Simple Summary
RNA interference (RNAi) has shown great potentials as a novel technology for insect pest management. However, numerous studies have shown that the efficiency of RNAi varies substantially among different insect species. For example, as a major insect pest of corn, the Asian corn borer (Ostrinia furnacalis) showed very low RNAi efficiency. Therefore, it is necessary to develop new strategies for enhancing RNAi efficiency in insects with low RNAi efficiency. In this study, six core RNAi pathway genes were identified and characterized from O. furnacalis transcriptome database. After dsEGFP was injected into O. furnacalis, the expression of the core RNAi pathway genes (OfDicer2 and OfAgo2) was significantly up-regulated in response to the exposure of dsEGFP. As a result, the RNAi efficiency against the target genes in certain tissues of O. furnacalis was significantly improved. These results suggest that RNAi efficiency can be improved by inducing the expression of key RNAi pathway genes in O. furnacalis.
Abstract
RNA interference (RNAi) is a sequence-specific gene silencing mechanism that holds great promise for effective management of agricultural pests. Previous studies have shown that the efficacy of RNAi varies among different insect species, which limits its wide spread application in the field of crop protection. In this study, we identified and characterized six core RNAi pathway genes including OfDicer1, OfDicer2, OfR2D2, OfAgo1, OfAgo2, and OfAgo3 from the transcriptomic database of the Asian corn borer (Ostrinia furnacalis). Domain analysis showed that the six deduced proteins contained the necessary functional domains. Insect developmental stage- and tissue-specific expression analysis showed that five genes were expressed in all the stages and tissues examined except OfAgo3, which showed low expression in larvae, and high expression in pupae and adults and in the midgut. RT-qPCR was performed to examine the response of these six genes to exogenous double-stranded RNA (dsRNA). Interestingly, the transcript levels of OfDicer2 and OfAgo2 were significantly enhanced after the injection of dsEGFP at different time points and tissues investigated. Consequently, the RNAi efficiency in targeting the insect endogenous genes can be greatly enhanced in the hemolymph or midgut. Taken together, our investigations suggest that RNAi efficiency can be enhanced by pre-injection of dsRNA to induce the RNAi core machinery genes, which could be a useful strategy to improving RNAi efficiency for studying gene functions under laboratory conditions.
1. Introduction
RNA interference (RNAi) has become a powerful tool for gene functional studies and next-generation insect pest control [1,2], since its discovery by Andrew Z. Fire and Craig C. Mello in 1998 [3]. Previously, a myriad of reports have shown robust and systemic RNAi responses in various insect species belonging to Coleoptera [2,4] and Orthoptera [1,5,6]. However, in some insect species of Diptera, Lepidoptera, and Hemiptera, RNAi efficiency appeared very poor [7,8,9,10,11]. This variability in RNAi efficiency among various insect species greatly limits the widespread use of RNAi technology in both basic research and field applications for pest management.
There are several potential factors affecting RNAi efficiency [12]: (1) Stability of dsRNA. Degradation of dsRNA by nucleases (dsRNase or REase) has been documented to be the primary factor in reducing RNAi efficiency in many insects, whether in Orthoptera (susceptible to RNAi by injection) or in Lepidoptera (insusceptible to RNAi) [8,13,14,15]. Studies have shown that suppression of nuclease (dsRNase or REase) gene expression with RNAi [13,15] or application of liposomes or nanomaterials to protect dsRNA [16,17] could improve the stability of dsRNA. (2) Cellular uptake of dsRNA. Internalization of dsRNA into cells is an essential step to generate high RNAi efficiency. The approaches for overcoming limitations in dsRNA uptake in insects include expressing dsRNA in recombinant microbes for repeated oral delivery to insects [18], genetically engineering symbiotic bacteria to express and deliver dsRNA intracellularly [19], and enhancing clathrin-dependent endocytosis using arachidonic acid or hydrogen peroxide as was done for B. dorsalis [20,21]. (3) RNAi core machinery. Even if dsRNA is internalized and reaches the core machinery of the RNAi pathway in the cytoplasm (i.e., reaching the core RNAi enzymes), differences in the expression and function of the core RNAi enzymes could contribute to differences in RNAi efficiency among insects [22]. In Leptinotarsa decemlineata, expression of the core siRNA enzymes peaks in young larvae, when RNAi efficiency is the highest [23]. In contrast, a study did find a correlation between low RNAi efficiency in reproductive tissues of Schistocerca gregaria and low expression of Dicer2 and Ago2 [24].
The Asian corn borer (Ostrinia furnalalis) is one of the most destructive pests of corn in Asia, South-east Asia, and Oceania. In China, despite numerous control measures, this pest causes an estimated yield loss of 6–9 million tons per year. At present, its control relies mainly on synthetic insecticides and transgenic crops producing Bacillus thuringiensis (Bt) toxins. However, the heavy use of insecticides and Bt corn has resulted in some severe problems such as environmental pollutions and insecticide resistance in insect populations [25]. To hinder these catastrophic effects, there is an urgent need to find alternate approaches for insect pest management. In recent years, RNAi technology has emerged as a promising tool for pest management. However, RNAi has proven difficult to achieve in O. furnalalis [8]. Previously, studies have shown that degradation of dsRNA by OfREase and OfdsRNase2 is the reason for the low RNAi efficiency in O. furnalalis [8,13]. However, there has been no research on relationship between the expression levels of core RNAi pathway genes and RNAi efficiency in O. furnalalis. In this study, we identified six core RNAi pathway genes (OfDicer1, OfDicer2, OfR2D2, OfAgo1, OfAgo2, and OfAgo3) from the transcriptomic databases of O. furnacalis and examined the relationship between the up-regulation of these genes and the RNAi efficiency against target genes.
2. Materials and Methods
2.1. Insect Rearing
The eggs of O. furnacalis were purchased from Keyun, Henan Province, China, and were continuously reared in the laboratory at the Institute of Plant Protection, Shanxi Academy of Agricultural Sciences (Taiyuan, Shanxi, China) as described in Fan et al. [8].
2.2. Identification and Sequence Analysis of Core RNAi Pathway Genes
The candidate core RNAi pathway genes were identified in the transcriptomic databases of O. furnacalis through local BLAST using the sequences of Bombyx mori Dicer1 (XP_028040138.1) and Ago1 (NP_001095931.1), and Ostrinia nubilali OnDicer2 (MT921812), OnR2D2 (MT981255), OnAgo2 (MT524717), and Drosophila melanogaster DmAgo3 (EF211827) as query sequences based on annotation information of cDNA sequences. The identities of the candidate genes were further confirmed using BLAST searches against the National Center for Biotechnology Information (NCBI) database (https://blast.ncbi.nlm.nih.gov, accessed on 30 November 2020. The obtained cDNAs were translated into protein sequences using the ExPASy-translational tool (https://web.expasy.org/translate/, accessed on 30 November 2020). SMART domain analysis tool (http://smart.embl-heidelberg.de/, accessed on 30 November 2020) was used to predict domain architecture.
