Sensitivity of Buff-Tailed Bumblebee (Bombus terrestris L.) to Insecticides with Different Mode of Action
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Maintenance of the Hives
2.2. Insecticides
2.3. Bioassays
2.4. Maximum Exposure of Bumblebee Workers in the Field
2.5. Statistical Analyses
3. Results
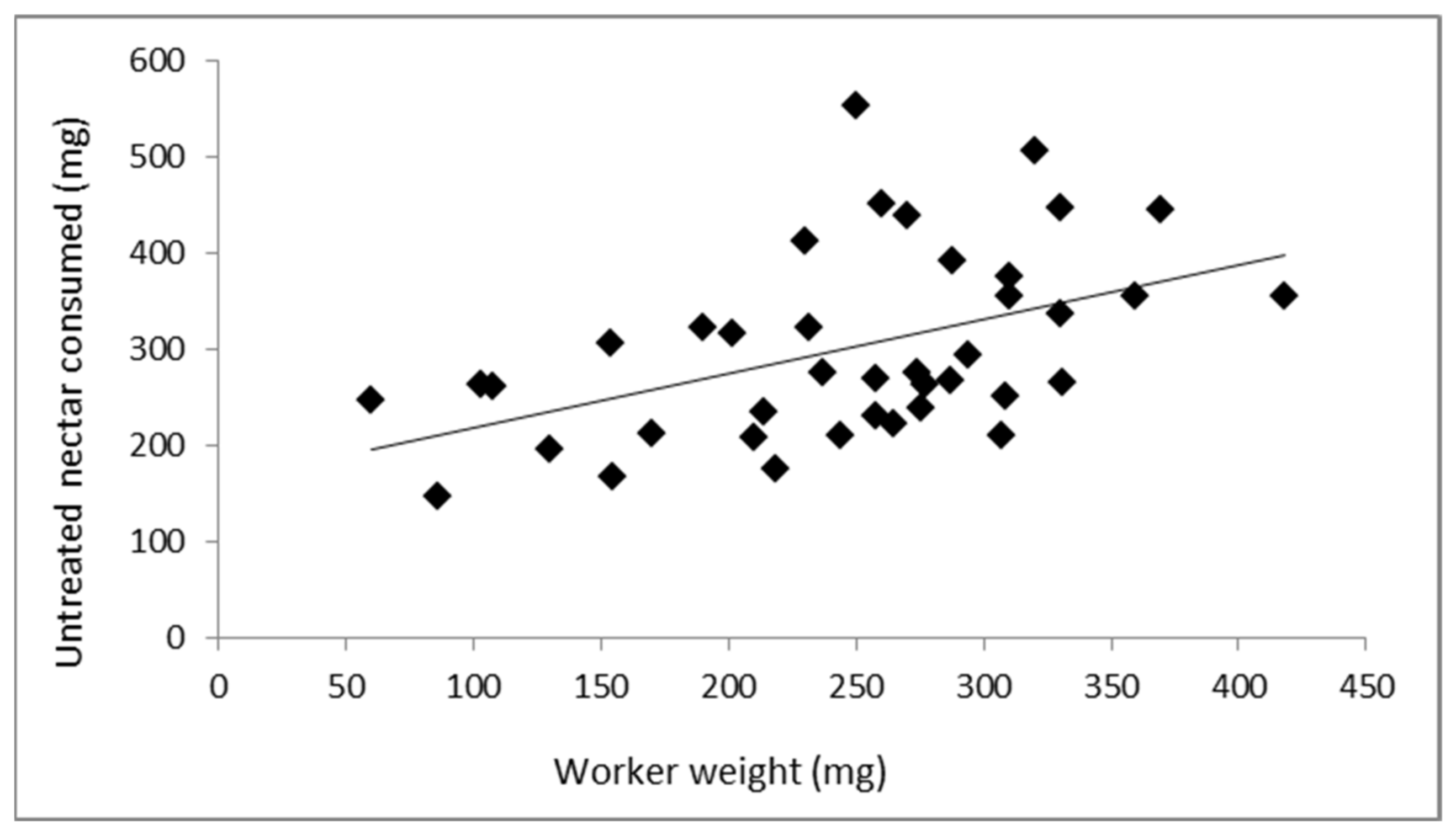
3.1. Relationship between Worker Weight and Nectar Consumed
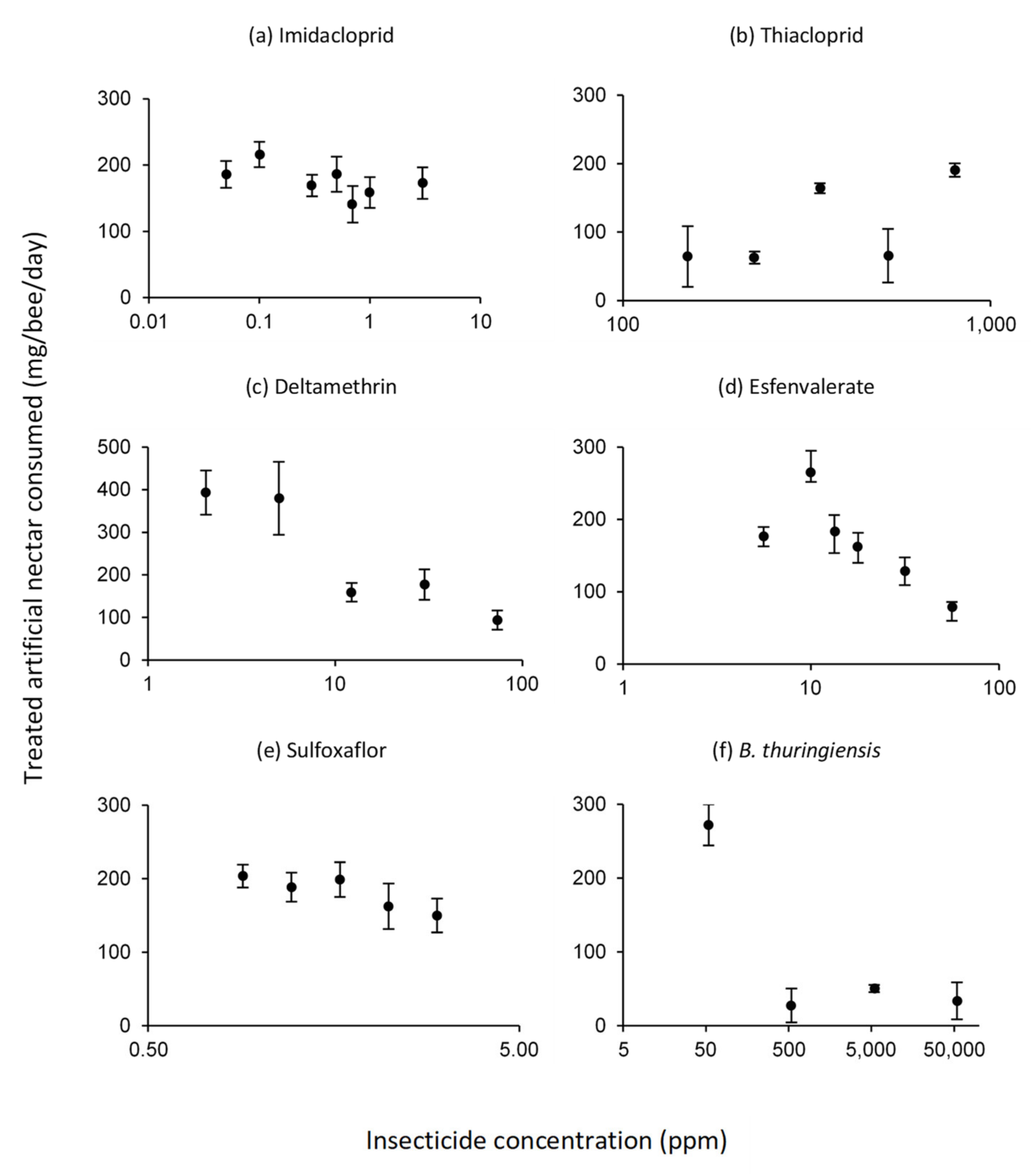
3.2. Intake of Treated Artificial Nectar
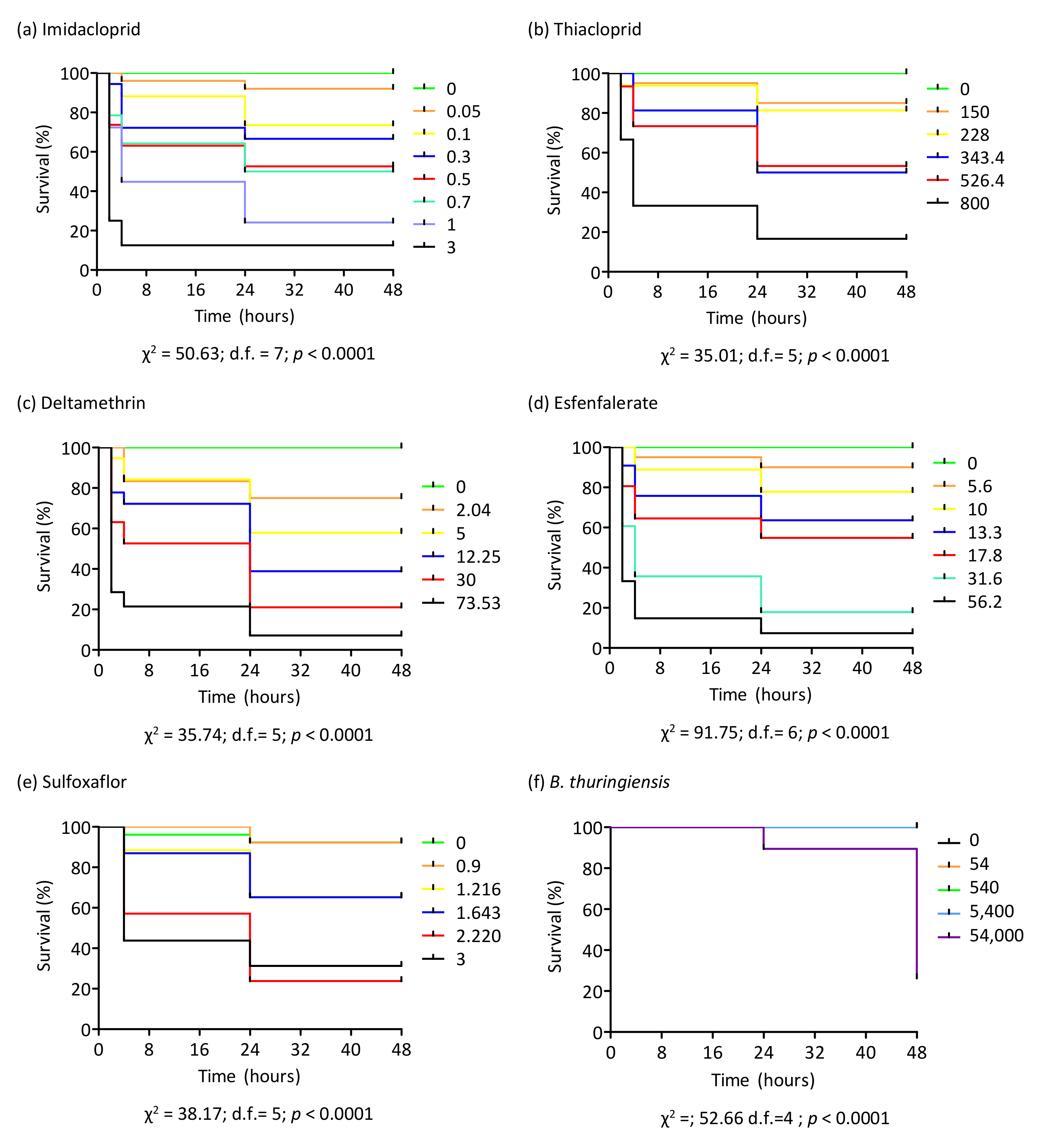
3.3. Toxicity of Insecticides on Bumblebee Workers
3.4. Survival Probability after Insecticide Treatment
3.5. Maximum Exposure of Bumblebee Workers in the Field
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hines, H.M. Historical Biogeography, Divergence Times, and Diversification Patterns of Bumble Bees (Hymenoptera: Apidae: Bombus). Syst. Biol. 2008, 57, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Velthuis, H.H.W.; Van Doorn, A. A century of advances in bumblebee domestication and the economic and environmental aspects of its commercialization for pollination. Apidologie 2006, 37, 421–451. [Google Scholar] [CrossRef] [Green Version]
- Trillo, A.; Montero-Castaño, A.; Vilà, M. Seasonality of bumblebee spillover between strawberry crops and adjacent pinewoods. Apidologie 2020, 51, 1051–1061. [Google Scholar] [CrossRef]
- Kleijn, D.; Winfree, R.; Bartomeus, I.; Carvalheiro, L.G.; Henry, M.; Isaacs, R.; Klein, A.; Kremen, C.; M’Gonigle, L.K.; Rader, R.; et al. Delivery of crop pollination services is an insufficient argument for wild pollinator conservation. Nat. Commun. 2015, 6, 7414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasmont, P.; Coppee, A.; Michez, D.; De Meulemeester, T. An overview of the Bombus terrestris (L. 1758) subspecies (Hymenoptera: Apidae). Annales de la Société entomologique de France (N.S.) 2008, 44, 243–250. [Google Scholar] [CrossRef] [Green Version]
- Biesmeijer, J.C.; Roberts, S.P.M.; Reemer, M.; Ohlemüller, R.; Edwards, M.; Peeters, T.; Schaffers, A.P.; Potts, S.G.; Kleukers, R.; Thomas, C.D.; et al. Parallel Declines in Pollinators and Insect-Pollinated Plants in Britain and the Netherlands. Science 2006, 313, 351–354. [Google Scholar] [CrossRef]
