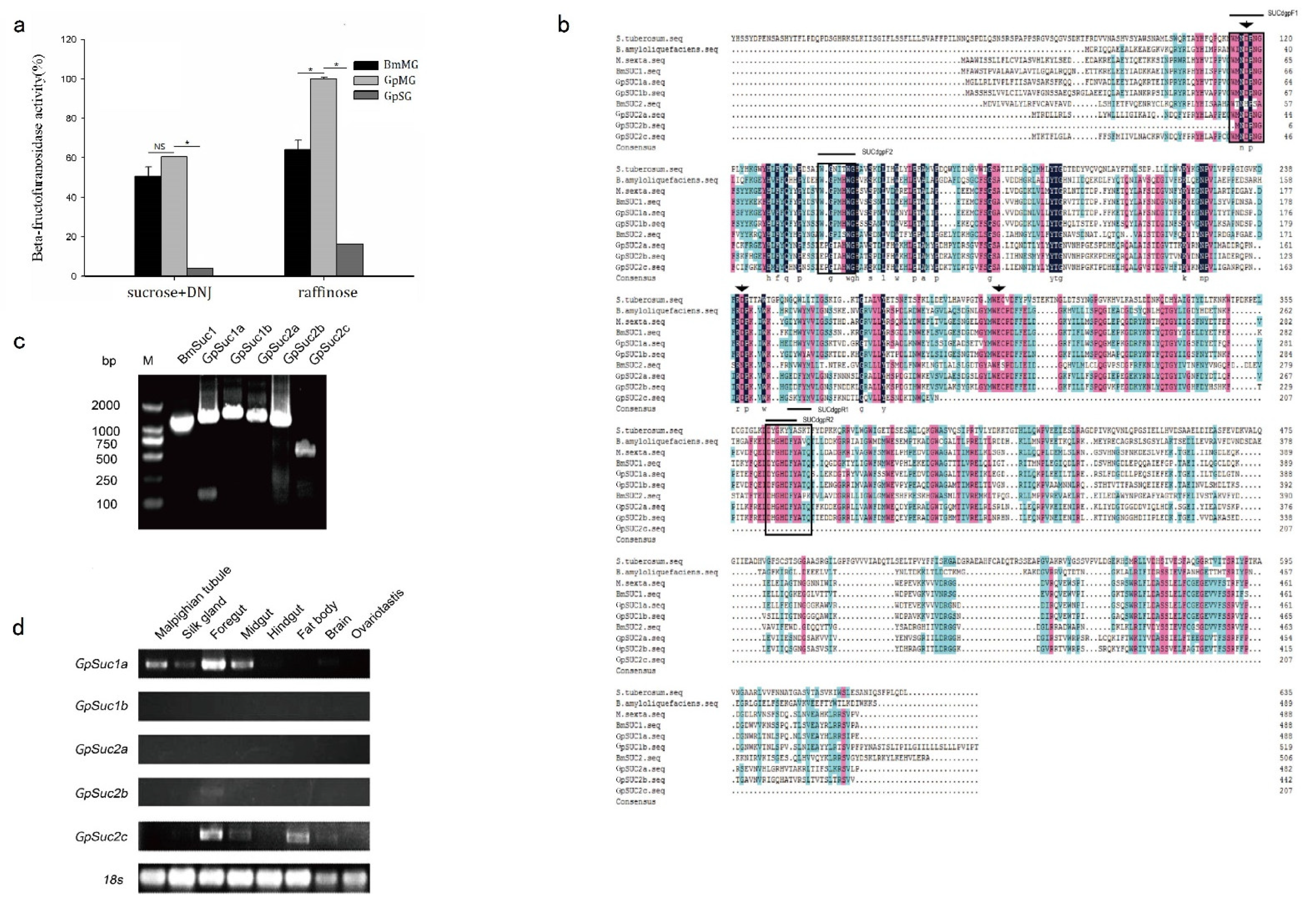
3.1. GpSuc1a Is a Homologous Gene of BmSuc1 Identified in G. pyloalis
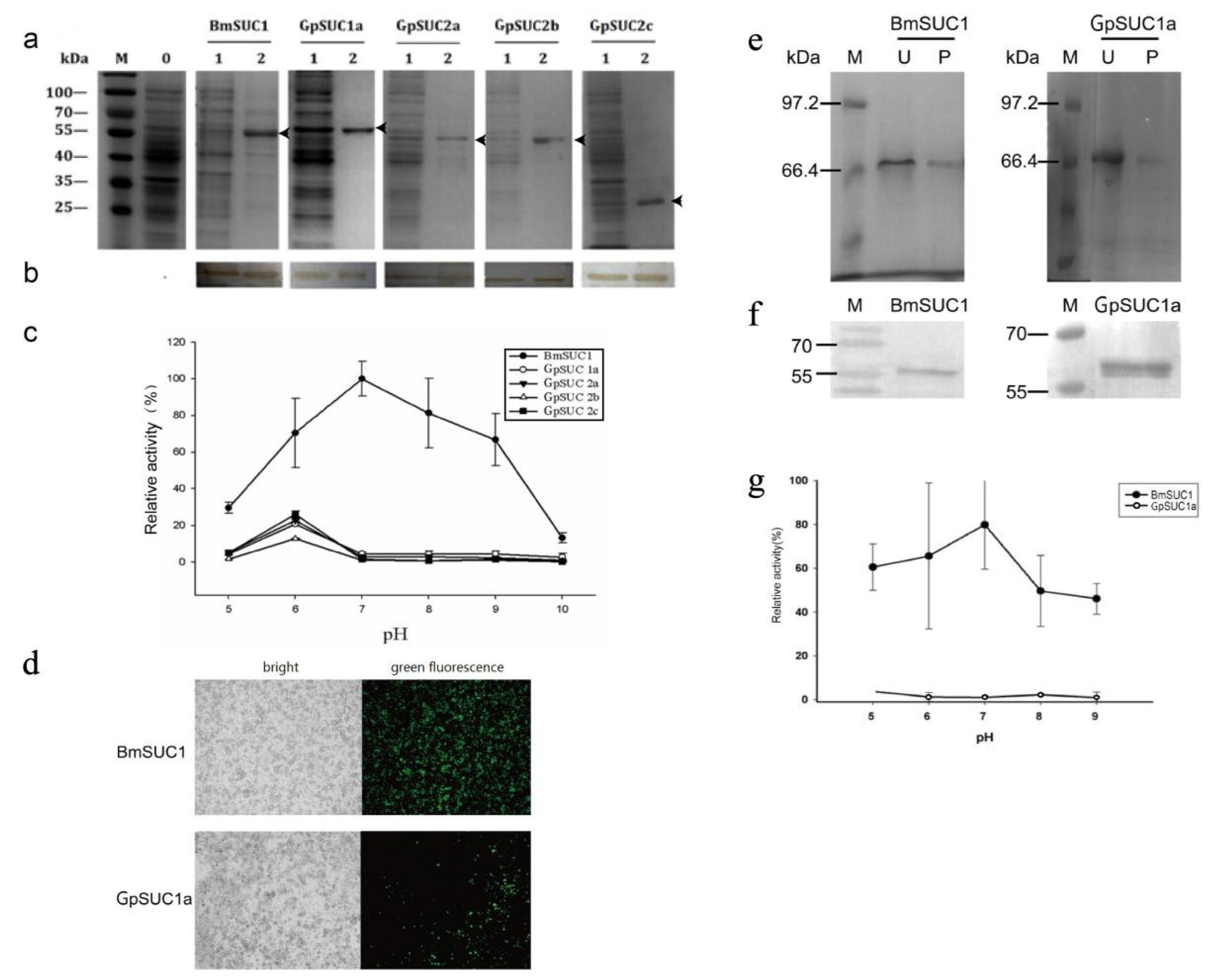
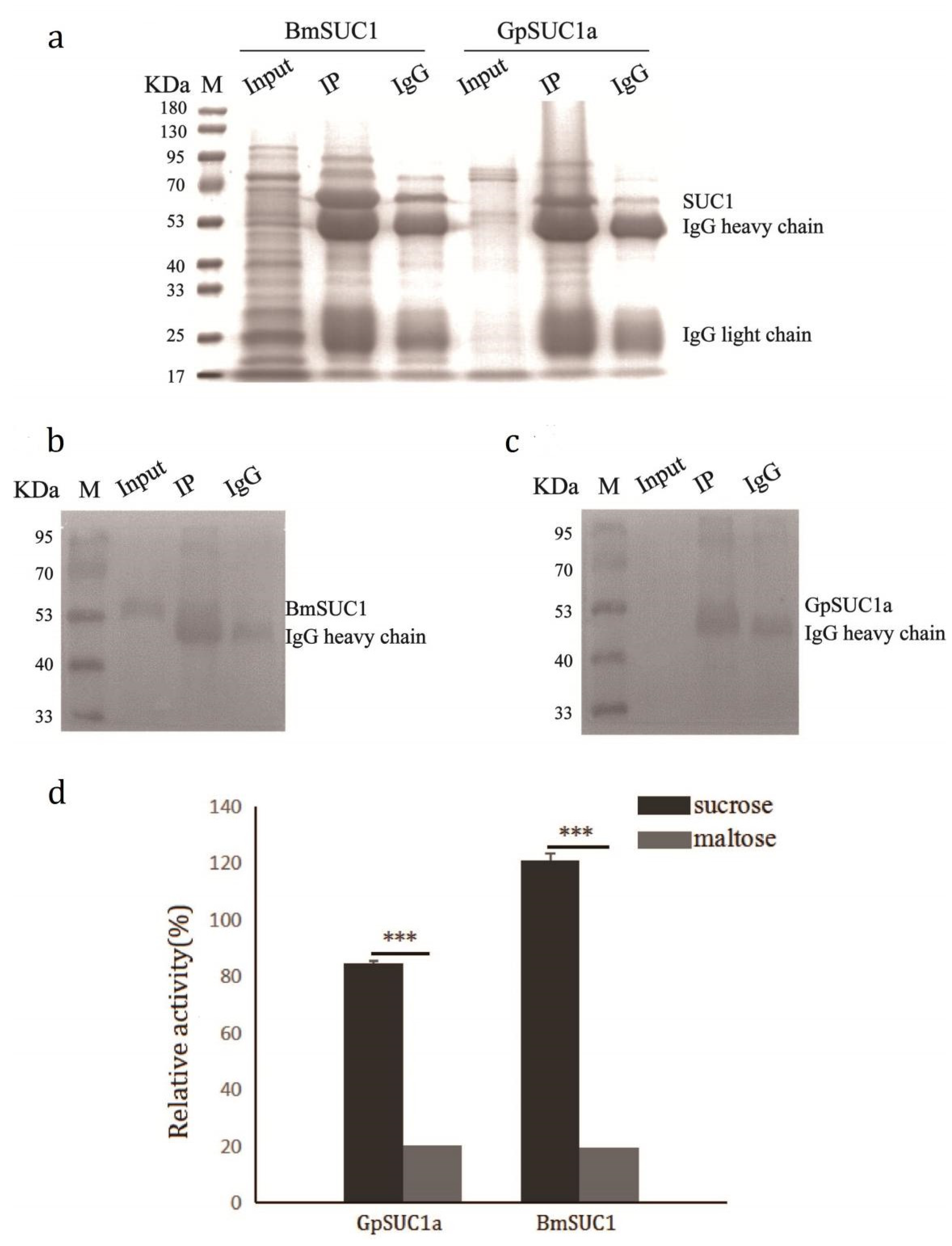
3.2. Recombinant GpSUC1a Showed Lower Activity Compared to BmSUC1
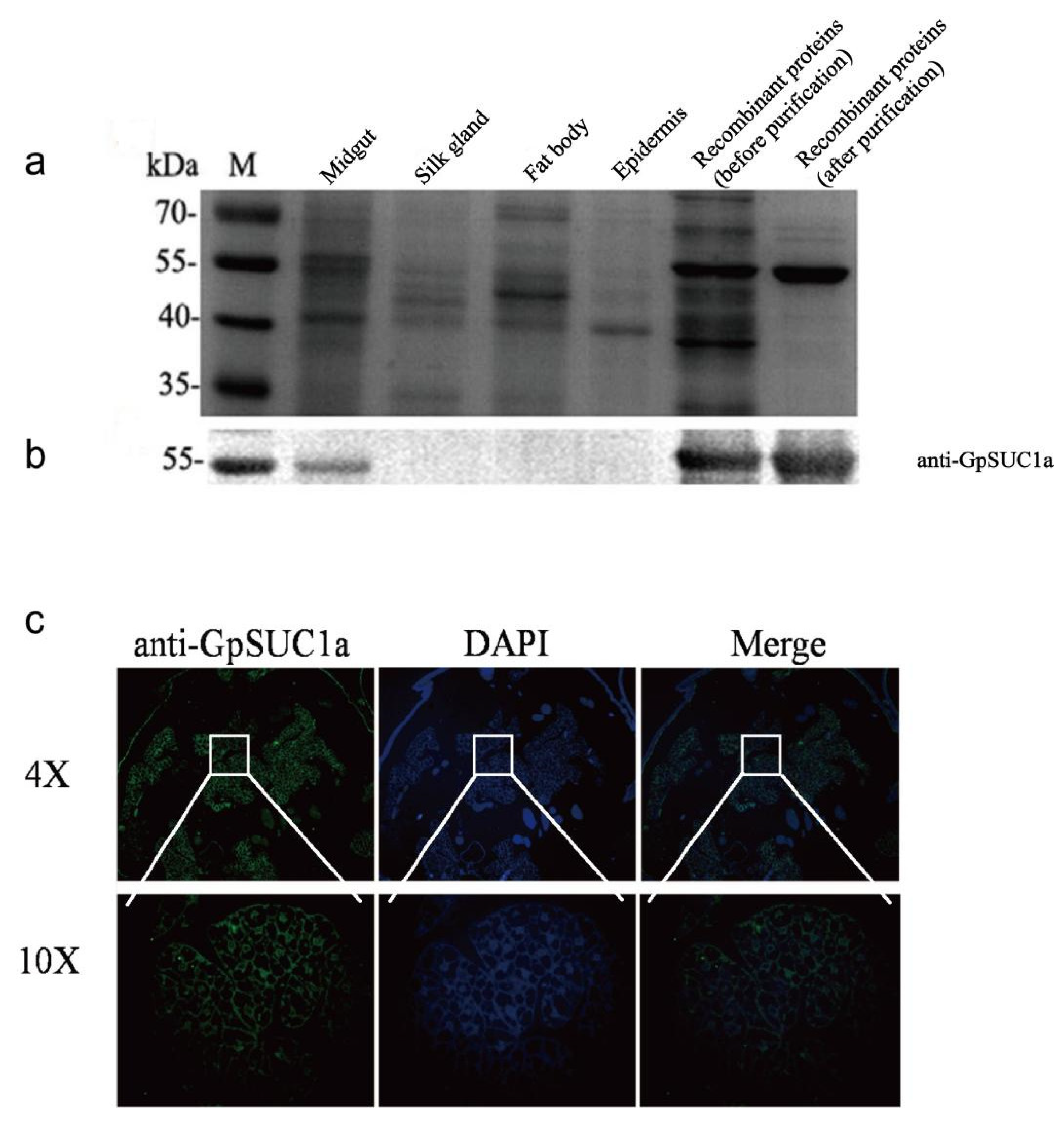
3.3. GpSuc1a Highly Expressed in the Midgut of G. pyloalis and Displayed obvious β-FFase Activity
3.4. Identification of Post-Translational Modifications (PTMs) of GpSUC1a and BmSUC1
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nishida, R. Sequestration of defensive substances from plants by Lepidoptera. Annu. Rev. Entomol. 2002, 47, 57–92. [Google Scholar] [CrossRef] [PubMed]
- Ponce de Leon, I.; Montesano, M. Activation of Defense Mechanisms against Pathogens in Mosses and Flowering Plants. Int. J. Mol. Sci. 2013, 14, 3178–3200. [Google Scholar] [CrossRef] [PubMed]
- Winter, P.S.; Bowman, C.E.; Villani, P.J.; Dolan, T.E.; Hauck, N.R. Systemic acquired resistance in moss: Further evidence for conserved defense mechanisms in plants. PLoS ONE 2014, 9, e101880. [Google Scholar]
- Zagrobelny, M. Cyanogenic glucosides and plant-insect interactions. Phytochemistry 2004, 65, 293–306. [Google Scholar] [CrossRef]
- Agrawal, A.A. Observation, natural history, and an early post-Darwinian view of plant-animal interactions. Am. Nat. 2014, 184, ii–iv. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.; Epping, J.; Gronover, C.S.; Fricke, J.; Aziz, Z.; Brillatz, T.; Swyers, M.; Köllner, T.G.; Vogel, H.; Hammerbacher, A. A Latex Metabolite Benefits Plant Fitness under Root Herbivore Attack. PLoS Biol. 2016, 14, e1002332. [Google Scholar] [CrossRef] [PubMed]
- Mithofer, A.; Boland, W. Plant defense against herbivores: Chemical aspects. Annu. Rev. Plant Biol. 2012, 63, 431–450. [Google Scholar] [CrossRef]
- Nawrot, R. Defense-related Proteins from Chelidonium majus L. as Important Components of its Latex. Curr. Protein Pept. Sci. 2017, 18, 864–880. [Google Scholar] [CrossRef]
