Effects of Fruit Sizes of Two Camellia Trees on the Larval Sizes of Curculio styracis (Roelofs, 1875): Testing the Endoparasitoid Body Size Hypothesis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study System
2.2. Sampling Methods
2.2.1. Dry Mass of Larvae in Fallen Fruits
2.2.2. Dry Mass of Larvae in Sleeve Experiments
2.3. The Model
- (1)
- The Richards equation,
- (2)
- The von Bertalanffy equation,
- (3)
- The Gompertz equation,
- (4)
- The logistic equation,
- (5)
- The negative exponential equation,
2.4. Data Analysis
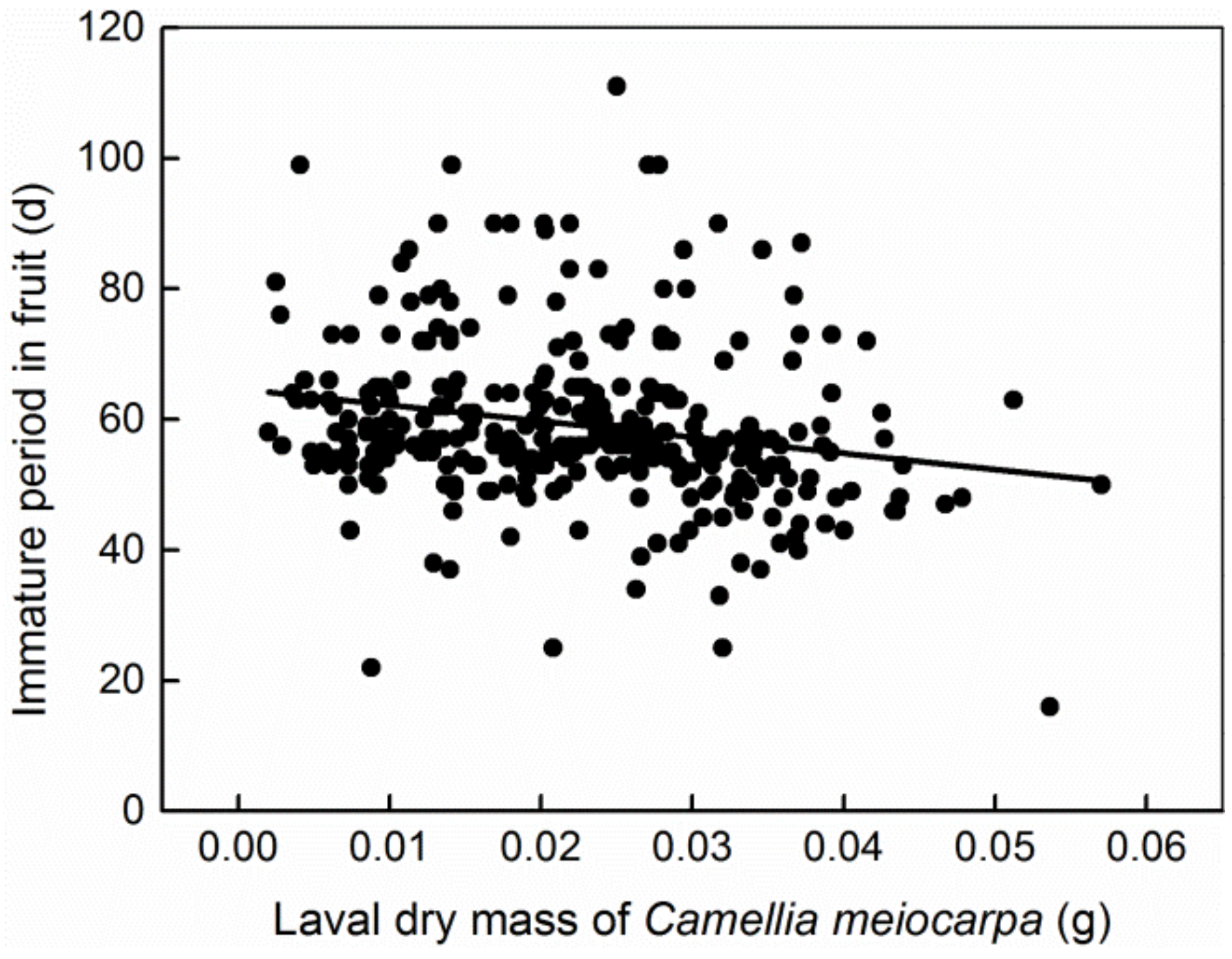
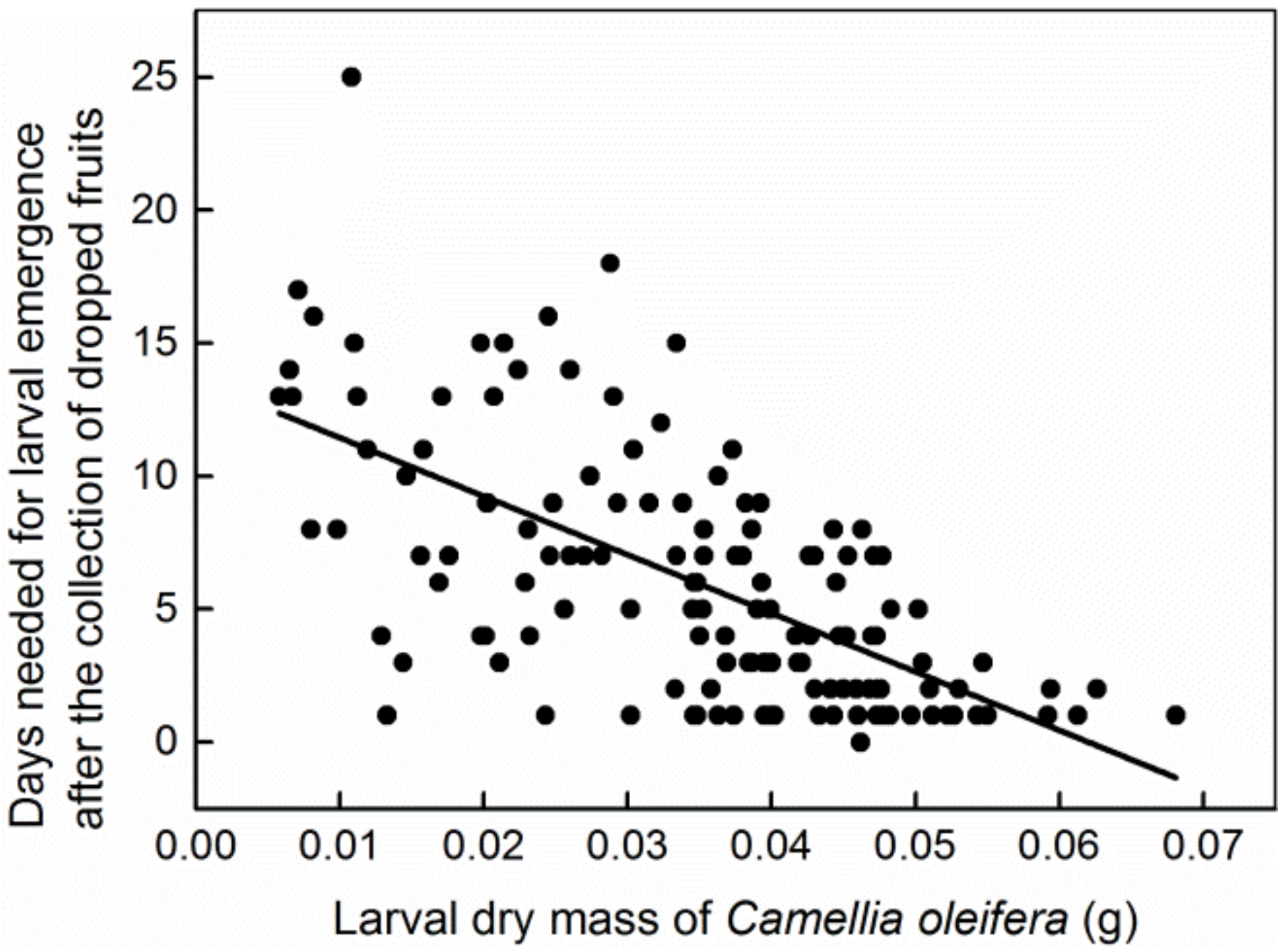
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Desouhant, E.; Debouzie, D.; Ploye, H.; Menu, F. Clutch size manipulations in the chestnut weevil, Curculio elephas: Fitness of oviposition strategies. Oecologia 2000, 122, 493–499. [Google Scholar] [CrossRef]
- Jervis, M.A.; Ferns, P.N.; Heimpel, G.E. Body size and the timing of egg production in parasitoid wasps: A comparative analysis. Funct. Ecol. 2003, 17, 375–383. [Google Scholar] [CrossRef]
- Hughes, J.; Vogler, A.P. Ecomorphological adaptation of acorn weevils to their oviposition site. Evolution 2004, 58, 1971–1983. [Google Scholar] [CrossRef]
- Bonal, R.; Espelta, J.M.; Vogler, A.P. Complex selection on life-history traits and the maintenance of variation in exaggerated rostrum length in acorn weevils. Oecologia 2011, 167, 1053–1061. [Google Scholar] [CrossRef]
- Muñoz, A.; Bonal, R.; Espelta, J.M. Acorn–weevil interactions in a mixed-oak forest: Outcomes for larval growth and plant recruitment. For. Ecol. Manag. 2014, 322, 98–105. [Google Scholar] [CrossRef]
- Fabian, D.; Flatt, T. Life history evolution. Nat. Educ. Knowl. 2012, 3, 24. [Google Scholar]
- Blanckenhorn, W.U. The evolution of body size: What keeps organisms small? Q. Rev. Biol. 2000, 75, 385–407. [Google Scholar] [CrossRef] [Green Version]