- Van Engelsdorp, D.; Hayes, J.; Underwood, R.M.; Pettis, J. A Survey of Honey Bee Colony Losses in the U.S., Fall 2007 to Spring 2008. PLoS ONE 2008, 3, e4071. [Google Scholar] [CrossRef]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef]
- Potts, S.G.; Imperatriz-Fonseca, V.; Ngo, H.T.; Aizen, M.; Biesmeijer, J.C.; Breeze, T.; Dicks, L.; Garibaldi, L.A.; Hill, R.; Settele, J.; et al. Safeguarding pollinators and their values to human well-being. Nature 2016, 540, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Bartomeus, I.; Ascher, J.S.; Gibbs, J.; Danforth, B.; Wagner, D.L.; Hedtke, S.M.; Winfree, R. Historical changes in northeastern US bee pollinators related to shared ecological traits. Proc. Natl. Acad. Sci. USA 2013, 110, 4656–4660. [Google Scholar] [CrossRef] [Green Version]
- Bommarco, R.; Lundin, O.; Smith, H.G.; Rundlöf, M. Drastic historic shifts in bumble-bee community composition in Sweden. Proc. Royal Soc. B 2011, 279, 309–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cameron, S.A.; Lozier, J.D.; Strange, J.P.; Koch, J.B.; Cordes, N.; Solter, L.F.; Griswold, T.L. Patterns of widespread decline in North American bumble bees. Proc. Natl. Acad. Sci. USA 2011, 108, 662–667. [Google Scholar] [CrossRef] [Green Version]
- Goulson, D.; Lye, G.C.; Darvill, B. Decline and Conservation of Bumble Bees. Annu. Rev. Èntomol. 2008, 53, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Powney, G.D.; Carvell, C.; Edwards, M.; Morris, R.K.A.; Roy, H.E.; Woodcock, B.A.; Isaac, N.J.B. Widespread losses of pollinating insects in Britain. Nat. Commun. 2019, 10, 1–6. [Google Scholar] [CrossRef] [PubMed]
- González-Varo, J.P.; Biesmeijer, J.C.; Bommarco, R.; Potts, S.G.; Schweiger, O.; Smith, H.G.; Steffan-Dewenter, I.; Szentgyörgyi, H.; Woyciechowski, M.; Vilà, M. Combined effects of global change pressures on animal-mediated pollination. Trends Ecol. Evol. 2013, 28, 524–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanbergen, A.J.; the Insect Pollinators Initiative. Threats to an ecosystem service: Pressures on pollinators. Front. Ecol. Environ. 2013, 11, 251–259. [Google Scholar] [CrossRef] [Green Version]
- Vanengelsdorp, D.; Evans, J.D.; Saegerman, C.; Mullin, C.; Haubruge, E.; Nguyen, B.K.; Frazier, M.; Frazier, J.; Cox-Foster, D.; Chen, Y.; et al. Colony Collapse Disorder: A Descriptive Study. PLoS ONE 2009, 4, e6481. [Google Scholar] [CrossRef] [PubMed]