- Asano, N.; Yamashita, T.; Yasuda, K.; Ikeda, K.; Ryu, K. Polyhydroxylated alkaloids isolated from mulberry trees (Morusalba L.) and silkworms (Bombyx mori L.). J. Agric. Food Chem. 2001, 49, 4208–4213. [Google Scholar] [CrossRef]
- Konno, K. Plant latex and other exudates as plant defense systems: Roles of various defense chemicals and proteins contained therein. Phytochemistry 2011, 72, 1510–1530. [Google Scholar] [CrossRef]
- Deng, M.J.; Lin, X.D.; Wen, C.W.; Dong, M.J.; Xu, J.P. Metabolic changes in the midgut of Eri silkworm after Oral administration of 1-deoxynojirimycin: A 1H-NMR-based metabonomic study. PLoS ONE 2017, 12, e0173213. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, C.; Konno, K.; Wasano, N.; Nakamura, M. Differential effects of sugar-mimic alkaloids in mulberry latex on sugar metabolism and disaccharidases of Eri and domesticated silkworms: Enzymatic adaptation of Bombyx mori to mulberry defense. Insect Biochem. Mol. Biol. 2007, 37, 1348–1358. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.W.; Lin, X.D.; Dong, M.J.; Deng, M.J. An Evaluation of 1-Deoxynojirimycin Oral Administration in Eri Silkworm through Fat Body Metabolomics Based on (1) H Nuclear Magnetic Resonance. BioMed Res. Int. 2016, 2016, 4676505. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Daimon, T.; Taguchi, T.; Meng, Y.; Katsuma, S.; Mita, K.; Shimada, T. Beta-fructofuranosidase genes of the silkworm, Bombyx mori: Insights into enzymatic adaptation of B. mori to toxic alkaloids in mulberry latex. J. Biol. Chem. 2008, 283, 15271–15279. [Google Scholar] [CrossRef]
- Konno, K.; Ono, H.; Nakamura, M.; Tateishi, K.; Hirayama, C.; Tamura, Y.; Hattori, M.; Koyama, A. Mulberry latex rich in antidiabetic sugar-mimic alkaloids forces dieting on caterpillars. Proc. Natl. Acad. Sci. USA 2006, 103, 1337–1341. [Google Scholar] [CrossRef]
- Carneiro, C.N.; Isejima, E.M.; Samuels, R.I.; Silva, C.P. Sucrose hydrolases from the midgut of the sugarcane stalk borer Diatraea saccharalis. J. Insect Physiol. 2004, 50, 1093–1101. [Google Scholar] [CrossRef]
- Krasikov, V.V.; Karelov, D.V.; Firsov, L.M. alpha-Glucosidases. Biochemistry. Biokhimiia 2001, 66, 267–281. [Google Scholar] [CrossRef]
