1. Introduction
Silk is a fibrous protein secreted by leaving organisms (insects, spiders, etc.) for housing, protection, or predation purposes. It is synthesized into the cells of specialized glands, accumulated into the lumen, and finally extruded to make extracorporeal filamentous structures (nets, cocoons, nests, etc.) [
1,
2]. The silks produced by
Lepidoptera (
Bombyx,
Antheraea,
Phylosamia genus) are an important source of starting materials for the textile industry [
3]. The so-called domesticated or mulberry silk, obtained from rearing
Bombyx mori silkworms and unraveling their cocoons, has been used for thousands of years to produce precious fabrics highly appreciated for their lustrous appearance, softness, and elegance. Silk production represents less than 0.1% of the global fiber market (estimated at about 110 million tons in the year 2020; source:
https://textileexchange.org, accessed on 15 November 2021) but is still a significant billion-dollar industry because silk is the leading fiber in fashion design (
https://fashionunited.com, accessed on 15 November 2021).
The first documented medical use of silk dates back to about 2200 years, when Claudius Galenus proposed to use silk as suture thread [
4]. Other similar applications appeared throughout the centuries, but only in the 20th century have silk sutures become commercially available for routine clinical use. A key step for the development of a wider range of medical applications has been the dissolution of native silk fibroin fibers and the obtainment of a liquid feedstock from which different regenerated silk-based materials such as films, hydrogels, porous scaffolds, nanofibers, particles, etc., could be prepared [
5]. Of the two main components of silk, fibroin and sericin, fibroin is the material that has attracted the most attention, although sericin has recently been carving out a significant space in the biomaterial field [
6]. However, every time silk is mentioned in this article, reference will be made solely to the fibroin component.
From the 1980s onwards, the scientific community’s interest in silk as a biomaterial has dramatically increased. Just to give an idea of the development of scientific interest in this area, a quick search in the Scopus database using “silk fibroin” as keyword returned about 50 papers published in the year 2000 and more than 800 in 2020, with an annual increase trend that is expected to continue in the future. While Japanese, European, and American research teams dominated the scientific production about silk at the beginning of the 21st century, the current panorama of the most prolific countries is led by China, whose scientific production is now three-times larger than that of America. It seems, therefore, that the millennial history of silk, which began in China and spread throughout the world via the Silk Road, has made a U-turn to return to where it originated, coupling the more traditional Chinese monopoly in silk production to the newly acquired scientific leadership.
The range of biomedical applications targeted by silk-based scaffolds is very wide, spanning from implantable devices for engineering and/or regeneration of soft and hard tissues to the development of tissue models, carriers for drug release, diagnostic devices, etc. [
4,
7,
8]. The reason for such a wide interest in silk lies in its intrinsic properties, including biocompatibility, controllable degradability, absence of toxicity of degradation products, structural stability, mechanical performance, wide choice of manufacturing options which makes it possible to design scaffolds with desirable features for specific applications [
5]. Different silk-based products have reached the pre-clinical stage and have been tested in small and large animal models, and in clinical trials with promising results [
4]. Even if the number of silk-based devices approved for clinical use is still very low, the number of companies involved in the development of silk-based medical products has been increasing over the last decade; thus, it is likely that, quite soon, many of them will undertake the regulatory path that leads to the approval of devices for use in the clinical setting [
4,
9].
The translational process that allows a silk-based material to go through the regulatory pathway that transforms the initial idea and the starting material into a product to be used in the therapeutic field is particularly long and complex. Although there might be differences depending on the different rules put in place by European (CE mark) or American (Food and Drug Administration-FDA approval) regulatory agencies, many basic steps are common. These include the acquisition of raw materials in accordance with well-established quality assurance programs, the rigorous control of the manufacturing process that must be robust and compliant in terms of product specification, the implementation and execution of on-bench and in vitro testing programs, the thorough biocompatibility evaluation of the product according to guidelines such as ISO 10993-1 (International Organization for Standardization), the execution of relevant in vivo functional tests using appropriate animal models, the access to clinical trials to evaluate safety and performance of the device and the submission of the dossier to a regulatory body for device approval [
9].