- Bass, C.; Denholm, I.; Williamson, M.S.; Nauen, R. The global status of insect resistance to neonicotinoid insecticides. Pestic. Biochem. Physiol. 2015, 121, 78–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeschke, P.; Nauen, R.; Schindler, M.; Elbert, A. Overview of the Status and Global Strategy for Neonicotinoids. J. Agric. Food Chem. 2011, 59, 2897–2908. [Google Scholar] [CrossRef]
- Simon-Delso, N.; Amaralrogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Chagnon, M.; Downs, C.; Furlan, L.; Gibbons, D.W.; Giorio, C.; Girolami, V.; et al. Systemic insecticides (neonicotinoids and fipronil): Trends, uses, mode of action and metabolites. Environ. Sci. Pollut. Res. 2015, 22, 5–34. [Google Scholar] [CrossRef] [PubMed]
- Blacquière, T.; Smagghe, G.; van Gestel, K.; Mommaerts, V. Neonicotinoids in bees: A review on concentrations, side-effects and risk assessment. Ecotoxicol. 2012, 21, 973–992. [Google Scholar] [CrossRef] [Green Version]
- Cresswell, J.E.; Page, C.J.; Uygun, M.B.; Holmbergh, M.; Li, Y.; Wheeler, J.G.; Laycock, I.; Pook, C.J.; de Ibarra, N.H.; Smirnoff, N.; et al. Differential sensitivity of honey bees and bumble bees to a dietary insecticide (imidacloprid). Zoology 2012, 115, 365–371. [Google Scholar] [CrossRef]
- Henry, M.; Béguin, M.; Requier, F.; Rollin, O.; Odoux, J.-F.; Aupinel, P.; Aptel, J.; Tchamitchian, S.; Decourtye, A. A Common Pesticide Decreases Foraging Success and Survival in Honey Bees. Science 2012, 336, 348–350. [Google Scholar] [CrossRef]
- Sandrock, C.; Tanadini, L.G.; Pettis, J.S.; Biesmeijer, J.C.; Potts, S.G.; Neumann, P. Sublethal neonicotinoid insecticide exposure reduces solitary bee reproductive success. Agric. For. Èntomol. 2014, 16, 119–128. [Google Scholar] [CrossRef]
- Rundlöf, M.; Andersson, G.; Bommarco, R.; Fries, I.; Hederström, V.; Herbertsson, L.; Jonsson, O.; Klatt, B.; Pedersen, T.R.; Yourstone, J.; et al. Seed coating with a neonicotinoid insecticide negatively affects wild bees. Nature 2015, 521, 77–80. [Google Scholar] [CrossRef]