- Orikasa, Y.; Oda, Y. Molecular characterization of beta-fructofuranosidases from Rhizopus delemar and Amylomyces rouxii. Folia Microbiol. 2013, 58, 301–309. [Google Scholar] [CrossRef]
- Gan, Q.; Zhang, X.; Zhang, D.; Shi, L.; Zhou, Y.; Sun, T.; Jiang, S.; Gao, J.; Meng, Y. BmSUC1 is essential for glycometabolism modulation in the silkworm, Bombyx mori. Biochim. Biophys. Acta 2018, 1861, 543–553. [Google Scholar] [CrossRef]
- Kong, W.; Yang, J. The complete mitochondrial genome of Glyphodes pyloalis Walker (Lepidoptera: Crambidae). DNA. Part A, DNA Mapp. Seq. Anal. 2016, 27, 4044–4045. [Google Scholar]
- Zhu, B.J.; Liu, Q.N.; Dai, L.S.; Wang, L.; Sun, Y.; Lin, K.Z.; Wei, G.Q.; Liu, C.L. Characterization of the complete mitochondrial genome of Glyphodes pyloalis (Lepidoptera: Pyralididae). Gene 2013, 527, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Kurihara, Y.; Wang, Y.X.; Shimizu, T. Mulberry pyralid, Glyphodes pyloalis: Habitual host of nonoccluded viruses pathogenic to the silkworm, Bombyx mori. Invertebr. J. Invertebr. Pathol. 1988, 52, 401–408. [Google Scholar] [CrossRef]
- Yazdani, E.; Sendi, J.J.; Aliakbar, A.; Senthil-Nathan, S. Effect of Lavandula angustifolia essential oil against lesser mulberry pyralid Glyphodes pyloalis Walker (Lep: Pyralidae) and identifification of its major derivatives. Pestic. Biochem. Physiol 2013, 107, 250–257. [Google Scholar] [CrossRef]
- Daimon, T.; Katsuma, S.; Iwanaga, M.; Kang, W.; Shimada, T. The BmChi-h gene, a bacterial-type chitinase gene of Bombyx mori, encodes a functional exochitinase that plays a role in the chitin degradation during the molting process. Insect Biochem. Mol. Biol. 2005, 35, 1112–1123. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, S.; Anizon, F.; Peixoto, P.; David-Cordonnier, M.H.; Golsteyn, R.M.; Léonce, S.; Pfeiffer, B.; Prudhomme, M. Synthesis and biological activities of 7-aza rebeccamycin analogues bearing the sugar moiety on the nitrogen of the pyridine ring. Bioorg. Med. Chem. 2006, 14, 7551–7562. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, M.; Ghadamyari, M.; Sajedi, R.H.; Zavareh, M.; Sheikhnejad, H. Insecticidal effects of 4-hexylresorcinol on the lesser mulberry snout moth, Glyphodes pyloalis Walker. Arch. Phytopathol. Plant Prot. 2013, 46, 1–13. [Google Scholar] [CrossRef]