2.3. Expression Analysis of Core RNAi Pathway Genes
Total RNA was isolated from the whole bodies of O. furnacalis at seven developmental stages (first to fifth instar larva, pupa and adult), and three tissues (hemolymph, integument, and midgut) dissected from 2-day-old fifth-instar larvae. We chose these three tissues because integument and midgut are the main exposed tissues when dsRNA is delivered by spray and feeding, respectively, whereas hemolymph is a key exposed tissue when dsRNA is delivered by injection. TRIzol reagent (Takara, Dalian, China) was used to homogenize the whole body and different tissues harvested from the larvae of O. furnacalis. Five biological replicates were made, and three tissues or individuals were pooled for each biological replicate. The quality and concentration of total RNA were determined using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA), and 1 μg of total RNA was taken for reverse-transcription using reagents and enzymes from Takara (Dalian, China). The synthesized cDNA samples were diluted ten-fold for use as template for reverse transcription quantitative PCR (RT-qPCR) analysis. RT-qPCR was performed using SYBR ® Green Real-time PCR Master Mix (Promega, Madison, WI, USA) in a Light Cycler ® 480II (Roche, Basel, Switzerland). The total volume of RT-qPCR reaction was 20 μL, containing 10 μL of 2 × SYBR mix, 0.8 μL of primers (F/R), 4 μL cDNA templates, and 4.4 μL ddH2O. The cycling conditions were: 94 °C for 2 min, followed by 40 cycles of 94 °C for 15 s, 60 °C for 31 s. Primers used for quantification of mRNA levels of core RNAi pathway genes were designed using primer Premier 5.0 software (Supplemental Table S1). Ribosomal protein S3 gene (OfRpS3, EU275206) was used as an internal control. OfRpS3 has been proven a suitable reference gene and widely applied in RNAi research of O. furnacalis [26,27]. Melting curves were analyzed to confirm the specificity of amplification. Relative mRNA levels were calculated as expression of the target gene relative to the mean of the reference gene using the 2−∆Ct method [28].
2.4. Expression of Core RNAi Pathway Genes after dsRNA Injection
Double-stranded RNA (dsRNA) was synthesized using T7 RiboMAX™ Express RNAi System (Promega, Madison, WI, USA). Primers for dsRNA synthesis were designed using the E-RNAi web service (http://www.dkfz.de/signaling/e-rnai3/, accessed on 30 January 2020). The 2× Taq Master Mix kit (Takara, Dalian, China) was used to synthesize the templates containing a T7 promoter sequence at each end. The amplified products were visualized by agarose gel electrophoresis (1%), and the PCR products were purified using the E.Z.N.ATM Gel Extraction Kit (Omega Bio-Tek, Norcross, GA, USA). PCR products were used directly as templates for the synthesis of dsRNA. The synthesized dsRNA was dissolved in nuclease-free water, and the final concentration of the dsRNA was adjusted to 2.5 μg/μL.
To investigate transcriptional responses of the core RNAi pathway genes at different times after injection of dsRNA, 2-day-old fifth-instar larvae (L5D2) of O. furnacalis were injected with dsEGFP at 5 μg/larva and then placed on diets. The larvae injected with ddH2O served as controls. Three individuals were collected as a sample at each of four time points (2, 4, 6, and 12 h) for expression analysis with RT-qPCR.
To investigate transcriptional responses of OfDicer2 and OfAgo2 in different tissues after the injection of dsRNA, each of L5D2 larvae was injected with 5 μg dsEGFP and then placed on the artificial diet. Larvae injected with ddH2O were used as controls. After 2 h, hemolymph, integument, and midgut were dissected and collected in ice-cold 1.5-mL microcentrifuge tubes. Total RNA was extracted and RT-qPCR was performed to detect the expression of OfDicer2 and OfAgo2 as described above. Five biological replicates were made and three tissues were pooled as a biological replicate.
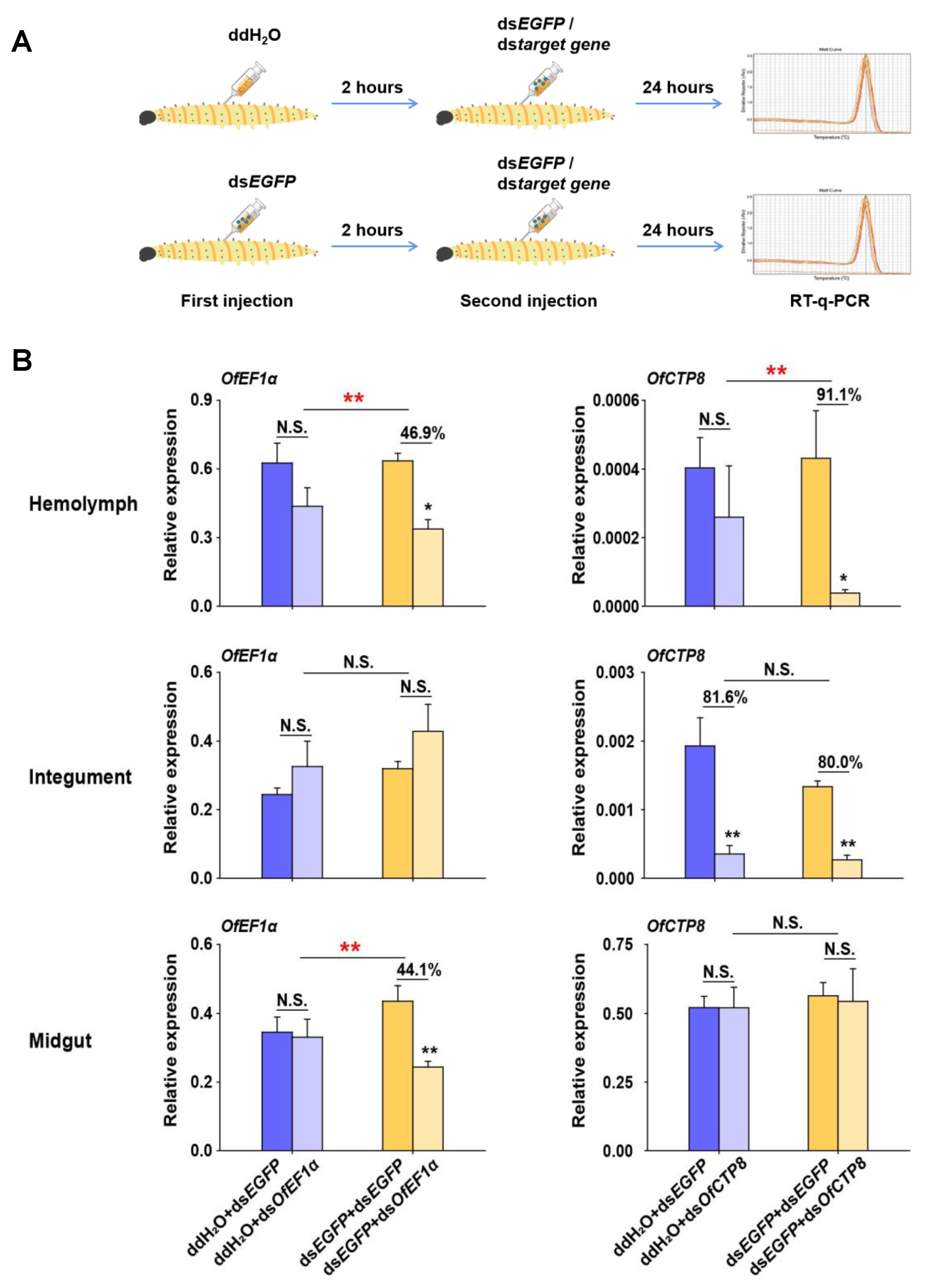
2.5. RNAi Efficiency Detection after Injection of dsRNA
The experimental design consisted of six different treatments, including ddH2O+dsEGFP, dsEGFP+dsEGFP, ddH2O+dsOfEF1α, dsEGFP+dsOfEF1α, ddH2O+dsOfCTP8, and dsEGFP+dsOfCTP8. A total of 90 L5D2 larvae of O. furnacalis were collected and divided into six groups with an average of 15 larvae per group. Each larva was first injected with a dose of 5 μg of dsEGFP (concentration: 2.5 μg/μL) or ddH2O. After 2 h of first injection each larva was reinjected with a dose of 10 μg (concentration: 4 μg/μL) of dsRNA. Twenty-four hours after the second injection, each larva was dissected, and hemolymph, integument, and midgut tissues were collected in ice-cold 1.5-mL microcentrifuge tubes for the extraction of total RNA. First-strand cDNA was synthesized from 1 μg of total RNA using M-MLV reverse transcriptase (Takara, Dalian, China). RT-qPCR was then performed to detect the relative expression of target genes. Specifically, the transcript levels of OfEF1α were determined in the treatments of ddH2O+dsEGFP, ddH2O+dsOfEF1α, dsEGFP+dsEGFP, and dsEGFP+dsOfEF1α, whereas the transcript levels of OfCTP8 were determined in the treatments of ddH2O+dsEGFP, ddH2O+dsOfCTP8, dsEGFP+dsEGFP, and dsEGFP+dsOfCTP8.