- Gotthard, K. Growth strategies and optimal body size in temperate Pararginii butterflies. Integr. Comp. Biol. 2004, 44, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Gotthard, K. Growth strategies of ectothermic animals in temperate environments. In Environment and Animal Development; Atkinson, D., Thorndyke, M., Eds.; BIOS Scientific Publishers: Oxford, UK, 2001; pp. 287–304. [Google Scholar]
- Roff, D.A. The Evolution of Life Histories: Theory and Analysis; Chapman and Hall: New York, NY, USA, 1992. [Google Scholar]
- Stearns, S.C. The Evolution of Life Histories; Oxford University Press: Oxford, UK, 1992. [Google Scholar]
- Stearns, S.C. Trade-offs in life-history evolution. Funct. Ecol. 1989, 3, 259–268. [Google Scholar] [CrossRef] [Green Version]
- Lafferty, K.D.; Kuris, A.M. Trophic strategies, animal diversity and body size. Trends Ecol. Evol. 2002, 17, 507–513. [Google Scholar] [CrossRef]
- Toju, H.; Sota, T. Imbalance of predator and prey armament: Geographic clines in phenotypic interface and natural selection. Am. Nat. 2006, 167, 105–117. [Google Scholar] [CrossRef]
- Toju, H. Weevils and camellias in a Darwin’s race: Model system for the study of eco-evolutionary interactions between species. Ecol. Res. 2011, 26, 239–251. [Google Scholar] [CrossRef]
- Toju, H.; Ueno, S.; Taniguchi, F.; Sota, T. Metapopulation structure of a seed-predator weevil and its host plant in arms race coevolution. Evolution 2011, 65, 1707–1722. [Google Scholar] [CrossRef]
- Bezemer, T.J.; Mills, N.J. Clutch size decisions of a gregarious parasitoid under laboratory and field conditions. Anim. Behav. 2003, 66, 1119–1128. [Google Scholar] [CrossRef] [Green Version]