- Babiker, E.E.; Azakami, H.; Ogawa, T.; Kato, A. Immunological characterization of recombinant soy protein allergen produced by Escherichia coli expression system. J. Agric. Food Chem. 2000, 48, 571–575. [Google Scholar] [CrossRef]
- Hillebrecht, J.R.; Chong, S. A comparative study of protein synthesis in vitro systems: From the prokaryotic reconstituted to the eukaryotic extract-based. BMC Biotechnol. 2000, 8, 58. [Google Scholar] [CrossRef]
- Pal Roy, M.; Mazumdar, D.; Dutta, S.; Saha, S.P.; Ghosh, S. Cloning and Expression of Phytase appA Gene from Shigella sp. CD2 in Pichia pastoris and Comparison of Properties with Recombinant Enzyme Expressed in E. coli. PLoS ONE 2016, 11, e0145745. [Google Scholar] [CrossRef]
- Sangolgi, P.B.; Balaji, C.; Dutta, S.; Jindal, N.; Jarori, G.K. Cloning, expression, purification and characterization of Plasmodium spp. glyceraldehyde-3-phosphate dehydrogenase. Protein Expr. Purif. 2016, 117, 17–25. [Google Scholar] [CrossRef]
- Zhao, N.; Yao, H.; Lan, L.; Cao, C.; Umashankar, M.L.; Lu, X.; Wu, X.; Wang, B.; Cui, W.; Cenis, J.L. Efficient production of canine interferon-alpha in silkworm Bombyx mori by use of a BmNPV/Bac-to-Bac expression system. Appl. Microbiol. Biotechnol. 2008, 78, 221–226. [Google Scholar]
- Porowinska, D.; Wujak, M.; Roszek, K.; Komoszynski, M. Prokaryotic expression systems. Postepy Hig. I Med. Dosw. 2013, 67, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Pitson, S.M.; ANDREA, D.; Richard, J.; Vandeleur, L.; Moretti, P.A.; Xia, P.; Gamble, J.R.; Vadas, M.A.; Wattenberg, B.W. Human sphingosine kinase: Purification, molecular cloning and characterization of the native and recombinant enzymes. Biochem. J. 2000, 350 Pt 2, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Rutschmann, C.; Baumann, S.; Cabalzar, J.; Luther, K.B.; Hennet, T. Recombinant expression of hydroxylated human collagen in Escherichia coli. Appl. Microbiol. Biotechnol. 