- Woodcock, B.A.; Bullock, J.M.; Shore, R.F.; Heard, M.S.; Pereira, M.G.; Redhead, J.W.; Ridding, L.; Dean, H.; Sleep, D.; Henrys, P.A.; et al. Country-specific effects of neonicotinoid pesticides on honey bees and wild bees. Science 2017, 356, 1393–1395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, D.S.W.; Raine, N.E. Population decline in a ground-nesting solitary squash bee (Eucera pruinosa) following exposure to a neonicotinoid insecticide treated crop (Cucurbita pepo). Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Goulson, D. REVIEW: An overview of the environmental risks posed by neonicotinoid insecticides. J. Appl. Ecol. 2013, 50, 977–987. [Google Scholar] [CrossRef]
- Van der Sluijs, J.P.; Simon-Delso, N.; Goulson, D.; Maxim, L.; Bonmatin, J.-M.; Belzunces, L.P. Neonicotinoids, bee disorders and the sustainability of pollinator services. Curr. Opin. Environ. Sustain. 2013, 5, 293–305. [Google Scholar] [CrossRef]
- Sgolastra, F.; Medrzycki, P.; Bortolotti, L.; Maini, S.; Porrini, C.; Simon-Delso, N.; Bosch, J. Bees and pesticide regulation: Lessons from the neonicotinoid experience. Biol. Conserv. 2020, 241, 108356. [Google Scholar] [CrossRef]
- EFSA Panel on Plant Protection Products and their Residues (PPR). Scientific Opinion on the science behind the development of a risk assessment of Plant Protection Products on bees (Apis mellifera, Bombus spp. and solitary bees). EFSA J. 2012, 10, 2668. [Google Scholar] [CrossRef]
- Rortais, A.; Arnold, G.; Dorne, J.-L.; More, S.J.; Sperandio, G.; Streissl, F.; Szentes, C.; Verdonck, F. Risk assessment of pesticides and other stressors in bees: Principles, data gaps and perspectives from the European Food Safety Authority. Sci. Total Environ. 2017, 587-588, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Franklin, E.L.; Raine, N.E. Moving beyond honeybee-centric pesticide risk assessments to protect all pollinators. Nat. Ecol. Evol. 2019, 3, 1373–1375. [Google Scholar] [CrossRef]
- European Commission. Commission Implementing Regulation (EU) No 485/2013 of 24 May 2013 amending Implementing Regulation (EU) No 540/2011, as regards the conditions of approval of the active substances clothianidin, thiamethoxam and imidacloprid, and prohibiting the use and sale of seeds treated with plant protection products containing those active substances. Off. J. 2013, 139, 12–26. Available online: http://data.europa.eu/eli/reg_impl/2013/485/oj (accessed on 13 November 2021).
