Sizing the Knowledge Gap in Taxonomy: The Last Dozen Years of Aphidiinae Research
Abstract
Simple Summary
Abstract
1. Prologue
2. What Has Been Accomplished in the Last Dozen Years?
2.1. Bookworm on the World Wide Web
2.2. New Taxa in the Old World and All Other Worlds
2.3. Aliens in Europe
2.4. Shrinking the Gap by Revising the Knowledge
2.5. Keys to Unlock an Easier Scientific Existence
3. Looking in and through the Mirror—Current Situation and Future Prospects
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Economic Forum. The Global Risks Report. Available online: https://www.weforum.org/reports/the-global-risks-report-2020 (accessed on 10 June 2021).
- May, R.M. Why Worry about How Many Species and Their Loss? PLoS Biol. 2011, 9, e1001130. [Google Scholar] [CrossRef] [PubMed]
- Meier, R.; Blaimer, B.; Buenaventura, E.; Hartop, E.; von Rintelen, T.; Srivathsan, A.; Yeo, D. A re-analysis of the data in Sharkey et al.’s (2021) minimalist revision reveals that BINs do not deserve names, but BOLD Systems needs a stronger commitment to open science. Cladistics 2021, 1–12. [Google Scholar] [CrossRef]
- Michener, C.D.; Corliss, J.O.; Cowan, R.S.; Raven, P.H.; Sabrosky, C.W.; Squires, D.S.; Wharton, G.W. Systematics in Support of Biological Research; Division of Biology and Agriculture, National Research Council: Washington, DC, USA, 1970; pp. 1–25. [Google Scholar]
- de Carvalho, M.R.; Bockmann, F.A.; Amorim, D.S.; Brandão, C.R.F. Systematics must embrace comparative biology and evolution, not speed and automation. Evol. Biol. 2008, 35, 150–157. [Google Scholar] [CrossRef]
- Engel, M.S.; Ceríaco, L.M.P.; Daniel, G.M.; Dellapé, P.M.; Löbl, I.; Marinov, M.; Reis, R.E.; Young, M.T.; Dubois, A.; Agarwal, I.; et al. The taxonomic impediment: A shortage of taxonomists, not the lack of technical approaches. Zool. J. Linn. Soc. 2021, 193, 381–387. [Google Scholar] [CrossRef]
- Chapman, A. Numbers of Living Species in Australia and the World; Australian Biological Resources Study: Canberra, Australia, 2009; pp. 1–80. [Google Scholar]
- Stork, N. How many species of insects and other terrestrial arthropods are there on Earth? Annu. Rev. Entomol. 2018, 63, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Hebert, P.D.; Ratnasingham, S.; Zakharov, E.V.; Telfer, A.C.; Levesque-Beaudin, V.; Milton, M.A.; Pedersen, S.; Jannetta, P.; DeWaard, J.R. Counting animal species with DNA barcodes: Canadian insects. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150333. [Google Scholar] [CrossRef]
- Morinière, J.; Balke, M.; Doczkal, D.; Geiger, M.F.; Hardulak, L.A.; Haszprunar, G.; Hausmann, A.; Hendrich, L.; Regalado, L.; Rulik, B.; et al. A DNA barcode library for 5200 German flies and midges (Insecta: Diptera) and its implications for metabarcoding-based biomonitoring. Mol. Ecol. Resour. 2019, 19, 900–928. [Google Scholar] [CrossRef]
- Forbes, A.A.; Bagley, R.K.; Beer, M.A.; Hippee, A.C.; Widmayer, H.A. Quantifying the unquantifiable: Why Hymenoptera, not Coleoptera, is the most speciose animal order. BMC Ecol. 2018, 18, 21. [Google Scholar] [CrossRef]
- Boivin, G.; Hance, T.; Brodeur, J. Aphid parasitoids in biological control. Can. J. Plant Sci. 2012, 92, 1–12. [Google Scholar] [CrossRef]
- Mackauer, M.; Starý, P. World Aphidiidae (Hym. Ichneumonoidea); Le Francois: Paris, France, 1967; pp. 1–195. [Google Scholar]
- Linnæus, C. Systema Naturæ per Regna Tria Naturæ, Secundum Classes, Ordines, Genera, Species, Cum Characteribus, Differentiis, Synonymis, Locis; Laurenti Salvi, Holmiae; Biodiversity Heritage Library: Stockholm, Sweden, 1758; pp. 1–824. [Google Scholar]
- Žikić, V.; Lazarević, M.; Milošević, D. Host range patterning of parasitoid wasps Aphidiinae (Hymenoptera: Braconidae). Zool. Anz. 2017, 268, 75–83. [Google Scholar] [CrossRef]
- Tian, H.W.; Achterberg, K.V.; Chen, X.X. A new genus of the tribe Praini Mackauer (Hymenoptera: Braconidae: Aphidiinae) from China. Zootaxa 2017, 4362, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Mackauer, M.; Finlayson, T. Choreopraon totarae (Hymenoptera, Braconidae, Aphidiinae), a new parasitoid of Neophyllaphis totarae (Hemiptera, Aphidoidea, Drepanosiphidae) in New Zealand. N. Z. J. Zool. 2012, 39, 77–84. [Google Scholar] [CrossRef][Green Version]
- Mackauer, M. Aphidiidae. In Hymenopterorum Catalogus, 9th ed.; Ferriere, C., van der Vecht, J., Eds.; Dr. W. Junk: The Hague, The Netherlands, 1968; pp. 1–103. [Google Scholar]
- Yu, D.S.; van Achterberg, K.; Horstmann, K. Taxapad 2012. In Ichneumonoidea 2011–Database on Flash–Drive; ScienceOpen: Ottawa, ON, Canada, 2012. [Google Scholar]