2014, 98, 4445–4455. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wu, X.; Lan, L.; Liu, J. Expression of Trichoderma reesei endo-beta-glucanase II in silkworm, Bombyx mori L. by using BmNPV/Bac-to-Bac expression system and its bioactivity assay. Biotechnol. Lett. 2010, 32, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Ogiue-Ikeda, M.; Machida, K. Expression and Purification of SH2 Domains Using Baculovirus Expression System. Methods Mol. Biol. 2017, 1555, 183–198. [Google Scholar]
- Michard, C.; Doublet, P. Post-translational modifications are key players of the Legionella pneumophila infection strategy. Front. Microbiol. 2015, 6, 87. [Google Scholar] [CrossRef]
- Hirata, Y.; Takahashi, M.; Morishita, T.; Noguchi, T.; Matsuzawa, A. Post-Translational Modifications of the TAK1-TAB Complex. Int. J. Mol. Sci. 2017, 18, 205. [Google Scholar] [CrossRef]
- Kar, U.P.; Dey, H.; Rahaman, A. Regulation of dynamin family proteins by post-translational modifications. J. Biosci. 2017, 18, 205. [Google Scholar] [CrossRef]
- Vallejo, D.; Codocedo, J.F.; Inestrosa, N.C. Posttranslational Modifications Regulate the Postsynaptic Localization of PSD-95. Mol. Neurobiol. 2017, 54, 1759–1776. [Google Scholar] [CrossRef]
- Imperiali, B.; O’Connor, S.E. Effect of N-linked glycosylation on glycopeptide and glycoprotein structure. Curr. Opin. Chem. Biol. 1999, 3, 643–649. [Google Scholar] [CrossRef]
- Patterson, M.C. Metabolic mimics: The disorders of N-linked glycosylation. Semin. Pediatr. Neurol. 2005, 12, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, N.; Ferreira, L.; Romão, T.P. N-glycosylation influences the catalytic activity of mosquito alpha-glucosidases associated with susceptibility or refractoriness to Lysinibacillus sphaericus. Insect Biochem. Mol. Biol. 2017, 81, 62–71. [Google Scholar] [CrossRef] [PubMed]
| Assay | Primer Name | Primer Sequence (5′ to 3′) |
|---|---|---|