- Bonal, R.; Hernández, M.; Ortego, J.; Muñoz, A.; Espelta, J.M. Positive cascade effects of forest fragmentation on acorn weevils mediated by seed size enlargement. Insect Conserv. Divers. 2012, 5, 381–388. [Google Scholar] [CrossRef]
- Honěk, A. Intraspecific variation in body size and fecundity in insects: A general relationship. Oikos 1993, 66, 483–492. [Google Scholar] [CrossRef]
- Hanks, L.M.; Millar, J.G.; Paine, T.D. Body size influences mating success of the Eucalyptus longhorned borer (Coleoptera: Cerambycidae). J. Insect Behav. 1996, 9, 369–382. [Google Scholar] [CrossRef] [Green Version]
- Blanckenhorn, W.U. Behavioral causes and consequences of sexual size dimorphism. Ethology 2005, 111, 977–1016. [Google Scholar] [CrossRef]
- Espelta, J.M.; Bonal, R.; Sánchez-Humanes, B. Pre-dispersal acorn predation in mixed oak forests: Interspecific differences are driven by the interplay among seed phenology, seed size and predator size. J. Ecol. 2009, 97, 1416–1423. [Google Scholar] [CrossRef]
- Fox, C.W.; Martin, J.D.; Thakar, M.S.; Mousseau, T.A. Clutch size manipulations in two seed beetles: Consequences for progeny fitness. Oecologia 1996, 108, 88–94. [Google Scholar] [CrossRef]
- Desouhant, E. Selection of fruits for oviposition by the chestnut weevil, Curculio elephas. Entomol. Exp. Appl. 1998, 86, 71–78. [Google Scholar] [CrossRef]
- Bonal, R.; Muñoz, A. Negative consequences of premature seed abscission on insect performance: Acorn growth suppression constrains Curculio elephas larval size. Ecol. Entomol. 2008, 33, 31–36. [Google Scholar]
- Bonal, R.; Muñoz, A. Seed weevils living on the edge: Pressures and conflicts over body size in the endoparasitic Curculio larvae. Ecol. Entomol. 2009, 34, 304–309. [Google Scholar] [CrossRef]
- Zhou, S.J. Biology and control of camellia weevil Curculio chinensis Chevrolat. Acta Entomol. Sin. 1981, 24, 48–52, (In Chinese with English abstract). [Google Scholar]