- European Commission. Commission Implementing Regulation (EU) 2018/783 of 29 May 2018, amending Implementing Regulation (EU) No 540/2011 as Regards the Conditions of Approval of the Active Substance Imidacloprid. Off. J. 2018, 132, 31–34. Available online: http://data.europa.eu/eli/reg_impl/2018/783/oj (accessed on 13 November 2021).
- European Commission. Commission Implementing Regulation (EU) 2018/784 of 29 May 2018, amending Implementing Regulation (EU) No 540/2011, as regards the conditions of approval of the active substance clothianidin). Off. J. 2018, 132, 35–39. Available online: http://data.europa.eu/eli/reg_impl/2018/784/oj (accessed on 13 November 2021).
- European Commission. Commission Implementing Regulation (EU) 2018/785 of 29 May 2018 amending Implementing Regulation (EU) No 540/2011 as regards the conditions of approval of the active substance thiamethoxam.). Off. J. 2018, 132, 40–44. Available online: http://data.europa.eu/eli/reg_impl/2018/785/oj (accessed on 13 November 2021).
- Kathage, J.; Castañera, P.; Alonso-Prados, J.L.; Gómez-Barbero, M.; Rodríguez-Cerezo, E. The impact of restrictions on neonicotinoid and fipronil insecticides on pest management in maize, oilseed rape and sunflower in eight European Union regions. Pest Manag. Sci. 2018, 74, 88–99. [Google Scholar] [CrossRef] [Green Version]
- Jactel, H.; Verheggen, F.; Thiéryc, D.; Escobar-Gutiérrez, A.J.; Gachete, E.; Desneuxf, N. Alternatives to neonicotinoids. Environ. Int. 2019, 129, 423–429. [Google Scholar] [CrossRef]
- Brown, M.J.; Dicks, L.V.; Paxton, R.J.; Baldock, K.C.; Barron, A.B.; Chauzat, M.-P.; Freitas, B.M.; Goulson, D.; Jepsen, S.; Kremen, C.; et al. A horizon scan of future threats and opportunities for pollinators and pollination. PeerJ 2016, 4, e2249. [Google Scholar] [CrossRef] [Green Version]
- EPA. Decision Memorandum Supporting the Registration Decision for New Uses of the Active Ingredient Sulfoxaflor on Alfalfa, Cacao, Citrus, Corn, Cotton, Cucurbits, Grains, Pineapple, Sorghum, Soybeans, Strawberries and Tree Plantations and Amendments to the Labels. 2019, Docket Number EPA-HQ-OPP-2010-0889-0570. Available online: https://www.regulations.gov/document/EPA-HQ-OPP-2010-0889-0570 (accessed on 3 December 2021).