- de Jong, Y.; Verbeek, M.; Michelsen, V.; de Place Bjørn, P.; Los, W.; Steeman, F.; Bailly, N.; Basire, C.; Chylarecki, P.; Stloukal, E.; et al. Fauna Europaea–all European animal species on the web. Biodivers. Data J. 2014, 2, e4034. [Google Scholar] [CrossRef]
- Starý, P. Aphid Parasitoids of the Czech Republic; Academia: Prague, Czech Republic, 2006; pp. 1–430. [Google Scholar]
- Gusenbauer, M.; Haddaway, N.R. Which academic search systems are suitable for systematic reviews or meta-analyses? Evaluating retrieval qualities of Google Scholar, PubMed, and 26 other resources. Res. Synth. Methods 2020, 11, 181–217. [Google Scholar] [CrossRef] [PubMed]
- Haddaway, N.R.; Bethel, A.; Dicks, L.V.; Koricheva, J.; Macura, B.; Petrokofsky, G.; Pullin, A.S.; Pullin, A.S.; Stewart, G.B. Eight problems with literature reviews and how to fix them. Nat. Ecol. Evol. 2020, 4, 1582–1589. [Google Scholar] [CrossRef] [PubMed]
- López-Cózar, E.D.; Orduña-Malea, E.; Martín-Martín, A. Google Scholar as a data source for research assessment. In Springer Handbook of Science and Technology Indicators; Glaenzel, W., Moed, H., Schmoch, U., Thelwall, M., Eds.; Springer: Cham, Switzerland, 2019; pp. 95–127. [Google Scholar]
- Gusenbauer, M. Google Scholar to overshadow them all? Comparing the sizes of 12 academic search engines and bibliographic databases. Scientometrics 2019, 118, 177–214. [Google Scholar] [CrossRef]
- Starý, P.; Rakhshani, E.; Tomanović, Ž.; Hoelmer, K.; Kavallieratos, N.G.; Yu, J.; Wang, M.; Heimpel, G.E. A new species of Lysiphlebus Förster 1862 (Hymenoptera: Braconidae: Aphidiinae) attacking soybean aphid, Aphis glycines Matsumura (Hemiptera: Aphididae) from China. J. Hymenop. Res. 2010, 19, 179–186. [Google Scholar]
- Davidian, E.M.; Gavrilyuk, A.V. A new species of the genus Areopraon Mackauer, 1959 (Hymenoptera: Aphidiidae) from Western Siberia. Russ. Entomol. J. 2011, 20, 247–249. [Google Scholar] [CrossRef]
- Starý, P.; Rakhshani, E.; Tomanović, Ž.; Kavallieratos, N.G.; Sharkey, M. Review and key to the world parasitoids (Hymenoptera: Braconidae: Aphidiinae) of Greenideinae aphids. Ann. Entomol. Soc. Am. 2010, 103, 307–321. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Khan, F.R.; Ramamurthy, V.V. Description of a new species of the genus Ephedrus (Hymenoptera: Braconidae: Aphidiinae) from India with an up to date checklist. Pan Pac. Entomol. 2011, 87, 98–105. [Google Scholar] [CrossRef]
- Rakhshani, E.; Tomanović, Ž.; Starý, P.; Kavalieratos, N.G.; Ilić, M.; Stanković, S.S.; Rajabi-Mazhar, N. Aphidiinae parasitoids (Hymenoptera: Braconidae) of Macrosiphoniella aphids (Hemiptera: Aphididae) in the western Palaearctic region. J. Nat. Hist. 2011, 45, 2559–2575. [Google Scholar] [CrossRef]
- Petrović, A.; Žikić, V.; Petrović-Obradović, O.; Mitrovski Bogdanović, A.; Kavallieratos, N.G.; Starý, P.; Tomanović, Ž. Two new species of aphid parasitoids (Hymenoptera, Braconidae, Aphidiinae) from the Balkan Peninsula. Zootaxa 2011, 2895, 58–64. [Google Scholar] [CrossRef]
- Pike, K.S.; Starý, P.; Graf, G.; Raworth, D.A.; Mathur, S.; Tanigoshi, L.K.; Murray, T. A new Aphidius Nees (Hymenoptera, Braconidae, Aphidiinae) of Ericaphis fimbriata (Richards) (Hemiptera, Aphididae) and key to parasitoids of blueberry aphid in the Pacific Northwest. Zootaxa 2011, 2802, 58–62. [Google Scholar] [CrossRef]
- Rakhshani, E.; Kazemzadeh, S.; Starý, P.; Barahoei, H.; Kavallieratos, N.G.; Ćetković, A.; Popović, A.; Bodlah, I.; Tomanović, Ž. Parasitoids (Hymenoptera: Braconidae: Aphidiinae) of northeastern Iran: Aphidiinae-aphid-plant associations, key and description of a new species. J. Insect Sci. 2012, 12, 143. [Google Scholar] [CrossRef] [PubMed]
- Rakhshani, E.; Barahoei, H.; Ahmed, Z.; Tomanović, Ž.; Janković, M.; Petrović, A.; Starý, P. New species and additional evidence of aphid parasitoids (Hymenoptera: Braconidae: Aphidiinae) from India. Zootaxa 2012, 3397, 45–54. [Google Scholar] [CrossRef]
- Kos, K.; Trdan, S.; Petrović, A.; Starý, P.; Kavallieratos, N.G.; Petrović-Obradović, O.; Tomanović, Ž. Aphidiinae (Hymenoptera, Braconidae, Aphidiinae) from Slovenia, with description of a new Aphidius species. Zootaxa 2012, 3456, 36–50. [Google Scholar] [CrossRef]
- Hollingbery, E.N.; Pike, K.S.; Graf, G.; Graf, D. Parasitoids (Hymenoptera: Braconidae: Aphidiinae) of rabbitbrush aphids and linkage with agriculturally important pest aphids in Washington State, United States of America. Can. Entomol. 2012, 144, 621–634. [Google Scholar] [CrossRef]