The aim of this paper is to disclose how the clinical translation route works using the experience of the Italian start up Silk Biomaterials srl in the development of a silk-based medical device, the SILKBridge
® nerve conduit, as case study. The device is designed for the surgical repair of peripheral nerve discontinuities to support the regeneration of injured nerves [
10]. It is intended to act as a bridge to guide and structurally support axonal growth across the gap during the healing process. The conduit is entirely made of silk fibroin obtained from
Bombyx mori silkworms. During the manufacturing process, sericin is completely removed by degumming to leave pure silk fibroin. The conduit has a tubular structure with a three-layered wall comprising an intermediate textile layer intimately and firmly coupled with two electrospun layers, one in the inner and the other in the outer parts of the device wall [
10]. This design allowed for optimizing both mechanical and biological properties, because the electrospun layers have biomimetic characteristics that enhance cell’s adhesion and integration with the surrounding tissues, while the textile layer was designed to provide the required mechanical resistance during implantation (suture retention strength) and in vivo functioning (compression strength) [
11]. The conduit displayed a slow degradation rate both in vitro [
12] and in vivo [
11]. As degradation occurred, the load bearing responsibility was transferred to the new tissue ingrowth such that mechanical integrity was maintained at the implantation site and a complete morphological and functional recovery of the transected nerve was achieved [
11].
The results reported here complement those already published [
10,
11,
12] about the synergic efficacy of the use of silk as biomaterial and of the design of a three-layered wall architecture for manufacturing the nerve conduit; moreover, they specifically tackle the following issues of the regulatory process: demonstration of the robustness of the production process through the evaluation of the functional parameters of the device defined in the design phase, verification of potential sources of risk that may emerge from the chemicals used for production through toxicological analysis, evaluation of biological safety as required by regulations in force for medical devices and start of the first phase of clinical trials.
4. Discussion
The development of an implantable medical device as the SILKBridge® nerve conduit must undergo a comprehensive series of control tests before being released for use to ensure that the manufacturing process is under control and that the device performs as expected. The on-bench testing framework complies with the design controls requirements set out according to FDA and EU/MDR regulations, and comprises a list of functional parameters and test methods inspired by the ISO 10993-18 standard, which can be used for the identification and evaluation of the physical, chemical, morphological, and topographical properties of materials in finished medical devices, all having a strong impact in terms of verification and validation of the target performance characteristics.
The results of the tests reported in
Table 1 (morphological and geometrical characteristics),
Table 2 (physical and structural characteristics), and
Table 3 (mechanical characteristics) demonstrate that the comparison of three production lots gave satisfactory results. All the measured characteristics appeared highly reproducible, with a very low level of batch-to-batch variability, thus, ensuring the robustness of the manufacturing process. With reference to the suitability of the device for the intended use, the following discussion will provide deeper insights into the set of functional specifications that were defined during the design controls to comply with end-use requirements.
The inner diameter of the device is a geometrical characteristic determined by the foreseen clinical use. The transversal dimension of the conduit must be large enough to accommodate the distal and proximal extremities of the severed nerve and to allow their fixation by suturing during surgery. The size of the nerve conduits currently produced is 1.5–2.0 mm inner diameter, which is optimized for repairing transected digital nerves [
16]. To repair larger nerves located in other anatomical positions, larger devices will be required. Usually, marketed nerve conduits cover a range of discrete sizes from 1 mm to 10 mm.
The wall thickness can be a more critical characteristic. A relationship between the formation of neuromas in regenerated nerves tissues and the thickness of the conduit wall has been reported [
17]. The wall-thickness of SILKBridge
® has been carefully designed considering the properties of the selected starting materials and the requirements in terms of mechanical performance, suturability, targeted degradation rate, and the permeability specifications [
18]. In general, wall thickness values lower that 1 mm are highly suggested for nerve guide application, with 0.6 mm as the optimum target for the maximum value [
19]. As shown in
Table 1, the final device falls within this optimal range.
Linear density, i.e., the weight per unit length, allows for evaluating the amount of material implanted into the body. Silk, as any other non-autologous biomaterial, is likely to elicit a foreign body response at the site of implantation [
7]. Moreover, it has been reported that silk-based devices implanted in soft tissues or with longer degradation times tend to induce a long-lasting inflammatory response than those with shorter degradation times or those located within hard tissues [
8]. On this basis, it has been decided to keep the linear density as low as possible to reduce the impact of the device in terms of local reactions. The choice of a macro-porous texture of the textile braid for the central layer of the wall allowed for keeping the linear density of the final device largely below the threshold of 10 mg/cm, as defined in the design stage (
Table 1).
The porosity of the wall of a nerve conduit is a key functional parameter. It must be optimized to maximize the influx of oxygen and nutrients from the interstitial fluid and to avoid the loss of neurotrophic factors secreted by Schwann cells at the distal stump. A wall porosity of about 80% or higher is considered ideal for peripheral nerve repair [
17,
18,
19]. This target has been achieved with SILKBridge
®, whose wall porosity higher than 80% results from the combination of the macro- and micro-porosity of the TEX and ES layers, respectively (
Table 1). With reference to the pore size, this characteristic is entirely determined by the two ES layers and does not exceed the 5 μm threshold on average (data not shown). This allows for the permeation of small nutrient molecules, while preventing the migration of inflammatory cells and the infiltration of fibrous tissue inside the lumen of the conduit [
18].