- Li, Z.W.; He, L.H.; Yang, L.J.; He, B.; Zeng, A.P. Oviposition strategy of the camellia weevil, Curculio chinensis (Coleoptera: Curculionidae), on oil tea (Camellia meiocarpa). Acta Entomol. Sin. 2015, 58, 981–988, (In Chinese with English abstract). [Google Scholar]
- Li, Z.W.; Sun, H.S. Interactions of Camellia meiocarpa, Curculio chinensis (Coleoptera: Curculionidae) and a rodent at a farm of Camellia meiocarpa of Yiyang City, Hunan Province. Acta Entomol. Sin. 2016, 59, 1123–1132, (In Chinese with English abstract). [Google Scholar]
- Yang, Y.; Chen, Y.Z.; Wang, R.; Peng, S.F.; Wang, X.N.; Chen, J.S.; Ma, L. Research progress on genetic variation characters of Camellia oleifera. Hunan For. Sci. Technol. 2010, 37, 19–23, (In Chinese with English abstract). [Google Scholar]
- Cai, S.P.; He, X.Y.; Li, Z.Z.; Xiong, Y.; Huang, J.S.; Zhou, S.Y. Study on damage of Curculio chinensis on Camellia oleifera fruit. J. Fujian For. Sci. Technol. 2011, 38, 14–16, (In Chinese with English abstract). [Google Scholar]
- Li, Z.W.; He, L.H.; Ma, L.; Xia, J.; Zeng, A.P. Influence of fruit size of Camellia meiocarpa on growth of oil tea weevil, Curculio chinensis (Coleoptera: Curculionidae). Chin. J. Appl. Ecol. 2014, 25, 3580–3586, (In Chinese with English abstract). [Google Scholar]
- He, L.H.; Li, Z.W.; Liu, J.J.; Si, J.Y.; Zeng, A.P. Correlation between damage of Curculio chinensis and fruit traits of Camellia meiocarpa. Sci. Silvae Sin. 2014, 50, 151–155, (In Chinese with English abstract). [Google Scholar]
- Xiao, Z.S.; Zhang, Z.B.; Krebs, C.J. Long-term seed survival and dispersal dynamics in a rodent-dispersed tree: Testing the predator satiation hypothesis and the predator dispersal hypothesis. J. Ecol. 2013, 101, 1256–1264. [Google Scholar] [CrossRef]