- Mitrovski Bogdanović, A.; Petrović, A.; Mitrović, M.; Ivanović, A.; Žikić, V.; Starý, P.; Vorburger, C.; Tomanović, Ž. Identification of two cryptic species within the Praon abjectum group (Hymenoptera: Braconidae: Aphidiinae) using molecular markers and geometric morphometrics. Ann. Entomol. Soc. Am. 2013, 106, 170–180. [Google Scholar] [CrossRef]
- Chaubet, B.; Derocles, S.A.P.; Hullé, M.; Le Ralec, A.; Outreman, Y.; Simon, J.-C.; Tomanović, Ž. Two new species of aphid parasitoids (Hymenoptera, Braconidae, Aphidiinae) from the high arctic (Spitsbergen, Svalbard). Zool. Anz. J. Comp. Zool. 2013, 252, 34–40. [Google Scholar] [CrossRef]
- Davidian, E.M. A new species of the genus Trioxys Mackauer (Hymenoptera: Aphidiidae) from Sverdlovsk Province of Russia. Proc. Russ. Entomol. Soc. 2014, 85, 157–159. (In Russian) [Google Scholar]
- Mitrovski Bogdanović, A.; Tomanović, Ž.; Mitrović, M.; Petrović, A.; Ivanović, A.; Žikić, V.; Starý, P.; Vorburger, C. The Praon dorsale-yomenae complex s. str. (Hymenoptera, Braconidae, Aphidiinae): Species discrimination using geometric morphometrics and molecular markers with description of a new species. Zool. Anz. 2014, 253, 270–282. [Google Scholar] [CrossRef]
- Davidian, E.M. Parasitoid wasps of the subgenus Pauesia Quilis s. str. (Hymenoptera, Aphidiidae) from Russia and neighboring countries. Entomol. Obozr. 2015, 94, 440–445. (In Russian) [Google Scholar] [CrossRef]
- Rakhshani, E.; Starý, P.; Perez Hidalgo, N.; Čkrkić, J.; Tomanović, S.; Petrović, A.; Tomanović, Ž. Revision of the world Monoctonia Starý, parasitoids of gall aphids: Taxonomy, distribution, host range, and phylogeny (Hymenoptera, Braconidae: Aphidiinae). Zootaxa 2015, 3905, 474–488. [Google Scholar] [CrossRef] [PubMed]
- Davidian, E.M. A new genus and new species of subfamily Trioxinae (Hymenoptera: Aphidiidae) from the Russian Far East. Zootaxa 2016, 4205, 475–479. [Google Scholar] [CrossRef] [PubMed]
- van Achterberg, C.; Ortiz de Zugasti Carrón, N.F. Revision of the genus Paralipsis Foerster, 1863 (Hymenoptera, Braconidae), with the description of two new species. ZooKeys 2016, 606, 25. [Google Scholar] [CrossRef]
- Petrović, A.; Kocić, K.; Kos, K.; Plećaš, M.; Žikić, V.; Kavallieratos, N.G.; Tomanović, Ž. High genetic diversity and a new cryptic species within the Ephedrus persicae species group (Hymenoptera, Braconidae). Biologia 2016, 71, 1386–1394. [Google Scholar] [CrossRef]
- Rakhshani, E.; Pons, X.; Lumbierres, B.; Havelka, J.; Pérez Hidalgo, N.; Tomanović, Ž.; Starý, P. A new parasitoid (Hymenoptera, Braconidae, Aphidiinae) of the invasive bamboo aphids Takecallis spp. (Hemiptera, Aphididae) from Western Europe. J. Nat. Hist. 2017, 51, 1237–1248. [Google Scholar] [CrossRef]
- Kula, R.R.; Johnson, P.J.; Heidel-Baker, T.T.; Boe, A. A new species of Acanthocaudus Smith (Braconidae: Aphidiinae), with a key to species and new host and distribution records for aphidiines associated with Silphium perfoliatum L. (Asterales: Asteraceae). Zootaxa 2017, 4236, 543–552. [Google Scholar] [CrossRef]
- Davidian, E.M. A new species of the aphidiid wasp genus Dyscritulus Hincks, 1943 (Hymenoptera, Aphidiidae) from the Caucasus. Entomol. Rev. 2018, 98, 776–780. [Google Scholar] [CrossRef]
- Tian, H.-W.; van Achterberg, C.; Chen, X.-X. The genera Areopraon Mackauer, 1959 and Pseudopraon Starý, 1975 (Hymenoptera, Braconidae, Aphidiinae) from China, with keys to species. ZooKeys 2018, 780, 61–70. [Google Scholar] [CrossRef]
- Tomanović, Ž.; Mitrović, M.; Petrović, A.; Kavallieratos, N.G.; Žikić, V.; Ivanović, A.; Rakhshani, E.; Starý, P.; Vorburger, C. Revision of the European Lysiphlebus species (Hymenoptera: Braconidae: Aphidiinae) on the basis of COI and 28SD2 molecular markers and morphology. Arthropod Syst. Phylogeny 2018, 76, 179–213. [Google Scholar]
- Mitrović, M.; Starý, P.; Jakovljević, M.; Petrović, A.; Žikić, V.; Pérez Hidalgo, N.; Tomanović, Ž. Integrative taxonomy of root aphid parasitoids from the genus Paralipsis (Hymenoptera, Braconidae, Aphidiinae) with description of new species. ZooKeys 2019, 831, 49–69. [Google Scholar] [CrossRef]
- Kocić, K.; Petrović, A.; Čkrkić, J.; Mitrović, M.; Tomanović, Ž. Phylogenetic relationships and subgeneric classification of European Ephedrus species (Hymenoptera, Braconidae, Aphidiinae). ZooKeys 2019, 878, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Čkrkić, J.; Petrović, A.; Kocić, K.; Kavallieratos, N.G.; Hebert, P.D.N.; Tomanović, Ž. Review of the world Monoctonina Mackauer 1961 (Hymenoptera, Braconidae, Aphidiinae): Key for identification with a description of five new species. Zootaxa 2019, 4691, 359–385. [Google Scholar] [CrossRef] [PubMed]