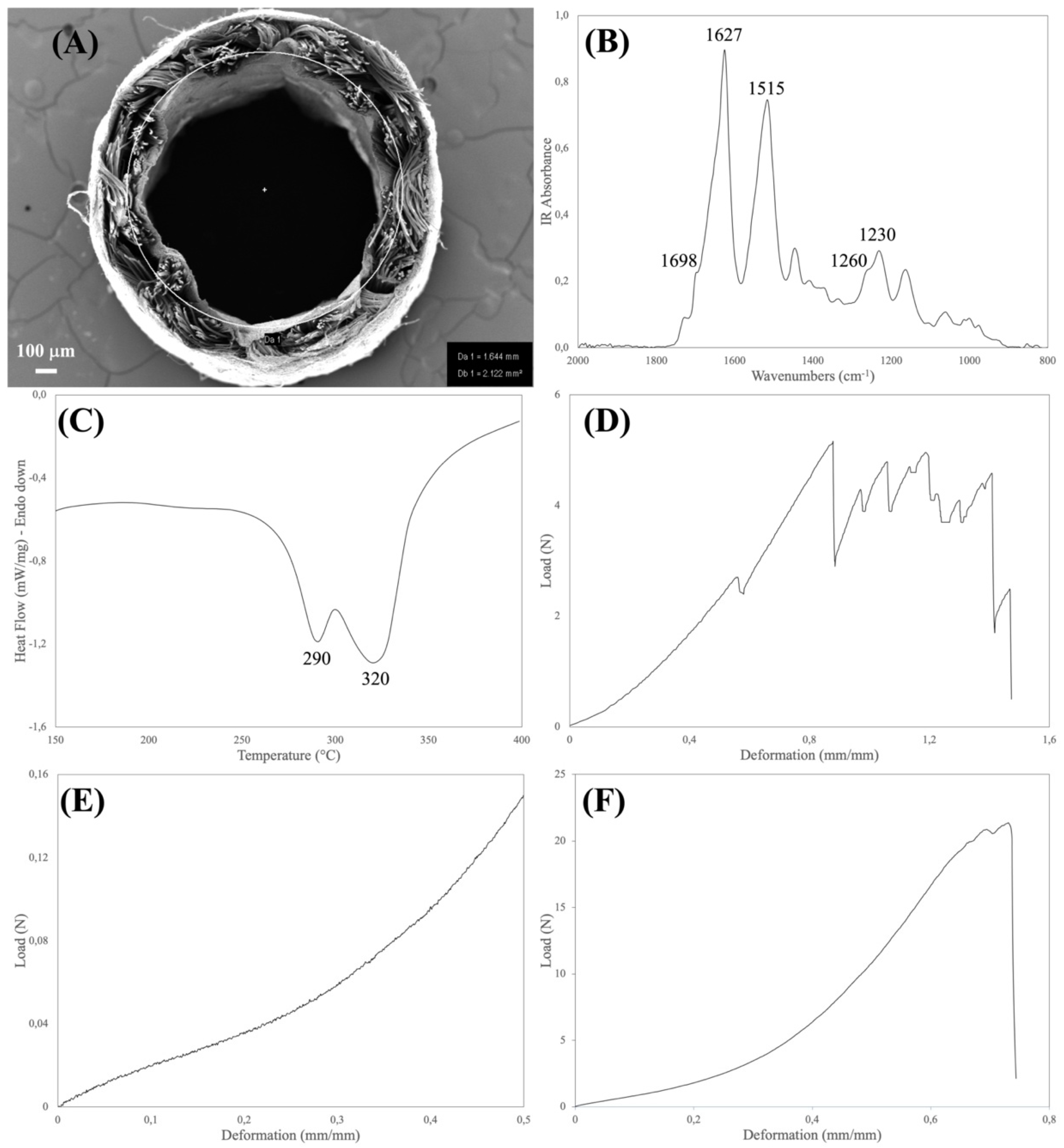
The physical and structural features of the device were explored by FTIR and DSC analyses (
Table 2). Both the spectroscopic and thermal properties of silk fibroin-based materials have been extensively investigated [
20,
21]. The type, position, and intensity of the bands in the IR spectra and of the thermal transitions in the DSC thermograms not only reflect the chemical structure of the silk materials but also provide information about the molecular conformation taken by the silk fibroin chains in native and regenerated formats.