| Degenerate PCR | SUCdgpF1 | TGGATIAAYGAYCCIAAYGG |
| SUCdgpR1 | GTGIGTIGCRTARAAITC | |
| SUCdgpF2 | TGGGGICCIATGCAYTGGGGICA | |
| SUCdgpR2 | GCRTARAARTCRTGICCRTRRTC | |
| RACE | GpSuc1a-5RAgsp | AGCACCACCGGAGAAACACATTTCTT |
| GpSuc1a-5RAngsp | GGGCTGGACACGTGGCCCCAGTGCAT | |
| GpSuc1a-3RAgsp | ACAGTTTACATGTGGGAATGCCCTGA | |
| GpSuc1a-3RAngsp | GGCATGGAACCCAAAGGCGATCGGTT | |
| GpSuc1b-5RAgsp | TCCACTGCCCGAGAAGCACTGTTCTT | |
| GpSuc1b-5RAngsp | GCGCTGGACGAGTGCCCCCAATGCAT | |
| GpSuc1b-3RAgsp | ATGGGGTACATGTGGGAGTGCCCTGA | |
| GpSuc1b-3RAngsp | GGAATGGCACCTCAGGGTGACAGGTA | |
| GPSuc2a-5RAgsp | AGCAATTGGTAGATGCTTCCAATGGAA | |
| GpSuc2a-5RAngsp | ATGCCCCCAATGAGCTATTCCAGGTT | |
| GpSuc2a-3RAgsp | GATGGCGTCACTACTAAAAAGTATCGG | |
| GpSuc2a-3RAngsp | CGCCTCAGGGTATAGAACCGGAAGGA | |
| GpSuc2b-5RAgsp | CTCCACTTTTGTCATATGCCTTGTCC | |
| GpSuc2b-5RAngsp | TCGCTGCTCATGGTCGGGCTTTTTG | |
| GpSuc2b-3RAgsp | CTTGGATACATGTGGGAATGTCCAGATT | |
| GpSuc2b-3RAngsp | CGCCTCAAGGTGTGAAACCTGAAGGA | |
| GpSuc2c-5RAgsp | TCTGTGACTTATGTGCCAAAATGCTG | |
| GpSuc2c-5RAngsp | TTAAGGGAATGCCATTCTCATCCGTT | |
| GpSuc2c-3RAgsp | CTTGGMTAYATGTGGGAATGTCCAGATT | |
| GpSuc2c-3RAngsp | CAAATGACAAAACAAACTGGCAAGAA | |
| RT-PCR | GpSuc1a Fw | ATTGTTGTACACTGGGCGACTAACC |
| GpSuc1a Re | TGGTGAGTCGTTCGGAGTGTACGTA | |
| GpSuc1b Fw | CGCAGCTATGAACAACTTGAGGC | |
| GpSuc1b Re | CTGACCACCACCATTTGTGCCAGT | |
| GpSuc2a Fw | GATGGTACAGGTGGGGATGATG | |
| GpSuc2a Re | CAGTGCTCCTTATTCCATCATGG | |
| GpSuc2b Fw | TGTCAGGGAACTTCGGTTGAAC | |
| GpSuc2b Re | GCCATTGAAAATACTTTTGGCGACT | |
| GpSuc2c Fw | CGAAGAGCACCAAGCTCTTGGTGTG | |
| GpSuc2c Re | TTGATTCATATAGTAGAACCTGAC | |
| BmSuc1 Fw | CGGACCCGTTTTACAACGAA | |
| BmSuc1 Re | CACGTAGGAGAGGACTGGAT | |
| Genomic PCR And pET-24b Expression Vectors Construction | GpSuc1a F | ATAGGATCCGATGGGGCTCCTAAGGCTAATCG |
| GpSuc1a R | CCGCTCGAGTTCAGGTATACTTCTTCTTAAATG | |
| GpSuc1b F | CTAGATCTCATATGATGGCGGTCTTCACATTCACTG | |
| GpSuc1b R | CCGCTCGAGCAATGTTGGGATAACTGG | |
| GpSuc2a F | GGACTTCCATATGATGACGAGAGATTTACTGAGGC | |
| GpSuc2a R | TATCTCGAGGGGCAAAACGGAACGCTTGAG | |