2.6. Statistical Analysis
Data were analyzed using one-way ANOVA followed by Tukey’s test for multiple comparisons when the data from more than two groups were compared. However, data were analyzed with a Student’s t-test when the data from two groups were compared. A p value < 0.05 was considered statistically significant (* p < 0.05, ** p < 0.01).
3. Results
3.1. Identification and Domain Analysis of Core RNAi Pathway Genes
A total of six core RNAi pathway genes, including two OfDicers (OfDicer1 and OfDicer2), one OfR2D2 and three OfAgos (OfAgo1, OfAgo2, and OfAgo3), were identified through local BLAST in the transcriptomic database of O. furnacalis (Table 1).

Table 1.
Characteristics of core RNAi pathway transcripts and their deduced proteins from O. furnacalis.
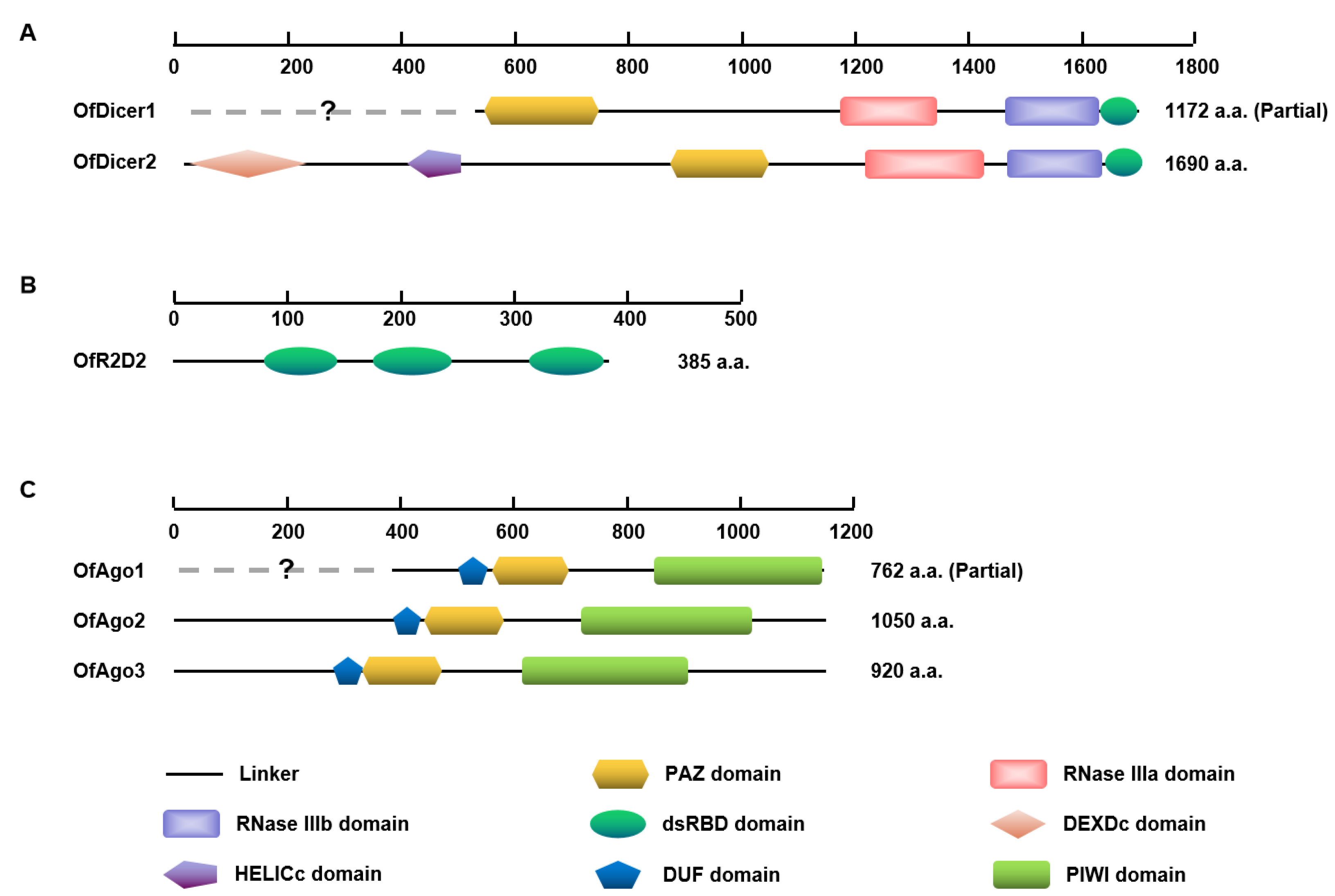
Domain analysis showed that almost all predicted OfAgo proteins contained common domains including DUF, PAZ, and PIWI. OfDicer2 contained each of DEXDc, HELICc, PAZ, RNase IIIa, RNase IIIb, and dsRBD domains, whereas the DEXDc and HELICc domains are absent in OfDicer1, probably due to the partial sequences in the database. In contrast, OfR2D2 contained three dsRBD domains (Figure 1). The PAZ domain are specialized to bind RNA ends, especially duplex ends with short (approximately 2 nt) 3′ overhangs in Ago proteins, and the PIWI domain is unique to the Argonaute superfamily. The PAZ and RNase III domains play central roles in excising siRNAs preferentially from ends of dsRNA molecules in Dicer proteins. The function of dsRBD is to bind dsRNA [29].
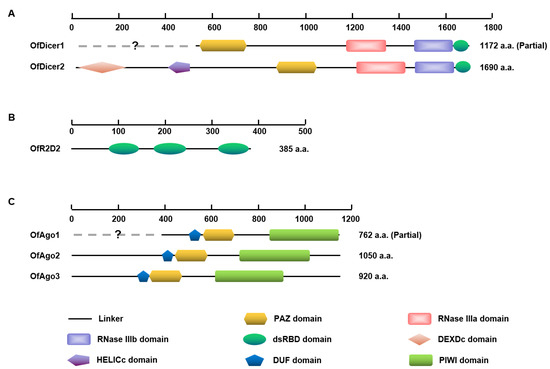
Figure 1.
Schematic diagram of deduced domains of Dicer1, Dicer2 (A), R2D2 (B), Ago1, Ago2, and Ago3 (C). Blank lines represent linker regions; yellow hexagons represent PAZ domains; pink quadrilaterals represent RNase IIIa domain; purple quadrilaterals represent RNase IIIb domain; green ellipses represent dsRBD domain; pink diamond represent DEXDc domain; purple pentagon represents HELICc domain; blue pentagons represent DUF domains and green quadrilaterals represent PIWI domain.