- Bailey, J.; Scott-Dupree, C.; Harris, R.; Tolman, J.; Harris, B. Contact and oral toxicity to honey bees (Apis mellifera) of agents registered for use for sweet corn insect control in Ontario, Canada. Apidologie 2005, 36, 623–633. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, B.K.; Saegerman, C.; Pirard, C.; Mignon, J.; Widart, J.; Thirionet, B.; Verheggen, F.J.; Berkvens, D.; De Pauw, E.; Haubruge, E. Does Imidacloprid Seed-Treated Maize Have an Impact on Honey Bee Mortality? J. Econ. Èntomol. 2009, 102, 616–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendriksma, H.; Küting, M.; Härtel, S.; Näther, A.; Dohrmann, A.B.; Steffan-Dewenter, I.; Tebbe, C.C. Effect of Stacked Insecticidal Cry Proteins from Maize Pollen on Nurse Bees (Apis mellifera carnica) and Their Gut Bacteria. PLoS ONE 2013, 8, e59589. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, H.T.; Jehle, J.A. Quantitative analysis of the seasonal and tissue-specific expression of Cry1Ab in transgenic maize Mon810. J. Plant Dis. Prot. 2007, 114, 82–87. [Google Scholar] [CrossRef]
- Duan, J.J.; Marvier, M.; Huesing, J.; Dively, G.; Huang, Z.Y. A Meta-Analysis of Effects of Bt Crops on Honey Bees (Hymenoptera: Apidae). PLoS ONE 2008, 3, e1415. [Google Scholar] [CrossRef] [PubMed]
- Székács, A.; Weiss, G.; Quist, D.; Takács, E.; Darvas, B.; Meier, M.; Swain, T.; Hilbeck, A. Inter-laboratory comparison of Cry1Ab toxin quantification inMON 810maize by enzyme-immunoassay. Food Agric. Immunol. 2012, 23, 99–121. [Google Scholar] [CrossRef]
- Morandin, L.A.; Winston, M.L. Effects of Novel Pesticides on Bumble Bee (Hymenoptera: Apidae) Colony Health and Foraging Ability. Environ. Èntomol. 2003, 32, 555–563. [Google Scholar] [CrossRef] [Green Version]
- Ramirez-Romero, R.; Desneux, N.; Decourtye, A.; Chaffiol, A.; Pham-Delègue, M. Does Cry1Ab protein affect learning performances of the honey bee Apis mellifera L. (Hymenoptera, Apidae)? Ecotoxicol. Environ. Saf. 2008, 70, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Mommaerts, V.; Jans, K.; Smagghe, G. Impact ofBacillus thuringiensisstrains on survival, reproduction and foraging behaviour in bumblebees (Bombus terrestris). Pest Manag. Sci. 2009, 66, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Siviter, H.; Brown, M.J.F.; Leadbeater, E. Sulfoxaflor exposure reduces bumblebee reproductive success. Nature 2018, 561, 109–112. [Google Scholar] [CrossRef]
- OECD. Test No. 247: Bumblebee, Acute Oral Toxicity Test, OECD Guidelines for the Testing of Chemicals, Section 2, No. 247; OECD Publishing: Paris, France, 2017. [Google Scholar] [CrossRef]
- Gill, R.J.; Ramos-Rodriguez, O.; Raine, N.E. Combined pesticide exposure severely affects individual- and colony-level traits in bees. Nature 2012, 491, 105–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Bayo, F.; Goka, K. Pesticide Residues and Bees—A Risk Assessment. PLoS ONE 2014, 9, e94482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.; Chen, J.; Zhao, C.; Tian, Y.; Zhang, Z.; Xu, H. Sulfoxaflor Residues in Pollen and Nectar of Cotton Applied through Drip Irrigation and Their Potential Exposure to Apis mellifera L. Insects 2020, 11, 114. [Google Scholar] [CrossRef] [Green Version]
- Robertson, J.L.; Russell, R.M.; Preisler, H.K.; Savin, N.E. Pesticide Bioassays with Arthropods, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Thompson, H.M. Assessing the exposure and toxicity of pesticides to bumblebees (Bombus sp.). Apidologie 2001, 32, 305–321. [Google Scholar] [CrossRef] [Green Version]
- Thompson, H.; Wilkins, S. Assessment of the synergy and repellency of pyrethroid/fungicide mixtures. Bull. Insectology 2003, 56, 131–134. [Google Scholar]
- Laycock, I.; Lenthall, K.M.; Barratt, A.T.; Cresswell, J.E. Effects of imidacloprid, a neonicotinoid pesticide, on reproduction in worker bumble bees (Bombus terrestris). Ecotoxicology 2012, 21, 1937–1945. [Google Scholar] [CrossRef] [PubMed]
- Thompson, H.M.; Wilkins, S.; Harkin, S.; Milner, S.; Walters, K.F. Neonicotinoids and bumblebees (Bombus terrestris): Effects on nectar consumption in individual workers. Pest Manag. Sci. 2015, 71, 946–950. [Google Scholar] [CrossRef] [PubMed]
- Kessler, S.C.; Tiedeken, E.J.; Simcock, K.L.; Derveau, S.; Mitchell, J.; Softley, S.; Radcliffe, A.; Stout, J.; Wright, G.A. Bees prefer foods containing neonicotinoid pesticides. Nature 2015, 521, 74–76. [Google Scholar] [CrossRef] [PubMed]