The aim of the FTIR analysis was to verify that the manufacturing process did not alter the chemical integrity of the constituent material, did not leave foreign matters or contaminants on the device, and allowed for achieving the desired degree of crystallinity of the regenerated electrospun layers. Loss of integrity and changes in the chemical and conformational structure of the constituent silk material may have a strong impact on the biodegradation rate of the device upon implantation. Moreover, the presence of contaminants may impact on the biocompatibility of the device. For the purpose of integrity and purity checks, the position and intensity of the IR bands found in the spectrum of the sample were compared with reference spectra, while the crystallinity index was calculated from the intensity ratio of the Amide III bands at 1260 cm
−1 (crystalline phase) and 1230 cm
−1 (amorphous phase) [
15].
The major thermal events characteristics of silk fibroin fall in the 150–400 °C temperature range (glass transition and melting/degradation). As with FTIR, the aim of the DSC analysis was to verify the structural integrity of the constituent silk fibroin materials to avoid any impact on the performance of the device. The DSC analysis allowed us to also calculate the mass ratio between the electrospun and textile layers, which present distinct endothermic peaks [
10]. This parameter is a very useful tool to further assess the robustness of the manufacturing process. The DSC and FTIR results listed in
Table 2 allowed for confirming the structural integrity of the silk fibroin materials comprising the device.
Medical devices must be designed with an optimal set of mechanical properties fitting the requirements of the specific clinical need. Nerve conduits must provide a balanced combination of strength and elasticity to withstand clinical operation stresses, such as manipulation and suturing during implantation, to resist deformation caused by the biomechanical stresses generated in vivo, and to avoid channel collapse, since compression can result in damage to the growing axon [
10,
11]. To verify that the manufacturing process allows for reaching the desired mechanical characteristics, the devices were analyzed for determining the tensile, suture retention, and compression properties (
Table 3).
To withstand physiological loads, the tensile properties of nerve conduits should at least approach the ones of natural nerves [
18,
22]. The device must be elastic enough to match the deformation of the natural nerve and strong enough to protect the growing axons from breaking. From the data reported in the literature, the stress and strain values of human nerves are about 7 MPa and 60%, respectively. Considering the geometry of the device, the stress value corresponds to a breaking load of about 15 N. As shown in
Table 3, the SILKBridge
® nerve conduit largely met the target values.
The suture retention test is intended to verify whether the device withstands the mechanical stresses applied by the surgeon during implantation and later during in vivo functioning. Suture failure may cause dramatic consequences to the patients, due to the loss of stability of the implant, lack of support for tissue regeneration, and need to re-operate. No threshold values for suture retention strength of nerve conduits have been reported in literature. On the contrary, limits were imposed for vascular grafts, a much more demanding application in terms of anastomotic strength, which requires that grafts are capable to exceed 2 N as suture retention strength [
23,
24,
25]. Thanks to the strength of the textile braid, which is the load-bearing element of the wall of the device, the target threshold of suture retention strength was achieved by the final device (
Table 3).
Finally, nerve conduits must withstand the mechanical compression stresses of surrounding tissues until complete nerve regeneration, avoiding collapse that may hinder the healing and cause pain to the patient [
18]. To set acceptable threshold values for the compression performance, reference was made to the limit pressure values reported for the human median nerve exposed to carpal tunnel pressure [
26]. In healthy individuals, carpal tunnel pressures are typically well below 10 mmHg, corresponding to a stress 1.33 kPa. Considering the geometry of the SILKBridge
®, this stress corresponds to a compression load of about 1.1 cN. The results listed in
Table 3 indicate that the compression resistance threshold was easily achieved, and that the device can withstand compression stresses and remain open to allow for a smooth progression of the nerve regeneration.
The evaluation of the potential toxicological risks associated with leachable substances released by a medical device in the surrounding tissues is another important step for the identification and quantification of the biological hazards related to its use. The ISO 10993-12 standard provides provisions for the preparation of samples for analysis. The ISO 10993-18 standard specifies a framework for the identification of leachable substances and for the quantitative determination of their potential release in the human body. The ISO 10993-17 standard guides the manufacturer through the process of estimation and control of the toxicological risks associated with the medical device use. The chemical analysis addressed all the processing aids used along the manufacturing process. Their amount was determined by applying suitable laboratory extraction conditions and advanced analytical methods.