- Li, Z.W.; Zhao, R.; Li, Y.Z. Population density and spatial distribution pattern of Curculio chinesis (Coleoptera: Curculionidae) in Hunan, China. Plant Prot. 2019, 45, 163–170, (In Chinese with English abstract). [Google Scholar]
- Li, Z.W.; He, L.H.; Xia, J.; Ma, L.; Zeng, A.P. Determination of larval instars of the camellia weevil, Curculio chinensis (Coleoptera: Curculionidae). Acta Entomol. Sin. 2015, 58, 181–189, (In Chinese with English abstract). [Google Scholar]
- Dong, Z.; Cao, L.L.; Yi, X.F. Adaptive strategies of weevil larvae in the superparasitized acorns of the oriental white oak, Quercus aliena (Fagaceae). Acta Entomol. Sin. 2012, 55, 825–831. [Google Scholar]
- Wang, X.; Xiao, Z.S.; Zhang, Z.B.; Pan, H.C. Insect seed predation and its relationship with seed crop and seed size of Quercus mongolica. Acta Entomol. Sin. 2008, 51, 161–165, (In Chinese with English abstract). [Google Scholar]
- Ueda, S. Theory of the growth of silkworm larvae and its application. JARQ 1982, 15, 180–184. [Google Scholar]
- Lu, S.L.; Xu, J.L. Logistic curve of silkworm larval growth. Acta Sericologica Sin. 1989, 15, 223–225. (In Chinese) [Google Scholar]
- Yin, R.G. A preliminary study on the s-shaped growth curve and its point of inflection of Artogeia rapae (L.). Acta Entomol. Sin. 1989, 32, 380–381, (In Chinese with English abstract). [Google Scholar]
- Xu, L.; Meng, X.M.; Su, G.M.; Qi, L.; Jiao, Y.; Che, M.Q. An analysis on larval growth and development regularity of different Antheraea pernyi varieties using animal growth curve models. Sci. Seric. 2013, 39, 0620–0623, (In Chinese with English abstract). [Google Scholar]
- Yuwatida, S.; Chun-I, C.; Soisunee, T.; Kittiya, L.; Kuntida, M. Modeling the growth of black soldier fly Hermetia illucens (Diptera: Stratiomyidae): An approach to evaluate diet quality. J. Econ. Entomol. 2020, 113, 742–751. [Google Scholar]