3.2. Expression Patterns of Core RNAi Pathway Genes
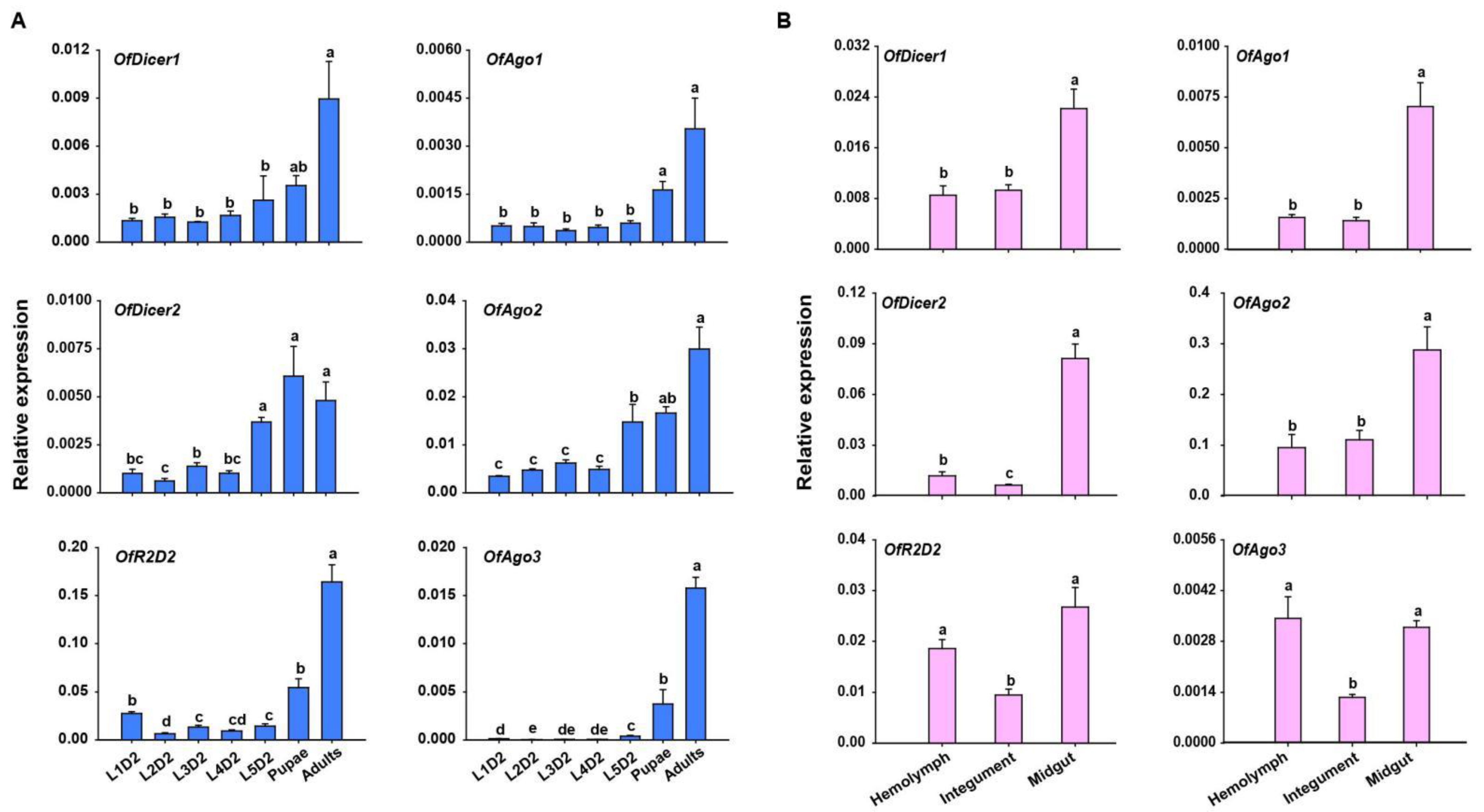
To examine the transcription levels of core RNAi pathway genes, RT-qPCR was performed in seven developmental stages of O. furnacalis. The expression profiles showed that almost all the candidate genes were expressed in all the developmental stages examined except for OfAgo3 which showed the expression only in pupal and adult stages, but not in the larval stage. Generally, the expression of almost all the candidate genes was lowest in the larval stage, and gradually increased in the rest of developmental stages. Four candidate genes, including OfDicer1, OfDicer2, OfAgo1, and OfAgo2, displayed higher expression in pupal stage. However, almost all the candidate genes showed the highest expression in the adult stage (Figure 2A).
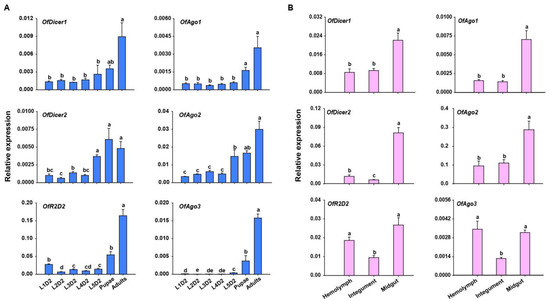
Figure 2.
RT-qPCR analyses of relative expression of core RNAi pathway genes in different developmental stages and tissues of O. furnacalis. (A) Relative expression of Dicer1, Dicer2, Ago1, Ago2, Ago3, and R2D2 at different developmental stages of O. furnacalis. L1D2-L5D2: Two-day-old first instar larvae to two-day-old fifth instar larvae. (B) Relative expression of Dicer1, Dicer2, Ago1, Ago2, Ago3, and R2D2 in different tissues including hemolymph (HE), integument (IN), and midgut (MG) in two-day-old fifth-instar larvae. Statistical significance was determined using a one-way ANOVA followed by Tukey’s post hoc test. Different letters (a–e) above the bars represent significant differences (n = 5) (p < 0.05).
Tissue-specific expression profiles in L5D2 larvae showed that core RNAi pathway genes were expressed in all tissues investigated. OfAgo1, OfAgo2, OfDicer1, and OfDicer2 were highly expressed in the midgut, whereas OfAgo3 and OfR2D2 displayed highest expression in the hemolymph and midgut (Figure 2B).
3.3. Transcriptional Responses of Core RNAi Pathway Genes to Injection of dsEGFP
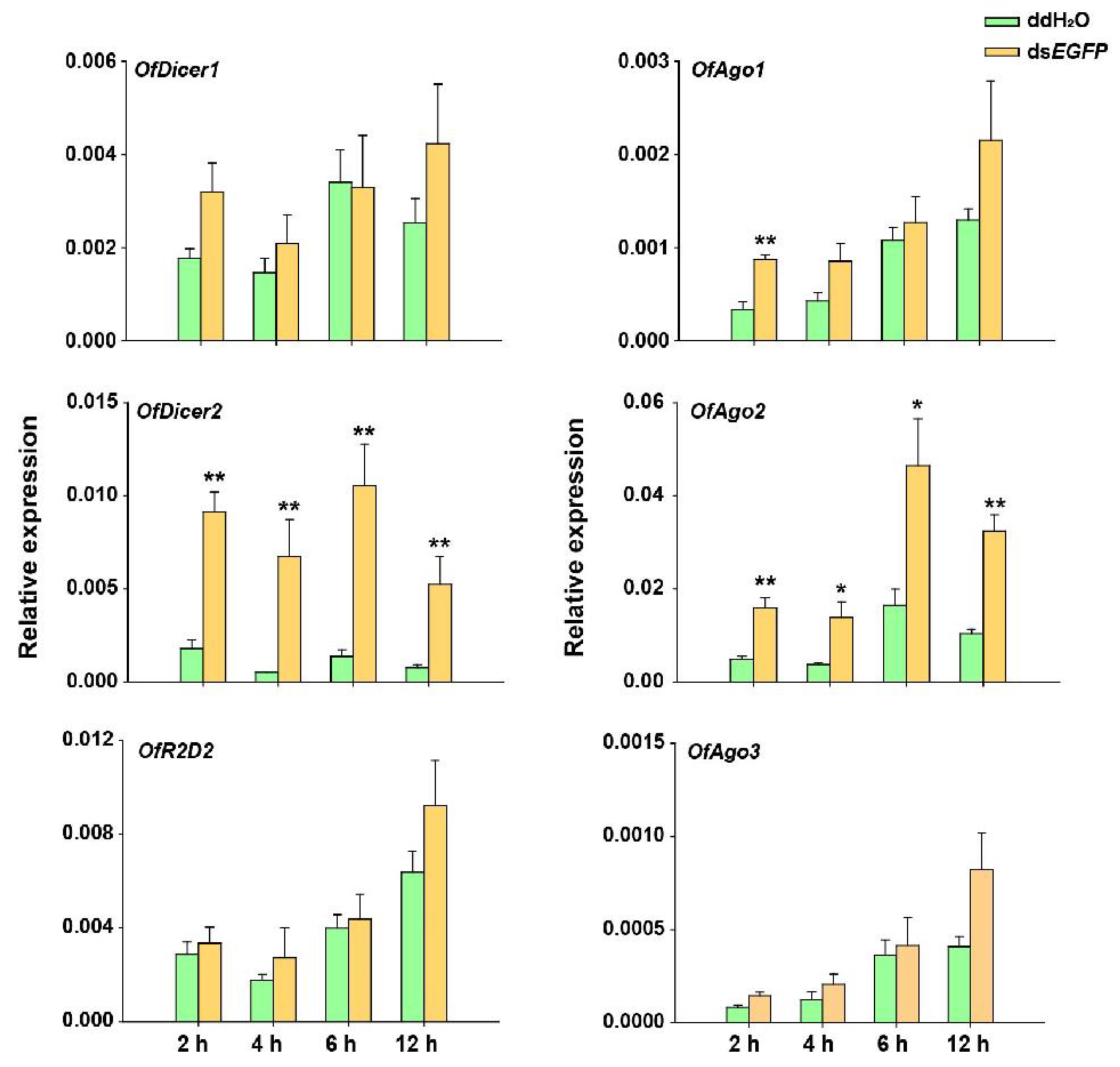
After introduction of dsEGFP into O. furnacalis, the response of RNAi pathway genes was investigated under two different experimental conditions. In the first experiment, larvae injected with 5 μg of dsEGFP in each were collected at 2, 4, 6, and 12 h and the expression levels of the respective core RNAi pathway genes were evaluated using RT-qPCR. The results demonstrated that there was no significant change in the relative expression of OfAgo3, OfDicer2, and OfR2D2 at any of these time points. However, OfAgo1 was up-regulated only at 2 h after dsRNA injection. To our surprise, the expression levels of OfAgo2 and OfDicer2 were significantly increased after the injection of dsEGFP compared with the injection of ddH2O as control at each time interval (Figure 3).