- Manjon, C.; Troczka, B.J.; Zaworra, M.; Beadle, K.; Randall, E.; Hertlein, G.; Singh, K.S.; Zimmer, C.T.; Homem, R.A.; Lueke, B.; et al. Unravelling the Molecular Determinants of Bee Sensitivity to Neonicotinoid Insecticides. Curr. Biol. 2018, 28, 1137–1143.e5. [Google Scholar] [CrossRef] [Green Version]
- Reid, R.J.; Troczka, B.J.; Kor, L.; Randall, E.; Williamson, M.S.; Field, L.; Nauen, R.; Bass, C.; Davies, T.E. Assessing the acute toxicity of insecticides to the buff-tailed bumblebee (Bombus terrestris audax). Pestic. Biochem. Physiol. 2020, 166, 104562. [Google Scholar] [CrossRef] [PubMed]
- Krupke, C.H.; Long, E.Y. Intersections between neonicotinoid seed treatments and honey bees. Curr. Opin. Insect Sci. 2015, 10, 8–13. [Google Scholar] [CrossRef]
- Iwasa, T.; Motoyama, N.; Ambrose, J.T.; Roe, R. Mechanism for the differential toxicity of neonicotinoid insecticides in the honey bee, Apis mellifera. Crop Prot. 2004, 23, 371–378. [Google Scholar] [CrossRef]
- Jacob, C.R.O.; Malaquias, J.B.; Zanardi, O.Z.; Silva, C.A.S.; Jacob, J.F.O.; Yamamoto, P. Oral acute toxicity and impact of neonicotinoids on Apis mellifera L. and Scaptotrigona postica Latreille (Hymenoptera: Apidae). Ecotoxicology 2019, 28, 744–753. [Google Scholar] [CrossRef]
- Decourtye, A.; Devillers, J. Ecotoxicity of Neonicotinoid Insecticides to Bees. Adv. Exp. Med. Biol. 2010, 683, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Botías, C.; David, A.; Horwood, J.; Abdulsada, A.K.; Nicholls, E.; Hill, E.M.; Goulson, D. Neonicotinoid Residues in Wildflowers, a Potential Route of Chronic Exposure for Bees. Environ. Sci. Technol. 2015, 49, 12731–12740. [Google Scholar] [CrossRef]
- Uhl, P.; Brühl, C.A. The Impact of Pesticides on Flower-Visiting Insects: A Review with Regard to European Risk Assessment. Environ. Toxicol. Chem. 2019, 38, 2355–2370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, D.S.W.; Prosser, R.S.; Rodríguez-Gil, J.L.; Raine, N.E. Assessment of risk to hoary squash bees (Peponapis pruinosa) and other ground-nesting bees from systemic insecticides in agricultural soil. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Girolami, V.; Mazzon, L.; Squartini, A.; Mori, N.; Marzaro, M.; Di Bernardo, A.; Greatti, M.; Giorio, C.; Tapparo, A. Translocation of Neonicotinoid Insecticides From Coated Seeds to Seedling Guttation Drops: A Novel Way of Intoxication for Bees. J. Econ. Èntomol. 2009, 102, 1808–1815. [Google Scholar] [CrossRef] [PubMed]
- Mörtl, M.; Takács, E.; Klátyik, S.; Székács, A. Appearance of Thiacloprid in the Guttation Liquid of Coated Maize Seeds. Int. J. Environ. Res. Public Heal. 2020, 17, 3290. [Google Scholar] [CrossRef] [PubMed]
- Girolami, V.; Marzaro, M.; Vivan, L.; Mazzon, L.; Greatti, M.; Giorio, C.; Marton, D.; Tapparo, A. Fatal powdering of bees in flight with particulates of neonicotinoids seed coating and humidity implication. J. Appl. Èntomol. 2011, 136, 17–26. [Google Scholar] [CrossRef]
- Bonmatin, J.-M.; Giorio, C.; Girolami, V.; Goulson, D.; Kreutzweiser, D.P.; Krupke, C.; Liess, M.; Long, E.; Marzaro, M.; Mitchell, E.A.D.; et al. Environmental fate and exposure; neonicotinoids and fipronil. Environ. Sci. Pollut. Res. 2015, 22, 35–67. [Google Scholar] [CrossRef] [PubMed]
- Zioga, E.; Kelly, R.; White, B.; Stout, J.C. Plant protection product residues in plant pollen and nectar: A review of current knowledge. Environ. Res. 2020, 189, 109873. [Google Scholar] [CrossRef] [PubMed]
- Kaila, L.; Ketola, J.; Toivonen, M.; Loukola, O.; Hakala, K.; Raiskio, S.; Hurme, T.; Jalli, M. Pesticide residues in honeybee-collected pollen: Does the EU regulation protect honeybees from pesticides? Environ. Sci. Pollut. Res. 2021, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Mommaerts, V.; Smagghe, G. Side-effects of pesticides on the pollinator bombus: An overview. In Pesticides in the Modern World-Pests Control and Pesticides Exposure and Toxicity Assessment; Stoytcheva, M., Ed.; In-Tech: Rijeka, Croatia, 2011; Volume 5, pp. 507–552. [Google Scholar]
- Baron, G.L.; Raine, N.; Brown, M.J.F. Impact of chronic exposure to a pyrethroid pesticide on bumblebees and interactions with a trypanosome parasite. J. Appl. Ecol. 2014, 51, 460–469. [Google Scholar] [CrossRef] [Green Version]
- Ceuppens, B.; Eeraerts, M.; Vleugels, T.; Cnops, G.; Roldan-Ruiz, I.; Smagghe, G. Effects of dietary lambda-cyhalothrin exposure on bumblebee survival, reproduction, and foraging behavior in laboratory and greenhouse. J. Pest Sci. 2015, 88, 777–783. [Google Scholar] [CrossRef]