| GpSuc2b F | CGCGGATCCGATGAATTGATCCGAACGGCTTCAG | |
| GpSuc2b R | TATCAAATGCGGCCGCAACAACAGAACGTGTCAATG | |
| GpSuc2c F | ATAGGATCCGATGACTAAAACTTTTTTAGGGC | |
| GpSuc2c R | CCGCTCGAGATTTACTTCTTGCCAGTTTG | |
| BmSuc1 F | CAGCTGTACATATGTTCGCCTGGAGCACAC | |
| BmSuc1 R | CGGCTCGAGAGCGGGTACACTTCTTCTCAATC | |
| pFastBac Dual Expression Vectors Construction | GFP F | CCGCTCGAGATGGTGAGCAAGGGC |
| GFP R | CGGGGTACCTTACTTGTACAGCTC | |
| pFB-BmSuc1 F | ATTTGCGGCCGCTATGTTCGCCTGGAGC | |
| pFB-BmSuc1 R | CCCAAGCTTTTAATGATGATGATGATGATGAGCGGG | |
| TACACT | ||
| pFB-GpSuc1a F | ATAGGATCCGATGGGGCTCCTAAGGCTAATCG | |
| pFB-GpSuc1a R | CCCAAGCTTTTCAATGATGATGATGATGATGGGTATA | |
| CTTCTTCTTAAATG |
| Protein | Mass (Da) | PI | Homology (%) | |||
|---|---|---|---|---|---|---|
| BmSUC1 BmSUC2 | M. sexta | B. amyloliquefaciens | S. tuberosum | |||
| GpSUC1a | 56,400.10 | 4.48 | 64.90 | 65.24 | 37.86 | 22.26 |
| 35.98 | ||||||
| GpSUC1b | 58,635.53 | 5.31 | 54.89 | 60.27 | 35.74 | 21.70 |
| 35.03 | ||||||
| GpSUC2a | 55,794.92 | 5.47 | 37.43 | 41.27 | 37.90 | 18.68 |
| 35.26 | ||||||
| GpSUC2b | 50,642.75 | 6.40 | 37.50 | 38.97 | 37.20 | 19.62 |
| 34.50 | ||||||
| GpSUC2c | 23,868.99 | 7.78 | 16.73 | 16.57 | 16.70 | 10.19 |
| 13.28 | ||||||
| Protein | Position (aa) | Amio Acid Sequences | Modifications |
|---|---|---|---|
| BmSUC1 | 21–47 | ALRQQNETTKRELEEYIADKKA | Methyl [K20]; Deamidated [R11] |
| EINPR | |||
| GpSUC1a | 20–45 | SFKQQFDNVADLEEYIAQKRTE | Deamidated [Q5; Q18;R20;R26] |
| INPR | |||
| BmSUC1 | 48–73 | YRPHYHISPPVGWMNDPNGFS | Methyl [R2; K24; K26]; |
| YYKEK | Deamidated [R2; N18] | ||
| GpSUC1a | 46–71 | YRLQYHVTPPVGWMNDPNGFS | Deamidated [Q4] |
| FYKGE | |||
| BmSUC1 | 162–183 | KYEGNPVLSYVPDNSADFRDPK | Methyl [R19; K22] |
| GpSUC1a | 160–181 | KYEGNPVLTYTPRPDFNDSDPK | Methyl [R19; K22]; |
| Deamidated [N13] | |||
| BmSUC1 | 187–202 | FKDHWYVVIGSSSNK · R | Methyl [K2]; Deamidated [N14] |
| GpSUC1a | 185–201 | HEDHWYVVIGSKTVDGR | No sites |
| BmSUC1 | 283–307 | TDKYFQELDYGHDFYAT | Methyl [K3; K25] |
| QTIQGDGK | |||
| GpSUC1a | 282–306 | PETEFQELDYGHDIYATQSLEK | No sites |