- Davidian, E.M. A New Species of the Aphidiid Wasp Genus Trioxys Haliday (Hymenoptera, Aphidiidae) from Orenburg Province of Russia. Entomol. Rev. 2020, 100, 1029–1032. [Google Scholar] [CrossRef]
- Kocić, K.; Petrović, A.; Čkrkić, J.; Kavallieratos, N.G.; Rakhshani, E.; Arnó, J.; Aparicio, Y.; Hebert, P.D.N.; Tomanović, Ž. Resolving the taxonomic status of potential biocontrol agents belonging to the neglected genus Lipolexis Förster (Hymenoptera, Braconidae, Aphidiinae) with descriptions of six new species. Insects 2020, 11, 667. [Google Scholar] [CrossRef] [PubMed]
- Tomanović, Ž.; Petrović, A.; Kocić, K.; Čkrkić, J.; Žikić, V. Two new morphologically interesting species of the genus Ephedrus Haliday (Hymenoptera, Braconidae, Aphidiinae). J. Hymenopt. Res. 2020, 77, 167–174. [Google Scholar] [CrossRef]
- Čkrkić, J.; Petrović, A.; Kocić, K.; Mitrović, M.; Kavallieratos, N.G.; van Acheterberg, C.; Hebert, P.D.N.; Tomanović, Ž. Phylogeny of the Subtribe Monoctonina (Hymenoptera, Braconidae, Aphidiinae). Insects 2020, 11, 160. [Google Scholar] [CrossRef]
- Čkrkić, J.; Petrović, A.; Kocić, K.; Tomanović, Ž. Insights into phylogenetic relationships between Trioxys Haliday, 1833 and Binodoxys Mackauer, 1960 (Hymenoptera, Braconidae, Aphidiinae), with a description of a new species of the genus Trioxys. Zoosystema 2021, 43, 145–154. [Google Scholar] [CrossRef]
- Petrović, A.; Pérez Hidalgo, N.; Michelena Saval, J.; Tomanović, Ž. A new Aphidius Nees species (Hymenoptera, Braconidae), a parasitoid of Acyrthosiphon malvae (Mosley, 1841) in Europe. Phytoparasitica 2021, 49, 93–101. [Google Scholar] [CrossRef]
- Petrović, A.; Kocić, K.; Čkrkić, J.; Tomanović, Ž. Additional data on Aphidiinae (Hymenoptera, Braconidae) fauna of Kyrgyzstan, with description of a new species. J. Hymenopt. Res. 2021, 82, 221–235. [Google Scholar] [CrossRef]
- Davidian, E.M.; Belokobylskij, S.A. Two new species of the genus Areopraon Mackauer, 1959 (Hymenoptera: Braconidae: Aphidiinae) from the Russian Far East. Zootaxa 2021, 4985, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Tomanović, Ž.; Yu, Y.; Sohn, J.H.; Han, Y.; Lee, G.; Kim, H. Three new species of the genus Aphidius (Hymenoptera, Braconidae, Aphidiinae) from South Korea. J. Hymenopt. Res. 2021, 86, 63–77. [Google Scholar] [CrossRef]
- Davidian, E.M.; Gavrilyuk, A.V. A new species of the genus Aphidius Nees (Hymenoptera: Aphidiidae) from Western Siberia. Rus. Entomol. J. 2010, 19, 119–121. [Google Scholar] [CrossRef]
- Takada, H. Redescription of Papilloma luteum and description of a new Praon species (Hymenoptera: Braconidae; Aphidiinae) parasitic on K urisakia querciphila (Homoptera: Aphididae: Thelaxinae) in J apan, with notes on synonymy and life cycle of P. luteum. Entomol. Sci. 2014, 17, 86–95. [Google Scholar] [CrossRef]
- Martens, A.P.; Buffington, M.L.; Quicke, D.L.J.; Raweearamwong, M.; Butcher, B.A.; Johnson, P.J. Ishtarella thailandica Martens, new genus, new species (Hymenoptera: Braconidae: Aphidiinae) of aphid parasitoid from Thailand, with a country checklist of Aphidiinae. Insecta Mundi 2021, 0904, 1–6. [Google Scholar]
- Holman, J. Host Plant Catalog of Aphids; Springer: Dordrecht, The Netherlands, 2009; pp. 1–1216. [Google Scholar]
- Obradović, O.P.; Ilić Milošević, M.; Stanković, S.S.; Žikić, V. Nine species of aphids (Hemiptera: Aphididae) new to the fauna of Serbia. Acta Entomol. Serbica 2020, 25, 13–19. [Google Scholar]
- Petrović-Obradović, O. Asian apricot aphid, Myzus mumecola (Matsumura, 1917) (Hemiptera: Aphididae), found in Serbia. Acta Entomol. Serbica 2021, 26, 19–26. [Google Scholar]
- Tomanović, Ž.; Žikić, V.; Petrović, A. Fauna of Parasitoid Wasps (Hymenoptera, Braconidae, Aphidiinae) of Serbia; Serbian Academy of Sciences and Arts: Belgrade, Serbia, 2021; pp. 1–262. [Google Scholar]
- Favret, C. Aphid Species File, Version 5.0/5.0; Available online: http://Aphid.SpeciesFile.org (accessed on 10 June 2021).
- Brightwell, R.; Dransfield, R.D. Check-List of North American Aphids. Available online: https://influentialpoints.com/aphid/Nearctic-aphid-checklist--plant-lice-of-north-america-usa-canada-mexico-greenland.htm (accessed on 10 June 2021).
- Marsh, P.M. Braconidae. In Catalog of Hymenoptera in America North of Mexico. Symphyta and Apocrita (Parasitica); Krombein, K.V., Hurd, P.D., Jr.;Smith, D.R., Burks, B.D., Eds.; Smithsonian Institution Press: Washington, DC, USA, 1979; Volume 1, pp. 144–295. [Google Scholar]