- Zhu, Q.; Yang, Y.; Zhong, Y.; Lao, Z.; O’Neill, P.; Hong, D.; Zhang, K.; Zhao, S. Synthesis, insecticidal activity, resistance, photodegradation and toxicity of pyrethroids (A review). Chemosphere 2020, 254, 126779. [Google Scholar] [CrossRef] [PubMed]
- Siviter, H.; Horner, J.; Brown, M.J.F.; Leadbeater, E. Sulfoxaflor exposure reduces egg laying in bumblebees Bombus terrestris. J. Appl. Ecol. 2019, 57, 160–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linguadoca, A.; Rizzi, C.; Villa, S.; Brown, M.J. Sulfoxaflor and nutritional deficiency synergistically reduce survival and fecundity in bumblebees. Sci. Total Environ. 2021, 795, 148680. [Google Scholar] [CrossRef] [PubMed]
- Azpiazu, C.; Bosch, J.; Bortolotti, L.; Medrzycki, P.; Teper, D.; Molowny-Horas, R.; Sgolastra, F. Toxicity of the insecticide sulfoxaflor alone and in combination with the fungicide fluxapyroxad in three bee species. Sci. Rep. 2021, 11, 1–9. [Google Scholar] [CrossRef]
- Mundy-Heisz, K.A.; Prosser, R.S.; Raine, N.E. Acute oral toxicity and risks of exposure to the neonicotinoid thiamethoxam, and other classes of systemic insecticide, for the Common Eastern Bumblebee (Bombus impatiens). bioRxiv 2020. [Google Scholar] [CrossRef]
- Babendreier, D.; Reichhart, B.; Romeis, J.; Bigler, F. Impact of insecticidal proteins expressed in transgenic plants on bumblebee microcolonies. Èntomol. Exp. Appl. 2008, 126, 148–157. [Google Scholar] [CrossRef]
- Klatt, B.K.; Rundlöf, M.; Smith, H.G. Maintaining the Restriction on Neonicotinoids in the European Union—Benefits and Risks to Bees and Pollination Services. Front. Ecol. Evol. 2016, 4, 4. [Google Scholar] [CrossRef] [Green Version]
- Carreck, N.L. A beekeeper’s perspective on the neonicotinoid ban. Pest Manag. Sci. 2017, 73, 1295–1298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Lethal Concentration | Lethal Dose | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Active Ingredient a | N b | d.f. | Slope (SE) | χ2 | LC50 (CI 95%) c | LC90 (CI 95%) c | LCR (LC50) (CI 95%) d | Slope (SE) | χ2 | LD50 (CI 95%) e | LD90 (CI 95%) e | LDR (LC50) (CI 95%) d |
| Imidacloprid (N) | 149 | 23 | 1.15 (0.23) | 28.9 | 0.38 (0.22–0.76) | 4.96 (1.85–58.6) | 1103 (608–2003) * | 1.2 (0.2) | 28.4 | 0.13 (0.08–0.24) | 1.31 (0.55–10.78) | 687.3 (352.5–1340.4) * |
| Thiacloprid (N) | 95 | 8 | 2.52 (0.64) | 9.2 | 424 (296–815) | 1366 (744–15338) | 1 | 1.8 (0.4) | 7.1 | 90.5 (58.8–172.7) | 554 (252–4330) | 1 |
| Deltamethrin (p) | 99 | 8 | 1.33 (0.32) | 5.1 | 7.1 (3.3–11.9) | 64.5 (31.2–382.9) | 60.1 (29.9–121) * | 1.8 (0.4) | 5.4 | 3.65 (2.19–5.11) | 15.5 (9.6–52.8) | 24.85 (12.86–47.65) * |
| Esfenvalerate (P) | 191 | 32 | 2.68 (0.38) | 36.5 | 17.8 (14.4–22.4) | 53.5 (38.3–96.1) | 23.8 (16.4–34.6) * | 3.2 (0.5) | 43.4 | 5.52 (4.55–6.70) | 12.5 (9.4–23.2) | 44.21 (12.7–155) * |
| Sulfoxaflor (S) | 126 | 19 | 3.73 (0.97) | 26.4 | 2.22 (1.66–3.85) | 4.90 (3.15–30.1) | 191 (162–289) * | 5.6 (1.5) | 22.8 | 0.71 (0.56–1.01) | 1.28 (0.93–5.30) | 123.3 (72.6–209) * |
| Max. Residue (ppb) a | LD50 (µg a.i./Bee) | Max. Exposure Dose (ng a.i./Bee/Day) c | No. Days a Worker Needs to Reach the LD50 (Worst Case Scenario) d | |
|---|---|---|---|---|
| Imidacloprid | 72.8 | 0.13 | 40.3 | 3.2 |
| Thiacloprid | 208.8 | 90.5 | 115.7 | 782.3 |
| Deltamethrin | 6.7 | 3.65 | 3.7 | 983.8 |
| Esfenvalerate | 0.7 b | 5.52 | 0.39 | 14,234.1 |
| Sulfoxaflor | 13.8 | 0.71 | 7.6 | 92.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabezas, G.; Farinós, G.P. Sensitivity of Buff-Tailed Bumblebee (Bombus terrestris L.) to Insecticides with Different Mode of Action. Insects 2022, 13, 184. https://doi.org/10.3390/insects13020184
Cabezas G, Farinós GP. Sensitivity of Buff-Tailed Bumblebee (Bombus terrestris L.) to Insecticides with Different Mode of Action. Insects. 2022; 13(2):184. https://doi.org/10.3390/insects13020184
Chicago/Turabian StyleCabezas, Guillermo, and Gema P. Farinós. 2022. "Sensitivity of Buff-Tailed Bumblebee (Bombus terrestris L.) to Insecticides with Different Mode of Action" Insects 13, no. 2: 184. https://doi.org/10.3390/insects13020184
APA StyleCabezas, G., & Farinós, G. P. (2022). Sensitivity of Buff-Tailed Bumblebee (Bombus terrestris L.) to Insecticides with Different Mode of Action. Insects, 13(2), 184. https://doi.org/10.3390/insects13020184