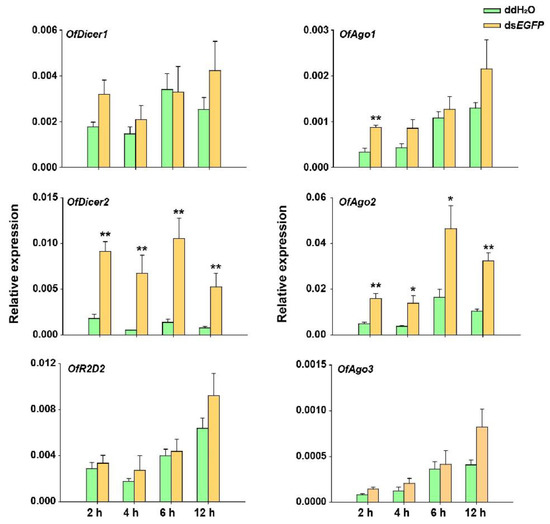
Figure 3.
RT-qPCR analyses of relative expression of Dicer1, Dicer2, Ago1, Ago2, Ago3, and R2D2 in O. furnacalis at different time-points after each larva was injected with 5 μg dsEGFP. ddH2O was injected in the negative control. Each treatment consisted of five replicates and three individuals were pooled in each replicate. Student’s t-test was used in statistical analysis of the data (*, p < 0.05, **, p < 0.01).
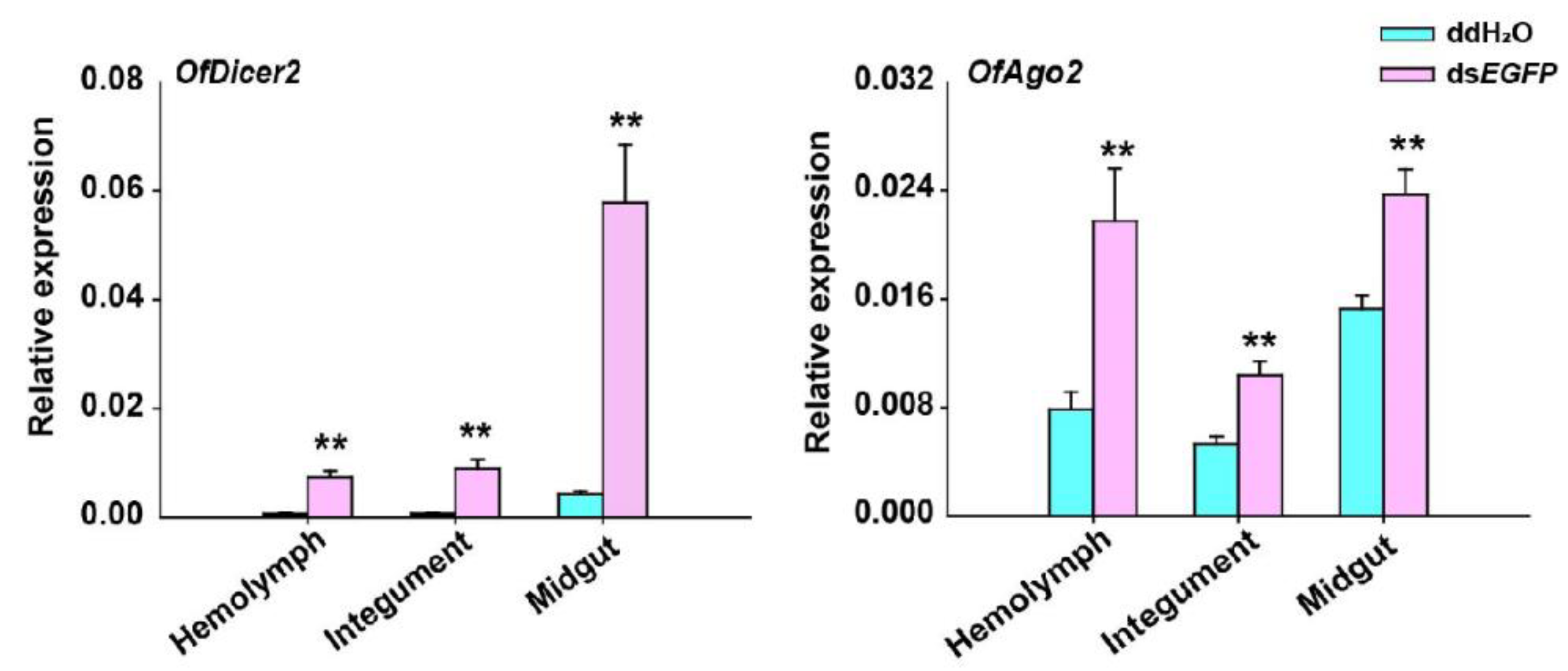
Since the expression of OfDicer2 and OfAgo2 was up-regulated for a long time after injection of dsEGFP, we evaluated the expression levels of OfDicer2 and OfAgo2 in three different tissues including hemolymph, integument, and midgut by RT-qPCR. Our results showed that the expression level for OfDicer2 in the hemolymph, integument, and midgut increased by 9.7-, 11.0-, and 13.5-fold, respectively, whereas the expression level of OfAgo2 increased in the hemolymph, integument, and midgut by 2.8-, 2.0-, and 1.5-fold, respectively (Figure 4).
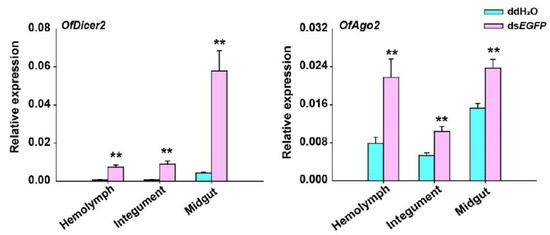
Figure 4.
RT-qPCR analyses of relative expression of Dicer2 and Ago2 in different tissues including hemolymph (HE), integument (IN), and midgut (MG) 2 h after injection of dsEGFP. ddH2O was injected in the negative control. Each treatment contained five replicates and three individuals were pooled in each replicate. Student’s t-test was used in statistical analysis of the data (**, p < 0.01).