- Zullinger, E.M.; Ricklefs, R.E.; Redford, K.H.; Mace, G.M. Fitting sigmoidal equations to mammalian growth curves. J. Mammal. 1984, 65, 607–636. [Google Scholar] [CrossRef]
- Beiki, H.; Pakdel, A.; Moradi-shahrbabak, M.; Mehrban, H. Evaluation of growth functions on Japanese quail lines. J. Poult. Sci. 2013, 50, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Kukhi, H.D.; Kebreab, E.; Lopez, S.; France, J. An evaluation of different growth functions for describing the profile of live weight with time (age) in meat and egg strains of chicken. Poult. Sci. 2003, 82, 1536–1543. [Google Scholar]
- Pitman, J.C.; Hagen, C.A.; Robel, R.J.; Loughin, T.M.; Applegate, R.D. Gender identification and growth of juvenile lesser prairie-chickens. Condor 2005, 107, 87–96. [Google Scholar] [CrossRef]
- Aggrey, S.E.; Ankra-Badu, G.A.; Marks, H.L. Effect of long-term divergent selection on growth characteristics in Japanese quail. Poult. Sci. 2003, 82, 538–542. [Google Scholar] [CrossRef]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control. 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Zhang, X.L.; Meng, L.; Li, B.P. Effects of Pieris rapae pupa mass on offspring number, sex ratio, and body size of gregarious parasitoid Pteromalus puparum (Hymenoptera: Pteromalidae). Chin. J. Ecol. 2009, 28, 677–680, (In Chinese with English abstract). [Google Scholar]
- Matsuo, Y. Cost of prolonged diapause and its relationship to body size in a seed predator. Funct. Ecol. 2006, 20, 300–306. [Google Scholar] [CrossRef]
- Toquenaga, Y. Contest and scramble competitions in Callosobruchus maculatus (Coleoptera: Bruchidae) II. Larval competition and interference mechanisms. Res. Popul. Ecol. 1993, 35, 57–68. [Google Scholar] [CrossRef]