| DGT | |||
| BmSUC1 | 337–344 | ELQLIGTR | No sites |
| GpSUC1a | 336–343 | EIKLEGDR | Methyl [K3] |
| BmSUC1 | 360–376 | SVHNGDLEPQQAIEFGP | No sites |
| GpSUC1a | 359–375 | SLFDGDLLPEQSIEFEK | Deamidated [Q11] |
| BmSUC1 | 424–436 | QVEWVPIGKTSWR | No sites |
| GpSUC1a | 359–375 | QVEWNPIGSQSWR | Deamidated [Q1] |
| BmSUC1 | 467–483 | VKNSSPQTLSVEAYRLR | Deamidated [Q7] |
| GpSUC1a | 467–483 | LTNLSPQNLSVEAYHLR | Deamidated [N3; N8] |
| BmSUC1 | 484–488 | RSVPA | No sites |
| GpSUC1a | 484–505 | RSIPEMDVFVTITENRLNSGFK | Deamidated [R1; N18] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Yang, L.; Chen, Y.; Zhang, X.; Li, J.; Liang, D.; Jiang, S.; Gao, J.; Meng, Y. A Comparative Analysis of Bombyx mori (Lepidoptera: Bombycidae) β-fructofuranosidase Homologs Reveals Different Post-Translational Regulations in Glyphodes pyloalis Walker (Lepidoptera: Pyralidae). Insects 2022, 13, 410. https://doi.org/10.3390/insects13050410
Zhao Y, Yang L, Chen Y, Zhang X, Li J, Liang D, Jiang S, Gao J, Meng Y. A Comparative Analysis of Bombyx mori (Lepidoptera: Bombycidae) β-fructofuranosidase Homologs Reveals Different Post-Translational Regulations in Glyphodes pyloalis Walker (Lepidoptera: Pyralidae). Insects. 2022; 13(5):410. https://doi.org/10.3390/insects13050410
Chicago/Turabian StyleZhao, Yue, Liangli Yang, Yu Chen, Xinwei Zhang, Jing Li, Dan Liang, Song Jiang, Junshan Gao, and Yan Meng. 2022. "A Comparative Analysis of Bombyx mori (Lepidoptera: Bombycidae) β-fructofuranosidase Homologs Reveals Different Post-Translational Regulations in Glyphodes pyloalis Walker (Lepidoptera: Pyralidae)" Insects 13, no. 5: 410. https://doi.org/10.3390/insects13050410
APA StyleZhao, Y., Yang, L., Chen, Y., Zhang, X., Li, J., Liang, D., Jiang, S., Gao, J., & Meng, Y. (2022). A Comparative Analysis of Bombyx mori (Lepidoptera: Bombycidae) β-fructofuranosidase Homologs Reveals Different Post-Translational Regulations in Glyphodes pyloalis Walker (Lepidoptera: Pyralidae). Insects, 13(5), 410. https://doi.org/10.3390/insects13050410