3.4. RNAi Efficiency Can Be Improved by Pre-Injection of dsEGFP
To ascertain whether an increase in the transcript levels of OfDicer2 and OfAgo2 could lead to an increase in the RNAi efficiency, two target genes, OfEF1α (elongation factor 1α) and OfCTP8 (chymotrypsins 8) were selected as marker genes. In the control group, L5D2 larvae were first injected with ddH2O. After 2 h, dsEGFP or dsRNA of each of the target genes was injected into the larvae. Twenty-four hours later, RT-qPCR was carried out to analyze the relative expression levels of the target genes. Our results demonstrated that the transcript levels for OfEF1α remained constant and no significant change was observed in the hemolymph, integument, and midgut, while the expression level for OfCTP8 was efficiently silenced (81.6%) only in the integument, but not in the hemolymph and the midgut. When L5D2 larvae in the treatment group were injected with dsEGFP and then with the dsRNA of the target genes, the transcript level of OfEF1α was suppressed by 46.9% and 44.1% in the hemolymph and the midgut, respectively. In contrast, the transcript level for OfCTP8 was suppressed by 91.9% and 80.0% in the hemolymph and the integument, respectively (Figure 5). These results suggest that RNAi efficiency can be improved in some tissues of O. furnacalis by pre-injecting dsEGFP to induce the expression of RNAi core machinery genes.
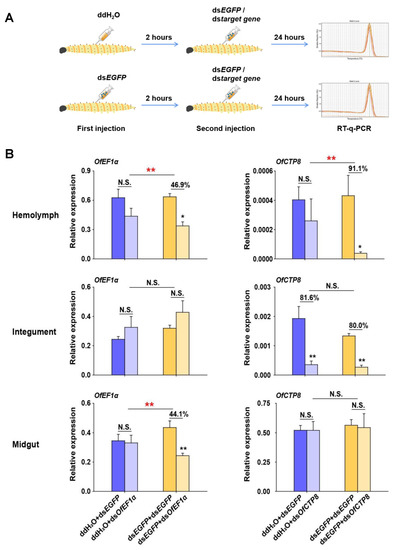
Figure 5.
Enhanced RNAi efficiency by pre-injection of dsEGFP in different tissues of O. furnacalis. (A) Overview of the experimental setup. ddH2O or dsEGFP was pre-injected into larvae (L5D2). After 2 h, dsEGFP or dsOfEF1α/dsOfCTP8 was injected into the larvae again. After 24 more hours, RT-qPCR was performed to examine the expression of OfEF1α or OfCTP8 in different tissues of O. furnacalis. (B) Different combinations of dsRNA or ddH2O injection were performed for this assay. The statistical significance between control and treatment results was assessed by Student’s t-test. Bars show mean ± SE (* p < 0.05; ** p < 0.01; N.S., nonsignificant) (n = 5).
4. Discussion
RNAi is a highly conserved, post-transcriptional gene-silencing mechanism in which small RNA molecules are utilized by RNAi core machinery that involves several core RNAi pathway genes to degrade complementary RNA molecules in a sequence-specific nature [12]. Core RNAi pathway genes are critical to RNAi efficiency. A previous study conducted by Tomoyasu et al. [30] has shown that two Ago2 (TcAgo2a and TcAgo2b) genes identified from the T. castaneum genome are involved in systemic RNAi response of RNAi. Similarly, both LmAgo2a and LmAgo2b have been found to contribute to the robust RNAi response in L. migratoria [31]. In L. decemlineata, two Dicer2 genes (Dicer2a and Dicer2b) and two Ago2 genes (Ago2a and Ago2b) were identified in L. decemlineata transcriptome database [23], which support the hypothesis that the number of core RNAi pathway genes may contribute to the variability of RNAi efficiency in insects. However, in the present study, only a single transcript of OfDicer2 and OfAgo2 was identified in O. furnacalis. It is speculated that this may be one of the reasons for the low RNAi efficiency in O. furnacalis.
In the present study, our results showed that the six core RNAi pathway genes (OfDicer1, OfDicer2, OfR2D2, OfAgo1, OfAgo2, and OfAgo3) were expressed in almost all the developmental stages and different tissues examined. In particular, the highest transcript level for the core RNAi pathway genes was observed at pupae and adult stages and in the midgut. Our results are in agreement with Cooper et al. [28] and Xie et al. [32] which showed that the expression patterns for Dicer2, R2D2, and Ago2 in O. nubilalis and B. dorsalis. Nevertheless, little has been known about differential expressions of core RNAi pathway genes during different developmental stages and in different tissues in relation to RNAi efficiency in insects. Because induced expression of core RNAi pathway genes can increase RNAi efficiency as we showed in this study, we hypothesize that RNAi-mediated suppression of a target gene is relatively easy to achieve when and where the core RNAi pathway genes are highly expressed. This information provides the fundamental basis to investigate the mechanism of RNA interference in various developmental stages and tissues of O. furnacalis.
Many previous studies have reported that RNAi acts as an RNA-based immune system against viral infections in insects, and plays a crucial role in regulating endogenous genes when insects are infected with a virus. To combat the viral infection, the RNAi machinery’s activity can enhance gradually by up-regulating some core RNAi components such as Dicer2 and Ago2 [32,33,34]. Similarly, non-specific dsRNAs have also been found in implicating as a trigger to induce the RNAi activity by up-regulating the expression of core RNAi pathway genes in certain insect species [23,35,36]. For example, it was found that dsLdSAHase-induced suppression of LdSAHase and the associated larval mortalities were influenced by pre-exposure to dsEGFP in Leptinotarsa decemlineata [23]. Similarly, pre-exposure of Acyrthosiphon pisum to dsGFP (600 ng) led to significant silencing of hunchback gene when the aphid was exposed to 60 ng of dshunchback, a dose which cannot lead to the suppression of hunchback expression without pre-exposure of the aphid to dsGFP [35].
In this study, we first investigated the possible association between RNAi efficiency and the expression levels of core RNAi pathway genes in O. furnacalis. Our result showed the mRNA levels of OfDicer2 and OfAgo2 were high at all of the insect developmental stages, particularly in the late larval (L5D2) stage, pupal and adult stages, and as well as in the tissues, particularly the midgut. Because Dicer2 and Ago2 are believed to be involved in the siRNA pathway as shown in Drosophila [37], high expression of OfDicer2 and OfAgo2 may be related to RNAi efficiency during these stages. Indeed, several studies have shown that sufficient silencing efficiency can be achieved during the wandering or pupal stages of lepidopterans, such as B. mori and Spodoptera litura [38,39], which may be related to high expression of Dicer2 and Ago2 during these stages. Similarly, high expression of the core RNAi pathway genes in certain tissues, particularly in the midgut, may also imply strong RNAi machinery in these tissues. However, up-regulation of OfAgo1 was observed 2 h post-injection of dsEGFP, which implies that OfAgo1 could also be involved in siRNA pathway at an early stage of dsRNA exposure in O. furnacalis. These results are similar to the functions of Ago1 in L. migratoria, which has been shown to be involved in siRNA-mediated RNAi pathway [31]. However, further research is needed to clarify the role of OfAgo1 in siRNA-mediated RNAi pathway.