- Messina, F.J. Predictable modification of body size and competitive ability following a host shift by a seed beetle. Evolution 2004, 58, 2788–2797. [Google Scholar] [CrossRef]
- Guedes, R.N.C.; Guedes, N.M.P.; Smith, R.H. Larval competition within seeds: From the behavior process to the ecological outcome in the seed beetle Callosobruchus maculatus. Austral Ecol. 2007, 32, 697–707. [Google Scholar] [CrossRef] [Green Version]
- Fox, C.W.; Messina, F.J. Evolution of larval competitiveness and associated life-history traits in response to host shifts in a seed beetle. J. Evol. Biol. 2018, 31, 302–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]


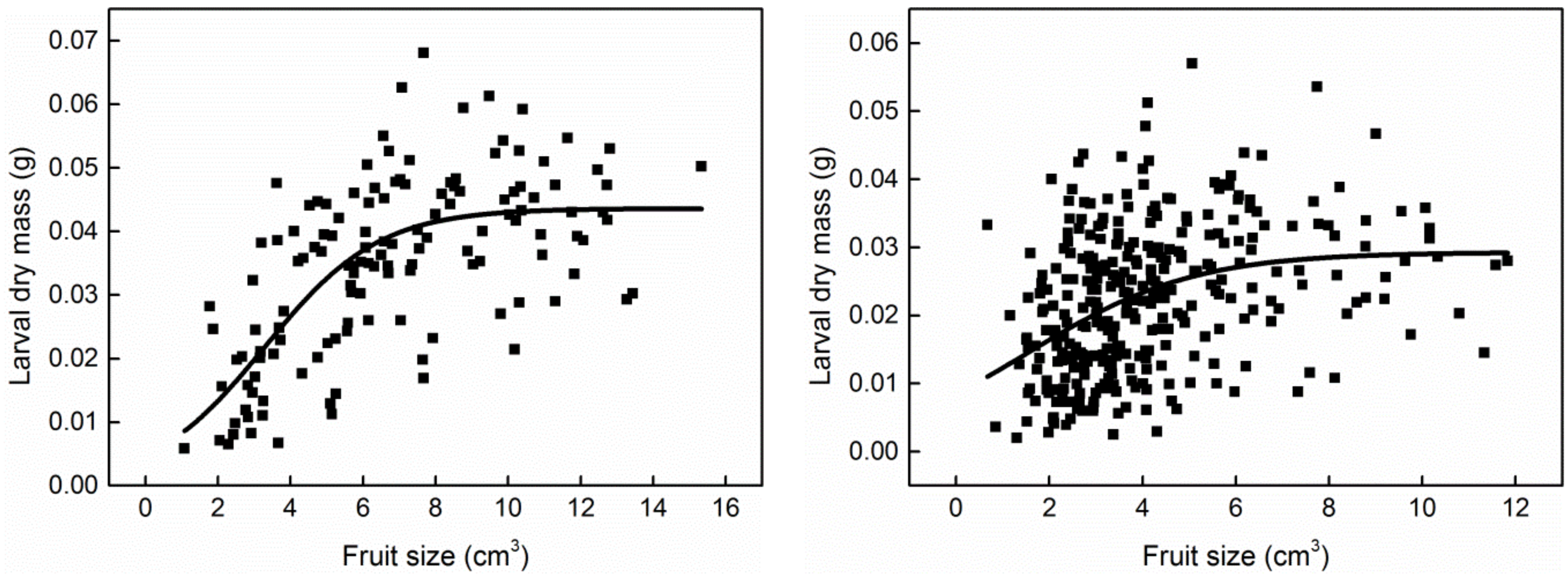
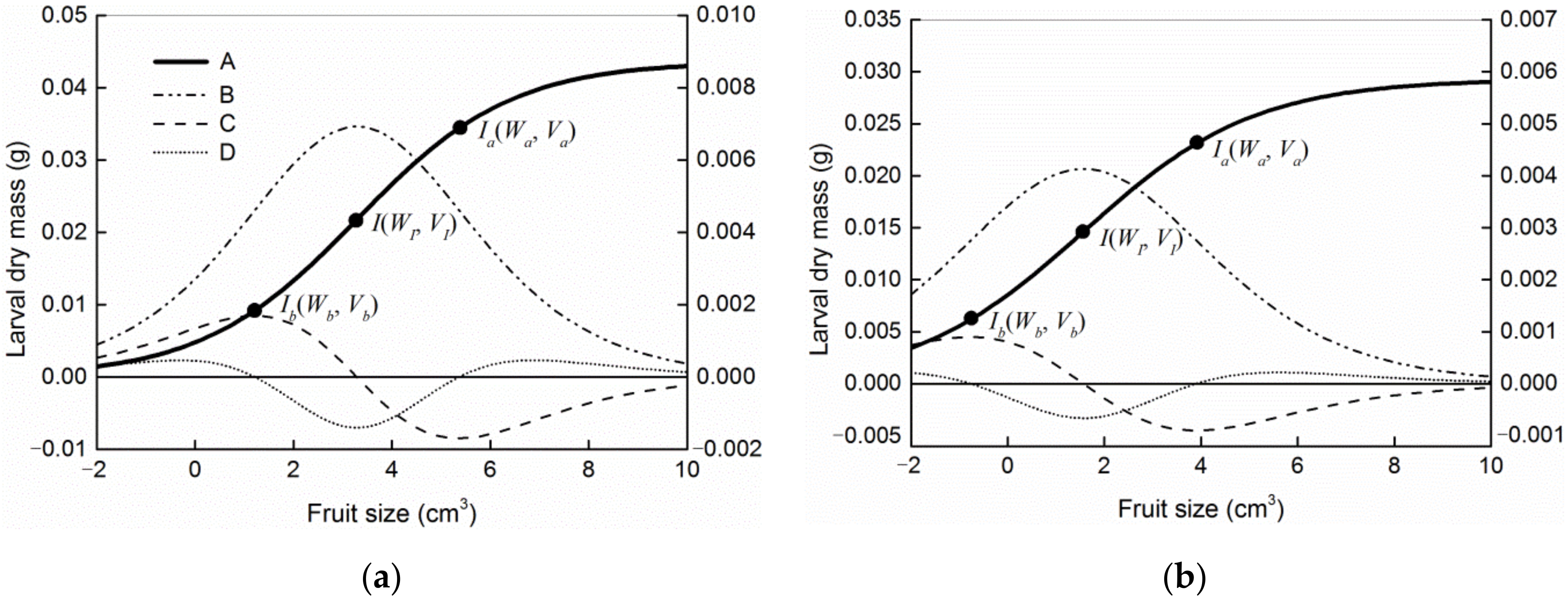
| Host | Model | Goodness-of-Fit | Parameters of Models | Coordinates of Ib and Ia | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MSE | AIC | p | Wm | K | VI/d | n | Ib (Vb, Wb) | Ia (Va, Wa) | ||
| Camellia oleifera | I | 1.063 × 10−4 | −1222.99 | <0.0001 | 0.0459 | 0.3077 | −9.0492 | |||
| II | 1.050 × 10−4 | −1223.71 | <0.0001 | 0.0434 | 0.7423 | 26.5858 | 1.7188 | (1.6644, 0.0123) | (5.7147, 0.0359) | |
| III | 1.050 × 10−4 | −1224.63 | <0.0001 | 0.0446 | 0.4281 | 2.1216 | (0.2635, 0.0008) | (3.9796, 0.0273) | ||
| IV | 1.047 × 10−4 | −1225.02 | <0.0001 | 0.0442 | 0.4825 | 2.4970 | (0.5025, 0.0032) | (4.4915, 0.0302) | ||
| V | 1.043 × 10−4 | −1225.61 | <0.0001 | 0.0436 | 0.6356 | 3.2885 | (1.2165, 0.0092) | (5.3606, 0.0344) | ||
| Camellia meiocarpa | I | 9.760 × 10−5 | −2952.09 | <0.0001 | 0.0306 | 0.3145 | −11.5828 | |||
| II | 9.722 × 10−5 | −2952.37 | <0.0001 | 0.0284 | 1.6766 | 5615.5633 | 10.0592 | (2.2433, 0.0175) | (5.3014, 0.0268) | |
| III | 9.741 × 10−5 | −2952.71 | <0.0001 | 0.0300 | 0.4013 | 0.4625 | (−1.5196, 0.0005) | (2.4446, 0.0184) | ||
| IV | 9.735 × 10−5 | −2952.92 | <0.0001 | 0.0297 | 0.4433 | 0.8100 | (−1.3612, 0.0022) | (2.9812, 0.0203) | ||