- Pike, K.S.; Starý, P.; Miller, T.; Graf, G.; Allison, D.; Boydston, L.; Miller, R. Aphid parasitoids (Hymenoptera: Braconidae: Aphidiinae) of Northwest USA. Proc. Entomol. Soc. Wash. 2000, 102, 688–740. [Google Scholar]
- Starý, P.; Remaudière, G. Complements to the aphid parasitoid fauna of Mexico (Hymenoptera, Aphidiidae). Ann. Soc. Entomol. Fr. 1983, 19, 113–116. [Google Scholar]
- Zamora Mejías, D.; Hanson, P.E.; Starý, P. Survey of the aphid parasitoids (Hymenoptera: Braconidae: Aphidiinae) of Costa Rica with information on their aphid (Hemiptera: Aphidoidea): Plant associations. Psyche J. Entomol. 2010, 2010, 278643. [Google Scholar] [CrossRef][Green Version]
- Starý, P. The Aphidiidae of Chile (Hymenoptera, Ichneumonoidea, Aphidiidae). Dtsch. Entomol. Z. 1995, 42, 113–138. [Google Scholar] [CrossRef]
- Starý, P.; Sampaio, M.V.; Bueno, V.H.P. Aphid parasitoids (Hymenoptera, Braconidae, Aphidiinae) and their associations related to biological control in Brazil. Rev. Bras. Entomol. 2007, 51, 107–118. [Google Scholar] [CrossRef]
- Ward, S.; Umina, P.A.; Polaszek, A.; Hoffmann, A.A. Study of aphid parasitoids (Hymenoptera: Braconidae) in Australian grain production landscapes. Austral Entomol. 2021, 60, 722–737. [Google Scholar] [CrossRef]
- Bulman, S.; Drayton, G.M.; Cameron, P.J.; Teulon, D.A.; Walker, G.P. Endemic New Zealand aphids (Hemiptera: Aphididae) parasitised by native Aphidiinae (Hymenoptera: Braconidae), not biological control parasitoids. Austral Entomol. 2021, 60, 713–721. [Google Scholar] [CrossRef]
- Starý, P.; Remaudiere, G.; Autrique, A. Les Aphidiides parasites de pucerons en region ethiopienne. In Contribution à L’écologie des Aphides Africains; Remaudiere, G., Autriquepp, A., Eds.; Etude FAO, Production Vegetale et Protectiondes Plantes: Rome, Italy, 1985; pp. 95–102. [Google Scholar]
- Starý, P.; Schmutterer, H. A review of aphid parasites (Hymenoptera: Aphidiidae) in Kenya. Z. Angew. Entomol. 1973, 74, 351–356. [Google Scholar] [CrossRef]
- Kfir, R.; van Rensburg, N.J.; Kirsten, F. Biological control of the black pine aphid Cinara cronartii (Homoptera: Aphididae) in South Africa. Afric. Entomol. 2003, 11, 117–121. [Google Scholar]
- Muller, L.; Kruger, K.; Kfir, R. First report of the aphid parasitoid Aphidius ervi Haliday (Hymenoptera, Braconidae, Aphidiinae) from South Africa. Afric. Entomol. 2014, 22, 214–215. [Google Scholar] [CrossRef]
- Starý, P. New species and a review of aphid parasitoids of Madagascar (Hym., Braconidae, Aphidiinae). Linzer biol. Beitr. 2005, 37, 1711–1718. [Google Scholar]
- Belokobylskij, S.A.; Samartsev, K.G.; Il’inskaya, A.S. Annotated catalogue of the Hymenoptera of Russia. Volume II. Apocrita: Parasitica. Proc. Zool. Inst. RAS 2019, 323 (Suppl. S8), 1–594. [Google Scholar] [CrossRef]
- Rakhshani, E.; Barahoei, H.; Ahmad, Z.; Starý, P.; Ghafouri-Moghaddam, M.; Mehrparvar, M.; Kavallieratos, N.G.; Čkrkić, J.; Tomanović, Ž. Review of Aphidiinae parasitoids (Hymenoptera: Braconidae) of the Middle East and North Africa: Key to species and host associations. Eur. J. Taxon. 2019, 552, 1–132. [Google Scholar] [CrossRef]
- Takada, H. Aphidiidae of Japan (Hymenoptera). Insecta Matsumurana 1968, 30, 67–124. [Google Scholar]
- Čkrkić, J.; Petrović, A.; Kocić, K.; Ye, Z.; Vollhardt, I.M.; Hebert, P.D.; Traugott, M.; Tomanović, Ž. Hidden in plain sight: Phylogeography of an overlooked parasitoid species Trioxys sunnysidensis Fulbright & Pike (Hymenoptera: Braconidae: Aphidiinae). Agric. For. Entomol. 2019, 21, 299–308. [Google Scholar]
- Westrum, K.; Klingen, I.; Hofsvang, T.; Hågvar, E.B. Checklist of primary parasitoids and hyperparasitoids (Hymenoptera, Apocrita) on aphids (Hemiptera, Aphididae) from Norway. Nor. J. Entomol. 2010, 57, 142–153. [Google Scholar]
- Belokobylskij, S.A.; Taeger, A.; van Achterberg, C.; Haeselbarth, E.; Riedel, M. Checklist of the Braconidae of Germany (Hymenoptera). Beitr. Entomol. 2003, 53, 341–435. [Google Scholar] [CrossRef]
- Broad, G.; Shaw, M.; Godfray, H. Checklist of British and Irish Hymenoptera–Braconidae. Biodivers. Data J. 2016, 4, e8151. [Google Scholar] [CrossRef]
- Tancoigne, E.; Ollivier, G. Evaluating the progress and needs of taxonomy since the Convention on Biological Diversity: Going beyond the rate of species description. Aust. Syst. Bot. 2017, 30, 326–336. [Google Scholar] [CrossRef]
- Roy, H.E.; Roy, D.B.; Roques, A. Inventory of terrestrial alien arthropod predators and parasites established in Europe. BioControl 2011, 56, 477–504. [Google Scholar] [CrossRef]