Nevertheless, our major focus of this study was on possible induced effects caused by injection of exogenous dsRNA (i.e., dsEGFP) on RNAi efficiency for attacking target genes in O. furnacalis. Since injection of dsEGFP could induce a strong response of OfDicer2 and OfAgo2, OfEF1α and OfCTP8 were selected as two target genes to determine whether the silencing efficiency against the two target genes could be improved by pre-injection of dsEGFP. Previous studies have shown that OfEF1α can be significantly silenced by tissue culture RNAi in O. furnacalis [40], and OfCTP8 can be significantly knocked down by injection of dsOfCTP8 in O. furnacalis [13]. As expected, OfEF1α could not be significantly silenced in any tissues (hemolymph, integument, and midgut) when O. furnacalis larvae were pre-injected with ddH2O. However, OfEF1α can be significantly silenced in the hemolymph and midgut, but not in the integument, when O. furnacalis larvae were pre-injected with dsEGFP. Furthermore, RNAi efficiency targeting OfCTP8 could be significantly improved in the hemolymph by pre-injection of dsEGFP compared with pre-injection of ddH2O. Although our results are highly encouraging for improving RNAi efficiency by inducing the activity of the core machinery of the siRNA-mediated RNAi pathway through the pre-injection of dsEGFP, the level of the improvement for different target genes and in different tissues still varies considerably. Presumably, many other factors may contribute to such variations, including dsRNA sequence difference, different efficiencies in dsRNA uptake in different tissues, and different intensities of responses of the RNAi core machinery genes (i.e., OfDicer2 and OfAgo2) to the injection of dsEGFP in O. furnacalis. Therefore, further study is needed to pinpoint the exact mechanisms causing such variations.
5. Conclusions
In the current study, we identified six core RNAi pathway genes (OfDicer1, OfDicer2, OfAgo1, OfAgo2, OfAgo3, and OfR2D2) in O. furnacalis. Expression profiles indicated that five genes were expressed in all the developmental stages and tissues examined except OfAgo3, which showed low expression in larvae. However, only OfDicer2 and OfAgo2 were upregulated in response to dsEGFP exposure at all four time points and tissues investigated. In addition, the RNAi efficiency of target genes can be significantly enhanced in the hemolymph or midgut by pre-injection of dsEGFP. Thus, this study provides new insights into developing useful strategies for improving RNAi efficiency in RNAi-refractory insect species, particularly in laboratory studies, where pre-exposure to an exogenous dsRNA in insects is feasible.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/insects13030274/s1, Table S1: Primers for PCR amplification, dsRNA synthesis and RT-qPCR analysis.
Author Contributions
Conceptualization, J.Z., K.Y.Z. and Y.F.; methodology, Y.F. and Y.W.; investigation, Y.F.; data curation, Y.F.; writing original draft preparation, Y.F. and M.A.; review and editing, K.Y.Z., J.Z., Y.W., H.S., E.M., M.A., T.L. and X.L.; supervision, J.Z., K.Y.Z. and E.M.; funding acquisition, J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by National Natural Science Foundation of China (Grant No. 31730074).
Institutional Review Board Statement
This work used arthropod species (the Asian corn borer) and was exempt from the Institutional Review Board.
Informed Consent Statement
Not applicable.
Data Availability Statement
Sequence data are available in a publicly accessible repository: GenBank accession numbers are shown in Table 1.
Acknowledgments
We thank our lab members for their valuable comments and suggestions during the preparation of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, J.; Liu, X.; Zhang, J.; Li, D.; Sun, Y.; Guo, Y.; Ma, E.; Zhu, K.Y. Silencing of two alternative splicing-derived mRNA variants of chitin synthase 1 gene by RNAi is lethal to the oriental migratory locust, Locusta migratoria manilensis (Meyen). Insect Biochem. Mol. Biol. 2010, 40, 824–833. [Google Scholar] [CrossRef]
- Zhu, F.; Xu, J.; Palli, R.; Ferguson, J.; Palli, S.R. Ingested RNA interference for managing the populations of the Colorado potato beetle, Leptinotarsa decemlineata. Pest Manag. Sci. 2011, 67, 175–182. [Google Scholar] [CrossRef]
- Fire, A.; Xu, S.Q.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef]
- Baum, J.A.; Bogaert, T.; Clinton, W.; Heck, G.R.; Feldmann, P.; Ilagan, O.; Johnson, S.; Plaetinck, G.; Munyikwa, T.; Pleau, M.; et al. Control of coleopteran insect pests through RNA interference. Nat. Biotechnol. 2007, 25, 1322–1326. [Google Scholar] [CrossRef]
- Abbas, M.; Fan, Y.H.; Shi, X.K.; Gao, L.; Wang, Y.L.; Li, T.; Cooper, A.M.W.; Silver, K.; Zhu, K.Y.; Zhang, J.Z. Identification of Rab family genes and functional analyses of LmRab5 and LmRab11A in the development and RNA interference of Locusta migratoria. Insect Sci. 2021. [Google Scholar] [CrossRef]
- Zhao, X.M.; Gou, X.; Liu, W.M.; Ma, E.B.; Moussian, B.; Li, S.; Zhu, K.Y.; Zhang, J.Z. The wing-specific cuticular protein LmACP7 is essential for normal wing morphogenesis in the migratory locust. Insect Biochem. Mol. Biol. 2019, 112, 103206. [Google Scholar] [CrossRef]
- Christiaens, O.; Smagghe, G. The challenge of RNAi-mediated control of hemipterans. Curr. Opin. Insect Sci. 2014, 6, 15–21. [Google Scholar] [CrossRef]
- Fan, Y.H.; Song, H.F.; Abbas, M.; Wang, Y.L.; Li, T.; Ma, E.B.; Cooper, A.M.W.; Silver, K.; Zhu, K.Y.; Zhang, J.Z. A dsRNA-degrading nuclease (dsRNase2) limits RNAi efficiency in the Asian corn borer (Ostrinia furnacalis). Insect Sci. 2021, 28, 1677–1689. [Google Scholar] [CrossRef]
- Joga, M.R.; Zotti, M.J.; Smagghe, G.; Christiaens, O. RNAi efficiency, systemic properties, and novel delivery methods for pest insect control: What we know so far. Front. Physiol. 2016, 7, 553. [Google Scholar] [CrossRef] [Green Version]
- Taning, C.N.T.; Christiaens, O.; Berkvens, N.; Casteels, H.; Maes, M.; Smagghe, G. Oral RNAi to control Drosophila suzukii: Laboratory testing against larval and adult stages. J. Pest Sci. 2016, 89, 803–814. [Google Scholar] [CrossRef]
- Terenius, O.; Papanicolaou, A.; Garbutt, J.S.; Eleftherianos, I.; Huvenne, H.; Kanginakudru, S.; Albrechtsen, M.; An, C.; Aymeric, J.L.; Barthel, A.; et al. RNA interference in Lepidoptera: An overview of successful and unsuccessful studies and implications for experimental design. J. Insect Physiol. 2011, 57, 231–245. [Google Scholar] [CrossRef] [Green Version]
- Cooper, A.M.; Silver, K.; Zhang, J.; Park, Y.; Zhu, K.Y. Molecular mechanisms influencing efficiency of RNA interference in insects. Pest Manag. Sci. 2019, 75, 18–28. [Google Scholar] [CrossRef] [Green Version]
- Guan, R.B.; Li, H.C.; Fan, Y.J.; Hu, S.R.; Christiaens, O.; Smagghe, G.; Miao, X.X. A nuclease specific to lepidopteran insects suppresses RNAi. J. Biol. Chem. 2018, 293, 6011–6021. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Wang, K.; Fu, W.; Sheng, C.; Han, Z. Biochemical comparison of dsRNA degrading nucleases in four different insects. Front. Physiol. 2018, 9, 624. [Google Scholar] [CrossRef] [Green Version]