With reference to the mechanism by which the chemical compounds may be released into the tissues surrounding the implant area, a combination of contributions can play a role. Simple diffusion from the device to the tissue may occur in the first period after implantation, when the device is still intact. Afterwards, when the device starts to degrade, swelling and fragmentation of the polymer texture may open new ways for the chemical to leach outside. However, it must be considered that the release will never occur in a bursting way, but gradually over time and in a more physiologically compliant manner, so that the local load is diluted over time. This assumption is supported by the fact that SILKBridge
® is made of silk fibroin materials characterized by a slow rate of degradation, i.e., from months to years for regenerated electrospun fibers and native microfibers, respectively [
12].
As shown in
Table 4, the results of the toxicological evaluation have demonstrated that the leachable substances coming from the manufacturing process were under control and that the cleaning procedures were effective in removing the greatest part of the processing aids from the final device. The residual amounts still present were largely below the threshold for toxicological concern, also considering the worst-case scenarios taken into account during the toxicological evaluation, i.e., the possibility of multiple implants (up to 10, one device for each finger), the ten-fold increase of the acceptable value of the Margin of Safety, and the assumption that the total amount is released in one day in a bursting way.
The targeted toxicological analysis here reported has been complemented with an untargeted one, which allowed to identify possible leachable compounds beyond those used for manufacturing, to include also unpredictable contaminants of environmental source, including laboratory materials, packaging, etc. (data not shown). It is worth noting that also this additional approach did not reveal the presence of unexpected contaminants likely to pose a health risk.
The evaluation of the toxicity profile of a degradable medical device such as SILKBridge
® cannot disregard the possible toxicity of the degradation products. This issue has been addressed in a previous study where the in vitro degradation profile and the cytotoxicity of the degradation products were reported [
12]. The bacterial protease type XIV from
Streptomyces griseus was used as hydrolytic agent at three different enzyme/substrate ratios and for incubation times as long as 91 days. Degradation of the device occurred by surface erosion. The mass spectrometry analysis of the degradation products showed that the silk fibroin polypeptides recovered in the incubation buffers were representative of the aminoacidic sequence of the fibroin light and heavy chains, indicating that virtually the entire sequence of the fibroin protein was degraded. More important, the incubation buffers containing the soluble degradation products were tested with human HEK293 cells and mouse neuroblastoma N2a cells to assess cytotoxicity. No detrimental effects on cell viability were observed, suggesting that the degradation products, consisting of amino acids and small peptides, did not show any toxic property and their more likely fate was to enter the metabolic pathways of the host tissue cells [
7]. Therefore, it is possible to conclude that the SILKBridge
® nerve conduits will not elicit any toxic effect due to the constituent materials and/or to the manufacturing process.
Biocompatibility is an essential requirement for the medical devices and the large amount of scientific data on the safety of silk is not sufficient on a regulatory prospective to allow the use of silk-based device since the manufacturing process can change the characteristics of the raw material and have an impact on the biocompatibility of the finished device [
8]. This perspective imposes that a comprehensive evaluation of the device biocompatibility is carried out to prevent any possible adverse effect for the patient’s health. Different parts of the ISO 10993 standard provide a framework for the evaluation of the biological safety of the device. A plan has been designed and a set of tests, including cytotoxicity, delayed hypersensitivity, intracutaneous reactivity, pyrogen test, LAL test, acute systemic toxicity, and genotoxicity has been carried out. The overall results of the biological response allowed confirming that SILKBridge
® is fully biocompatible (
Table 5). This is in good agreement with the numerous data reported in the scientific literature about the biocompatibility of silk materials [
8].
Finally, the evaluation of a medical device can’t exempt from carrying out performance tests to verify that the product achieve it’s intended use. In a previously published article [
11] the implantation of the SILKBridge
® in the median nerve of rats was discussed to demonstrate the regeneration of myelinated fibers along the conduit filling the gap previously created and leading to an effective morphological and functional recovery of the median nerve, like that observed with the reference autograft nerve reconstruction.
Altogether, the results reported here, and others previously published [
10,
11,
12] represent an important achievement towards the implementation of a clinical study aimed at investigating the safety and efficacy of the SILKBridge
® nerve conduit. The device has demonstrated an optimized balance of biomechanical and biological properties; it is intended as an “off-the-shelf” product ready to be used in the operating room as it is, without the need of adding neurotrophic and/or angiogenic factors or cells. The encouraging on-bench and pre-clinical results allowed us to proceed quickly towards the submission of a first in-human clinical study aimed at evaluating the reconstruction of digital nerve defects in humans (
ClinicalTrials.gov identifier: NCT03673449). The study has already started at the Department of Plastic Surgery and Hand Surgery of the University Hospital of Zurich. Four out of 15 patients have been enrolled so far and implanted with SILKBridge
® nerve conduit to repair a digital nerve gap, with very satisfactory outcome after one year in terms of functional recovery.