- Petrović, A.; Mitrović, M.; Ghailow, M.; Ivanović, A.; Kavallieratos, N.G.; Starý, P.; Tomanović, Ž. Resolving the taxonomic status of biocontrol agents belonging to the Aphidius eadyi species group (Hymenoptera: Braconidae: Aphidiinae): An integrative approach. Bull. Entomol. Res. 2019, 109, 342–355. [Google Scholar] [CrossRef]
- Žikić, V.; Stanković, S.S.; Ilić Milošević, M.; Petrović-Obradović, O.; Petrović, A.; Starý, P.; Tomanović, Ž. First detection of Lysiphlebus testaceipes (Cresson) (Hymenoptera: Aphidiinae) in Serbia: An introduced species invading Europe. North-West. J. Zool. 2015, 11, 97–101. [Google Scholar]
- Hughes, G.E.; Sterk, G.; Bale, J.S. Thermal biology and establishment potential in temperate climates of the aphid parasitoid, Lysiphlebus testaceipes. BioControl 2011, 56, 19–33. [Google Scholar] [CrossRef]
- Tepa-Yotto, G.T.; Hofsvang, T.; Godonou, I.; Sæthre, M.G. Host preference of Lysiphlebus testaceipes (Hymenoptera: Braconidae, Aphidiinae), an alien aphid parasitoid in Benin. Int. J. Trop. Insect Sci. 2013, 33, 127–135. [Google Scholar] [CrossRef]
- Woolley, V.C.; Tembo, Y.L.; Ndakidemi, B.; Obanyi, J.N.; Arnold, S.E.; Belmain, S.R.; Ndakidemi, P.A.; Ogendo, J.O.; Stevenson, P.C. The diversity of aphid parasitoids in East Africa and implications for biological control. Pest Manag. Sci. 2021. [Google Scholar] [CrossRef] [PubMed]
- Petrović, A.; Mitrović, M.; Starý, P.; Petrović-Obradović, O.; Tomanović, Ž.; Žikić, V.; Vorburger, C. Lysiphlebus orientalis, a new invasive parasitoid in Europe–evidence from molecular markers. Bull. Entomol. Res. 2013, 103, 451–457. [Google Scholar] [CrossRef]
- Petrović, A.; Čkrkić, J.; Jamhour, A.; Petrović-Obradović, O.; Mitrović, M.; Starý, P.; Nedstam, B.; Tomanović, Ž. First record of Aphidius ericaphidis (Hymenoptera, Braconidae) in Europe: North American hitchhiker or overlooked Holarctic citizen? J. Hymenopt. Res. 2017, 57, 143–153. [Google Scholar] [CrossRef]
- Rakhshani, E.; Saval, J.M.; Pérez Hidalgo, N.; Pons, X.; Kavallieratos, N.G.; Starý, P. Trioxys liui Chou & Chou, 1993 (Hymenoptera, Braconidae, Aphidiinae): An invasive aphid parasitoid attacking invasive Takecallis species (Hemiptera, Aphididae) in the Iberian Peninsula. ZooKeys 2020, 944, 99–114. [Google Scholar]
- Fulbright, J.L.; Pike, K.S.; Starý, P. A key to North American species of Trioxys Haliday (Hymenoptera: Braconidae: Aphidiinae), with a summary of the geographic distribution, hosts, and species diagnostic features. Proc. Entomol. Soc. Wash. 2007, 109, 779–790. [Google Scholar]
- Pyšek, P.; Hulme, P.E.; Simberloff, D.; Bacher, S.; Blackburn, T.M.; Carlton, J.T.; Dawson, W.; Essl, F.; Foxcroft, L.C.; Genovesi, P.; et al. Scientists’ warning on invasive alien species. Biol. Rev. 2020, 95, 1511–1534. [Google Scholar] [CrossRef]
- Kaiser, M.C.; Heimpel, G.E. Parasitoid-induced transgenerational fecundity compensation in an aphid. Entomol. Exp. Appl. 2016, 159, 197–206. [Google Scholar] [CrossRef]
- Tougeron, K.; Damien, M.; Le Lann, C.; Brodeur, J.; van Baaren, J. Rapid responses of winter aphid-parasitoid communities to climate warming. Front. Ecol. Evol. 2018, 6, 173. [Google Scholar] [CrossRef]
- Mayr, E. The Role of Systematics in Biology: The study of all aspects of the diversity of life is one of the most important concerns in biology. Science 1968, 159, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Milošević, M.; Petrović, A.; Stanković, S.S.; Čkrkić, J.; Starý, P.; Žikić, V.; Tomanović, Ž. Taxonomic position and phylogenetic relationships of the genera Euaphidius and Remaudierea (Hymenoptera: Braconidae: Aphidiinae) analyzed using molecular markers and geometric morphometrics. Ann. Entomol. Soc. Am. 2015, 108, 435–445. [Google Scholar]
- Stanković, S.S.; Petrović, A.; Ilić Milośević, M.; Starý, P.; Kavallieratos, N.G.; Žikić, V.; Tomanović, Ž. Morphological and molecular characterization of common European species of Adialytus (Hymenoptera: Braconidae: Aphidiinae) based on the mtCOI barcoding gene and geometric morphometrics of the forewings. Eur. J. Entomol. 2015, 112, 165–174. [Google Scholar] [CrossRef]
- Kos, K.; Petrović, A.; Starý, P.; Kavallieratos, N.G.; Ivanović, A.; Toševski, I.; Jakše, J.; Trdan, S.; Tomanović, Ž. On the Identity of Cereal Aphid Parasitoid Wasps Aphidius uzbekistanicus, Aphidius rhopalosiphi, and Aphidius avenaphis (Hymenoptera: Braconidae: Aphidiinae) by Examination of COI Mitochondrial Gene, Geometric Morphometrics, and Morphology. Anns. Entomol. Soc. Am. 2011, 104, 1221–1232. [Google Scholar] [CrossRef]