- Song, H.F.; Zhang, J.Q.; Li, D.Q.; Cooper, A.M.W.; Silver, K.; Li, T.; Liu, X.J.; Ma, E.B.; Zhu, K.Y.; Zhang, J.Z. A double-stranded RNA degrading enzyme reduces the efficiency of oral RNA interference in migratory locust. Insect Biochem. Mol. Biol. 2017, 86, 68–80. [Google Scholar] [CrossRef]
- Castellanos, N.L.; Smagghe, G.; Sharma, R.; Oliveira, E.E.; Christiaens, O. Liposome encapsulation and EDTA formulation of dsRNA targeting essential genes increase oral RNAi-caused mortality in the Neotropical stink bug Euschistus heros. Pest Manag. Sci. 2019, 75, 537–548. [Google Scholar] [CrossRef]
- He, B.; Chu, Y.; Yin, M.; Mullen, K.; An, C.; Shen, J. Fluorescent nanoparticle delivered dsRNA toward genetic control of insect pests. Adv. Mater. 2013, 25, 4580–4584. [Google Scholar] [CrossRef]
- Murphy, K.A.; Tabuloc, C.A.; Cervantes, K.R.; Chiu, J.C. Ingestion of genetically modified yeast symbiont reduces fitness of an insect pest via RNA interference. Sci. Rep. 2016, 6, 22587. [Google Scholar] [CrossRef] [Green Version]
- Whitten, M.; Dyson, P. Gene silencing in non-model insects: Overcoming hurdles using symbiotic bacteria for trauma-free sustainable delivery of RNA interference: Sustained RNA interference in insects mediated by symbiotic bacteria: Applications as a genetic tool and as a biocide. Bioessays 2017, 39, 1600247. [Google Scholar] [CrossRef]
- Dong, X.; Li, X.; Li, Q.; Jia, H.; Zhang, H. The inducible blockage of RNAi reveals a role for polyunsaturated fatty acids in the regulation of dsRNA-endocytic capacity in Bactrocera dorsalis. Sci. Rep. 2017, 7, 5584. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Zeng, B.; Ling, L.; Xu, J.; You, L.; Aslam, A.F.; Tan, A.; Huang, Y. Enhancement of larval RNAi efficiency by over-expressing Argonaute2 in Bombyx mori. Int. J. Biol. Sci. 2015, 11, 176–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, I.K.; Singh, S.; Mogilicherla, K.; Shukla, J.N.; Palli, S.R. Comparative analysis of double-stranded RNA degradation and processing in insects. Sci. Rep. 2017, 7, 17059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, W.C.; Fu, K.Y.; Yang, S.; Li, X.X.; Li, G.Q. Instar-dependent systemic RNA interference response in Leptinotarsa decemlineata larvae. Pestic. Biochem. Physiol. 2015, 123, 64–73. [Google Scholar] [CrossRef]
- Wynant, N.; Verlinden, H.; Breugelmans, B.; Simonet, G.; Broeck, V.J. Tissue-dependence and sensitivity of the systemic RNA interference response in the desert locust, Schistocerca gregaria. Insect Biochem. Mol. Biol. 2012, 42, 911–917. [Google Scholar] [CrossRef]
- Xu, L.N.; Wang, Y.Q.; Wang, Z.Y.; Hu, B.J.; Ling, Y.H.; He, K.L. Transcriptome differences between Cry1Ab resistant and susceptible strains of Asian corn borer. BMC Genom. 2015, 16, 173. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Zhang, H.; Liu, F.; Wu, Q.; Shen, X.; Yang, Q. Structural determinants of an insect beta-N-Acetyl-D-hexosaminidase specialized as a chitinolytic enzyme. J. Biol. Chem. 2011, 286, 4049–4058. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.; Yu, X.; Liu, J.; Zhao, H.; Wang, P.; Hu, S.; Chen, J.; Zhang, W.; Hu, J. Ostrinia furnacalis integrin beta1 may be involved in polymerization of actin to modulate spreading and encapsulation of plasmatocytes. Dev. Comp. Immunol. 2012, 37, 438–445. [Google Scholar] [CrossRef]
- Cooper, A.M.W.; Song, H.F.; Shi, X.K.; Yu, Z.T.; Lorenzen, M.; Silver, K.; Zhang, J.Z.; Zhu, K.Y. Characterization, expression patterns, and transcriptional responses of three core RNA interference pathway genes from Ostrinia nubilalis. J. Insect Physiol. 2021, 129, 104181. [Google Scholar] [CrossRef]
- Carthew, R.W.; Sontheimer, E.J. Origins and mechanisms of miRNAs and siRNAs. Cell 2009, 136, 642–655. [Google Scholar] [CrossRef] [Green Version]
- Tomoyasu, Y.; Miller, S.C.; Tomita, S.; Schoppmeier, M.; Grossmann, D.; Bucher, G. Exploring systemic RNA interference in insects: A genome-wide survey for RNAi genes in Tribolium. Genome Biol. 2008, 9, R10. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Wang, Y.L.; Fan, Y.H.; Abbas, M.; Ma, E.B.; Cooper, A.M.W.; Silver, K.; Zhu, K.Y.; Zhang, J.Z. Multiple Argonaute family genes contribute to the siRNA-mediated RNAi pathway in Locusta migratoria. Pestic. Biochem. Physiol. 2020, 170, 104700. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.F.; Niu, J.Z.; Jiang, X.Z.; Yang, W.J.; Shen, G.M.; Wei, D.; Smagghe, G.; Wang, J.J. Influence of various stressors on the expression of core genes of the small interfering RNA pathway in the oriental fruit fly, Bactrocera dorsalis. Insect Sci. 2017, 24, 418–430. [Google Scholar] [CrossRef] [PubMed]
- Marques, J.T.; Wang, J.P.; Wang, X.; de Oliveira, K.P.; Gao, C.; Aguiar, E.R.; Jafari, N.; Carthew, R.W. Functional specialization of the small interfering RNA pathway in response to virus infection. PLoS Pathog. 2013, 9, e1003579. [Google Scholar] [CrossRef]
- Niu, J.; Smagghe, G.; De Coninck, D.I.; Van Nieuwerburgh, F.; Deforce, D.; Meeus, I. In vivo study of Dicer-2-mediated immune response of the small interfering RNA pathway upon systemic infections of virulent and avirulent viruses in Bombus terrestris. Insect Biochem. Mol. Biol. 2016, 70, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; An, X.; Jiang, Y.D.; Ding, B.Y.; Shang, F.; Christiaens, O.; Taning, C.N.T.; Smagghe, G.; Niu, J.; Wang, J.J. Induction of RNAi core machinery’s gene expression by exogenous dsRNA and the effects of pre-exposure to dsRNA on the gene silencing efficiency in the pea aphid (Acyrthosiphon pisum). Front. Physiol. 2018, 9, 1906. [Google Scholar] [CrossRef] [Green Version]
- Garbutt, J.S.; Reynolds, S.E. Induction of RNA interference genes by double-stranded RNA; implications for susceptibility to RNA interference. Insect Biochem. Mol. Biol. 2012, 42, 621–628. [Google Scholar] [CrossRef]
- Lee, Y.S.; Nakahara, K.; Pham, J.W.; Kim, K.; He, Z.Y.; Sontheimer, E.J.; Carthew, R.W. Distinct roles for Drosophila Dicer-1 and Dicer-2 in the RNAi/miRNA silence pathway. Cell 2004, 117, 69–81. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Meng, Y.; Wang, Y.X.; Luo, J.; Katsuma, S.; Yang, C.W.; Banno, Y.; Kusakabe, T.; Shimada, T.; Xia, Q.Y. Vitellogenin receptor mutation leads to the oogenesis mutant phenotype “scanty vitellin” of the silkworm, Bombyx mori. J. Biol. Chem. 2013, 288, 13345–13355. [Google Scholar] [CrossRef] [Green Version]
- Shu, Y.H.; Wang, J.W.; Lu, K.; Zhou, J.L.; Zhou, Q.; Zhang, G.R. The first vitellogenin receptor from a Lepidopteran insect: Molecular characterization, expression patterns and RNA interference analysis. Insect Mol. Biol. 2011, 20, 61–73. [Google Scholar] [CrossRef]
- Fan, Y.H.; Song, H.F.; Abbas, M.; Wang, Y.L.; Liu, X.J.; Li, T.; Ma, E.B.; Zhu, K.Y.; Zhang, J.Z. The stability and sequence cleavage preference of dsRNA are key factors differentiating RNAi efficiency between migratory locust and Asian corn borer. Insect Biochem. Mol. Biol. 2022, 143, 103738. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).