| V | 9.716 × 10−5 | −2953.54 | <0.0001 | 0.0293 | 0.5649 | 1.5676 | (−0.7638, 0.0062) | (3.8989, 0.0231) | ||
| Measurements | Camellia oleifera | Camellia meiocarpa | Independent Samples t-Test or Pearson Chi-Square Test | |
|---|---|---|---|---|
| Fruit size (cm3) | 6.87 ± 0.27 | 4.14 ± 0.12 | t452 = 9.180 | p < 0.0001 |
| Larval dry mass (g) | 0.0348 ± 0.0012 | 0.0222 ± 0.0006 | ||
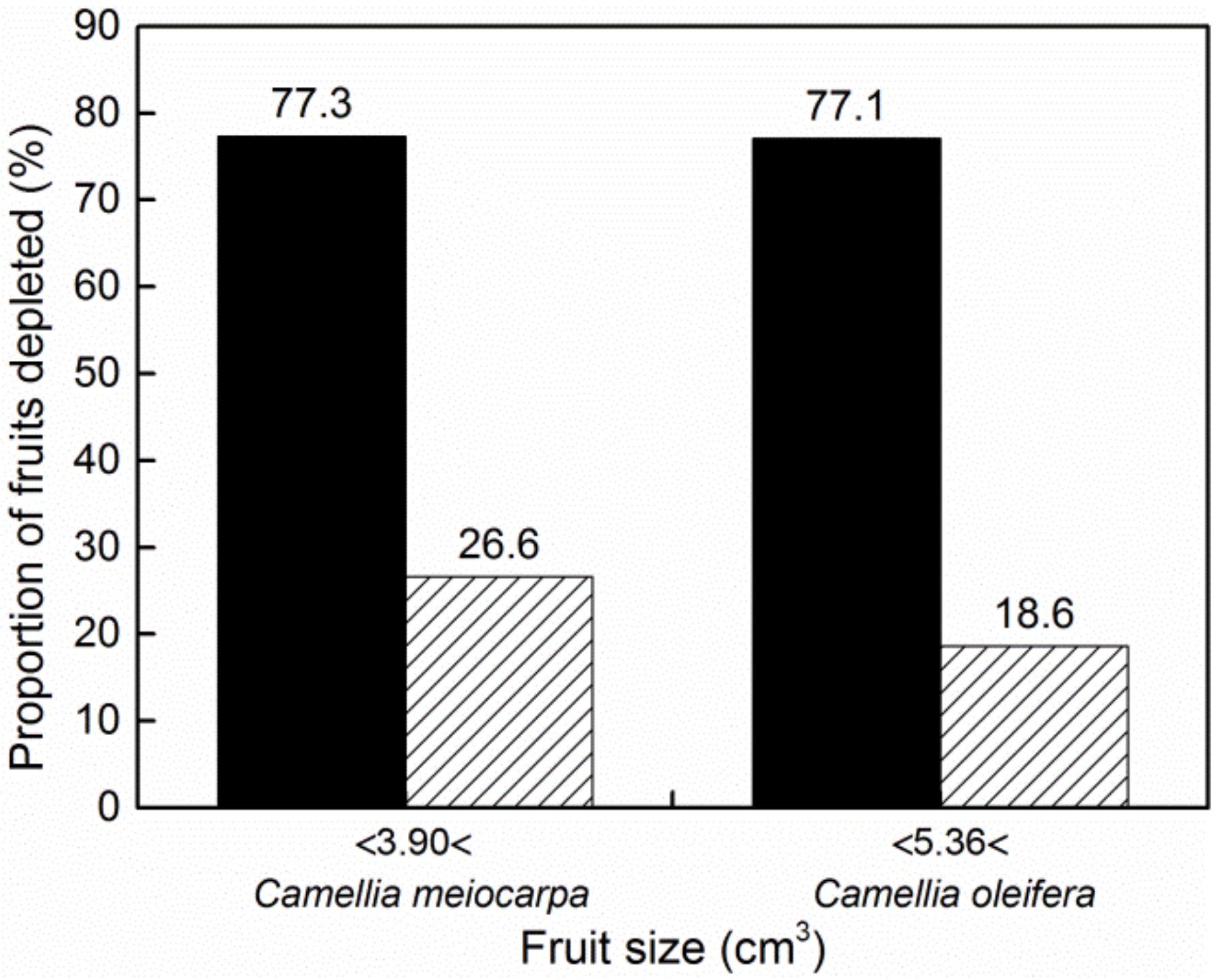
| Percentage of fruits depleted | 35.8% | 56.6% | χ21 = 16.255 | p < 0.0001 |
| Source of Variation | Larval Dry Mass(g) | ||
|---|---|---|---|
| d.f. | F | p | |
| Model | 3 | 86.552 | <0.0001 |
| Host species (H) | 1 | 30.628 | <0.0001 |
| Fruit size (F) | 1 | 116.591 | <0.0001 |
| H × F | 1 | 4.530 | 0.034 |
| Error | 450 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Yang, Z.; Li, Y. Effects of Fruit Sizes of Two Camellia Trees on the Larval Sizes of Curculio styracis (Roelofs, 1875): Testing the Endoparasitoid Body Size Hypothesis. Insects 2022, 13, 246. https://doi.org/10.3390/insects13030246
Li Z, Yang Z, Li Y. Effects of Fruit Sizes of Two Camellia Trees on the Larval Sizes of Curculio styracis (Roelofs, 1875): Testing the Endoparasitoid Body Size Hypothesis. Insects. 2022; 13(3):246. https://doi.org/10.3390/insects13030246
Chicago/Turabian StyleLi, Zhiwen, Zhongxia Yang, and Youzhi Li. 2022. "Effects of Fruit Sizes of Two Camellia Trees on the Larval Sizes of Curculio styracis (Roelofs, 1875): Testing the Endoparasitoid Body Size Hypothesis" Insects 13, no. 3: 246. https://doi.org/10.3390/insects13030246
APA StyleLi, Z., Yang, Z., & Li, Y. (2022). Effects of Fruit Sizes of Two Camellia Trees on the Larval Sizes of Curculio styracis (Roelofs, 1875): Testing the Endoparasitoid Body Size Hypothesis. Insects, 13(3), 246. https://doi.org/10.3390/insects13030246