- Jamhour, A.; Mitrović, M.; Petrović, A.; Starý, P.; Tomanović, Ž. Re-visiting the Aphidius urticae s. str. group: Re-description of Aphidius rubi Starý and A. silvaticus Starý (Hymenoptera: Braconidae: Aphidiinae). Zootaxa 2016, 4178, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Tomanović, Ž.; Petrović, A.; Mitrović, M.; Kavallieratos, N.G.; Starý, P.; Rakhshani, E.; Rakhshanipour, M.; Popović, A.; Shukshuk, A.H.; Ivanović, A. Molecular and morphological variability within the Aphidius colemani group with redescription of Aphidius platensis Brethes (Hymenoptera: Braconidae: Aphidiinae). Bull. Entomol. Res. 2014, 104, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Rakhshani, E.; Starý, P.; Davidian, E. A taxonomic review of the subgenus Pauesiella Sedlag & Starý, 1980 (Hym.: Braconidae: Aphidiinae). Plant Pest. Res. 2017, 7, 53–66. [Google Scholar]
- Tomanović, Ž.; Kos, K.; Petrović, A.; Starý, P.; Kavallieratos, N.G.; Žikić, V.; Jakše, J.; Trdan, S.; Ivanović, A. The relationship between molecular variation and variation in the wing shape of three aphid parasitoid species: Aphidius uzbekistanicus Luzhetzki, Aphidius rhopalosiphi De Stefani Perez and Aphidius avenaphis (Fitch)(Hymenoptera: Braconidae: Aphidiinae). Zool. Anz. 2013, 252, 41–47. [Google Scholar] [CrossRef]
- Bortolus, A. Error cascades in the biological sciences: The unwanted consequences of using bad taxonomy in ecology. AMBIO J. Hum. Environ. 2008, 37, 114–118. [Google Scholar] [CrossRef]
- Rakhshani, E.; Starý, P.; Tomanović, Ž.; Mifsud, D. Aphidiinae (Hymenoptera, Braconidae) aphid parasitoids of Malta: Review and key to species. Bull. Entomol. Soc. Malta 2015, 7, 121–137. [Google Scholar]
- Zumoffen, L.; Rodriguez, M.; Gerding, M.; Salto, C.E.; Salvo, A. Plants, aphids and parasitoids: Trophic interactions in agroecosystems in the province of Santa Fe, Argentina and a key to the identification of Aphidiinae and Aphelinidae (Hymenoptera) known to the region. Rev. Soc. Entomol. Argent. 2015, 74, 133–144. [Google Scholar]
- Huma, K.; Muhammad, A.; Muhammad, A.A.; Raza, A.B.M.; Muhammad, S.K.; Farghama, K. Biodiversity of Aphidiinae species (Braconidae: Hymenoptera) in Sargodha region with particular reference to citrus orchards. Munis Entomol. Zool. 2019, 14, 454–465. [Google Scholar]
- Bodlah, I.; Naeem, M.; Rakhshani, E. Genus Binodoxys Mackauer, 1960 (Hymenoptera: Braconidae: Aphidiinae) from Punjab Province of Pakistan. Pak. J. Zool. 2012, 44, 551–557. [Google Scholar]
- Zamora-Mejías, D.; Hanson, P.E. Key to the species of parasitoids and hyperparasitoids (Hymenoptera) of aphids (Hemiptera: Aphididae) of Costa Rica. Agron. mesoam. 2017, 28, 565–575. [Google Scholar] [CrossRef][Green Version]
- Tian, H.W. A Taxonomic Studies on the Tribes Ephedrini and Praini (Hymenoptera: Braconidae: Aphidiinae) from China. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2017. [Google Scholar]
- Kocić, K. Molecular Phylogeny, Subgeneric Classification and Cryptic Speciation of the European Species of Genus Ephedrus Haliday (Hymenoptera, Braconidae, Aphidiinae). Ph.D. Thesis, University of Belgrade, Belgrade, Serbia, 2019. [Google Scholar]
- Kavallieratos, N.G.; Tomanović, Ž.; Petrović, A.; Janković, M.; Starý, P.; Yovkova, M.; Athanassiou, C.G. Review and key for the identification of parasitoids (Hymenoptera: Braconidae: Aphidiinae) of aphids infesting herbaceous and shrubby ornamental plants in southeastern Europe. Ann. Entomol. Soc. Am. 2013, 106, 294–309. [Google Scholar] [CrossRef]
- Kavallieratos, N.G.; Tomanović, Ž.; Starý, P.; Žikić, V.; Petrović-Obradović, O. Parasitoids (Hymenoptera: Braconidae: Aphidiinae) attacking aphids feeding on Solanaceae and Cucurbitaceae crops in southeastern Europe: Aphidiine-aphid-plant associations and key. Ann. Entomol. Soc. Am. 2010, 103, 153–164. [Google Scholar] [CrossRef]
- Kavallieratos, N.G.; Tomanović, Ž.; Petrović, A.; Kocić, K.; Janković, M.; Starý, P. Parasitoids (Hymenoptera: Braconidae: Aphidiinae) of aphids feeding on ornamental trees in southeastern Europe: Key for identification and tritrophic associations. Ann. Entomol. Soc. Am. 2016, 109, 473–487. [Google Scholar] [CrossRef]
- Ghaliow, M.E.; Petrović, A.; Kocić, K.; Čkrkić, J.; Mitrovski Bogdanović, A.; Starý, P.; Kavallieratos, N.G.; Tomanović, Ž. Key for identification of the parasitoids (Hymenoptera: Braconidae: Aphidiinae) of aphids infesting alfalfa in Europe. Zootaxa 2018, 4378, 98–110. [Google Scholar] [CrossRef]
- Starý, P.; Rakhshani, E.; Tomanović, Ž.; Kavallieratos, N.G.; Petrović, A.; Žikić, V.; Havelka, J. Aphid-parasitoid Associations on the Impatiens Plants in Central Europe (Hemiptera, Aphididae; Hymenoptera, Braconidae, Aphidiinae). J. Entomol. Res. Soc. 2014, 16, 33–43. [Google Scholar]
- Havelka, J.; Tomanović, Ž.; Kos, K.; Kavallieratos, N.G.; Janeček, J.; Pons, X.; Rakhshani, E.; Starý, P. Mountain aphid and parasitoid guilds on Aconitum spp. in Europe. Bull. Insectol. 2014, 67, 57–61. [Google Scholar]
- Tomanović, Ž.; Starý, P.; Kavallieratos, N.G.; Gagić, V.; Plećas, M.; Janković, M.; Rakhshani, E.; Ćetković, A.; Petrović, A. Aphid parasitoids (Hymenoptera: Braconidae: Aphidiinae) in wetland habitats in western Palaearctic: Key and associated aphid parasitoid guilds. Ann. Soc. Entomol. Fr. 2012, 48, 189–198. [Google Scholar] [CrossRef]
- de Carvalho, M.R.; Bockmann, F.A.; Amorim, D.S.; Brandão, C.R.F.; de Vivo, M.; de Figueiredo, J.L.; Britski, H.A.; de Pinna, M.C.C.; Meneyes, N.A.; Marques, F.P.L.; et al. Taxonomic impediment or impediment to taxonomy? A commentary on systematics and the cybertaxonomic-automation paradigm. Evol. Biol. 2007, 34, 140–143. [Google Scholar] [CrossRef]
- Hebert, P.D.; Cywinska, A.; Ball, S.L.; DeWaard, J.R. Biological identifications through DNA barcodes. Proc. R. Soc. B 2003, 270, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Godfray, H.C.J. Linnaeus in the information age. Nature 2007, 446, 259–260. [Google Scholar] [CrossRef] [PubMed]
- Minteer, B.A.; Collins, J.P.; Love, K.E.; Puschendorf, R. Avoiding (re) extinction. Science 2014, 344, 260–261. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, M.J.; Janzen, D.H.; Hallwachs, W.; Chapman, E.G.; Smith, M.A.; Dapkey, T.; Brown, A.; Ratnasingham, S.; Naik, S.; Manjunath, R.; et al. Minimalist revision and description of 403 new species in 11 subfamilies of Costa Rican braconid parasitoid wasps, including host records for 219 species. ZooKeys 2021, 1013, 1–665. [Google Scholar] [PubMed]
- Ahrens, D.; Ahyong, S.T.; Ballerio, A.; Barclay, M.V.; Eberle, J.; Espeland, M.; Huber, B.A.; Mengual, X.; Pacheco, T.L.; Peters, R.S.; et al. Is it time to describe new species without diagnoses?—A comment on Sharkey et al. Zootaxa 2021, 5027, 151–159. [Google Scholar] [CrossRef]
- Borkent, A. Diagnosing diagnoses–can we improve our taxonomy? ZooKeys 2021, 1071, 43. [Google Scholar] [CrossRef]
- Mkenda, P.A.; Ndakidemi, P.A.; Stevenson, P.C.; Arnold, S.E.; Belmain, S.R.; Chidege, M.; Gurr, G.M.; Woolley, V.C. Characterization of Hymenopteran Parasitoids of Aphis fabae in An African Smallholder Bean Farming System Through Sequencing of COI “Mini-barcodes”. Insects 2019, 10, 331. [Google Scholar] [CrossRef]
- Mitrovski-Bogdanović, A.; Mitrović, M.; Milošević Ilić, M.; Žikić, V.; Jamhour, A.; Ivanović, A.; Tomanović, Ž. Molecular and morphological variation among the European species of the genus Aphidius Nees (Hymenoptera: Braconidae: Aphidiinae). Org. Divers. Evol. 2021, 21, 421–436. [Google Scholar] [CrossRef]
- Castalanelli, M.A.; Severtson, D.L.; Brumley, C.J.; Szito, A.; Foottit, R.G.; Grimm, M.; Munyard, K.; Groth, D.M. A rapid non-destructive DNA extraction method for insects and other arthropods. J. Asia Pac. Entomol. 2010, 13, 243–248. [Google Scholar] [CrossRef]
- Derocles, S.A.; Le Ralec, A.; Plantegenest, M.; Chaubet, B.; Cruaud, C.; Cruaud, A.; Rasplus, J.Y. Identification of molecular markers for DNA barcoding in the Aphidiinae (Hym. Braconidae). Mol. Ecol. Res. 2012, 12, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Higashiura, Y.; Maeto, K. Evaluation of easy, non-destructive methods of DNA extraction from minute insects. Appl. Entomol. Zool. 2017, 52, 349–352. [Google Scholar] [CrossRef]
- Saunders, T.E. Taxonomy at a crossroads: Communicating value, building capability, and seizing opportunities for the future. Megataxa 2020, 1, 063–066. [Google Scholar] [CrossRef]
- Swiss Re Institute. Biodiversity and Ecosystem Services: A Business Case for Re/Insurance; Swiss Re Management Ltd.: Zurich, Switzerland, 2020; pp. 1–60. [Google Scholar]
- Favret, C. Cybertaxonomy to accomplish big things in aphid systematics. Insect Sci. 2014, 21, 392–399. [Google Scholar] [CrossRef]

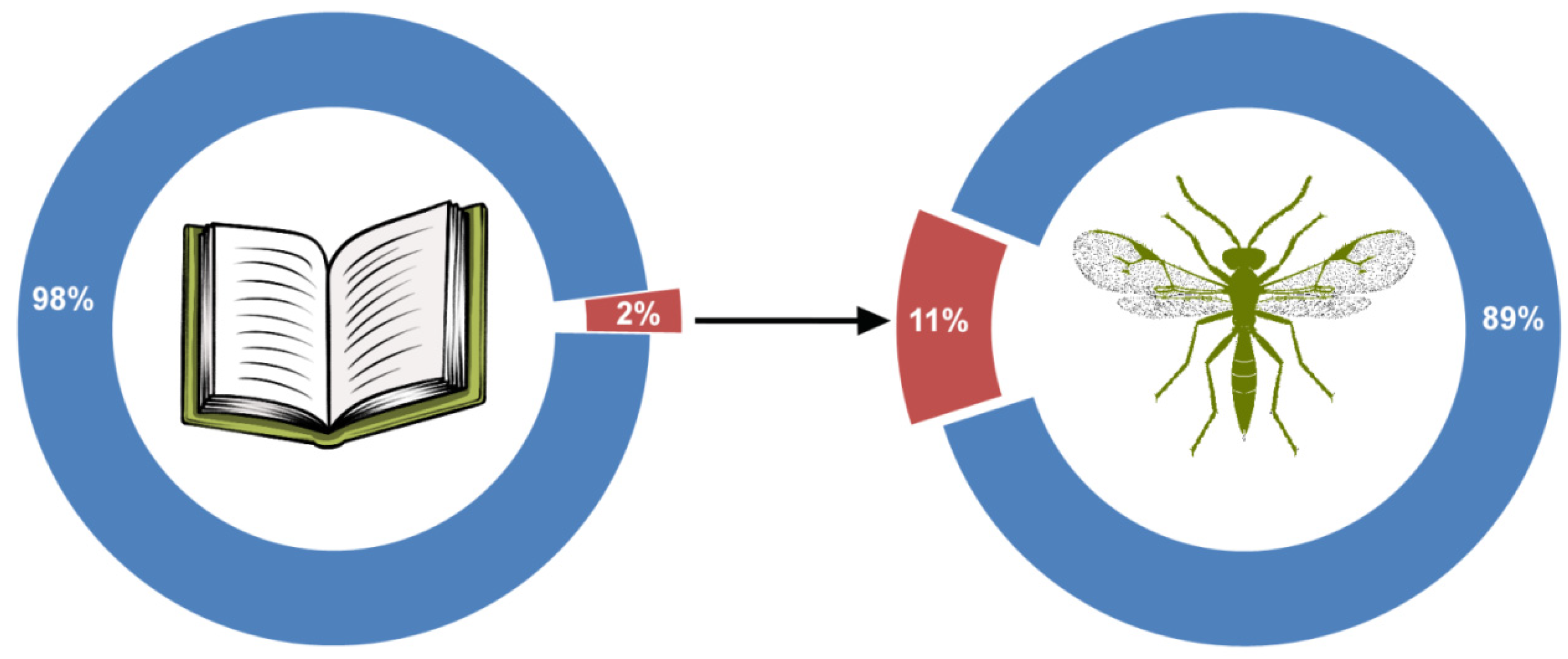
| Search System | Total Number of Results | Number of Relevant Results | Number of Unique Results Included in Analysis |
|---|---|---|---|
| GS | 3570 | 1753 | 1752 |
| Scopus | 874 | 803 | 125 |
| WoS | 1082 | 654 | 25 |
| Total | 5526 | 3209 | 1902 |
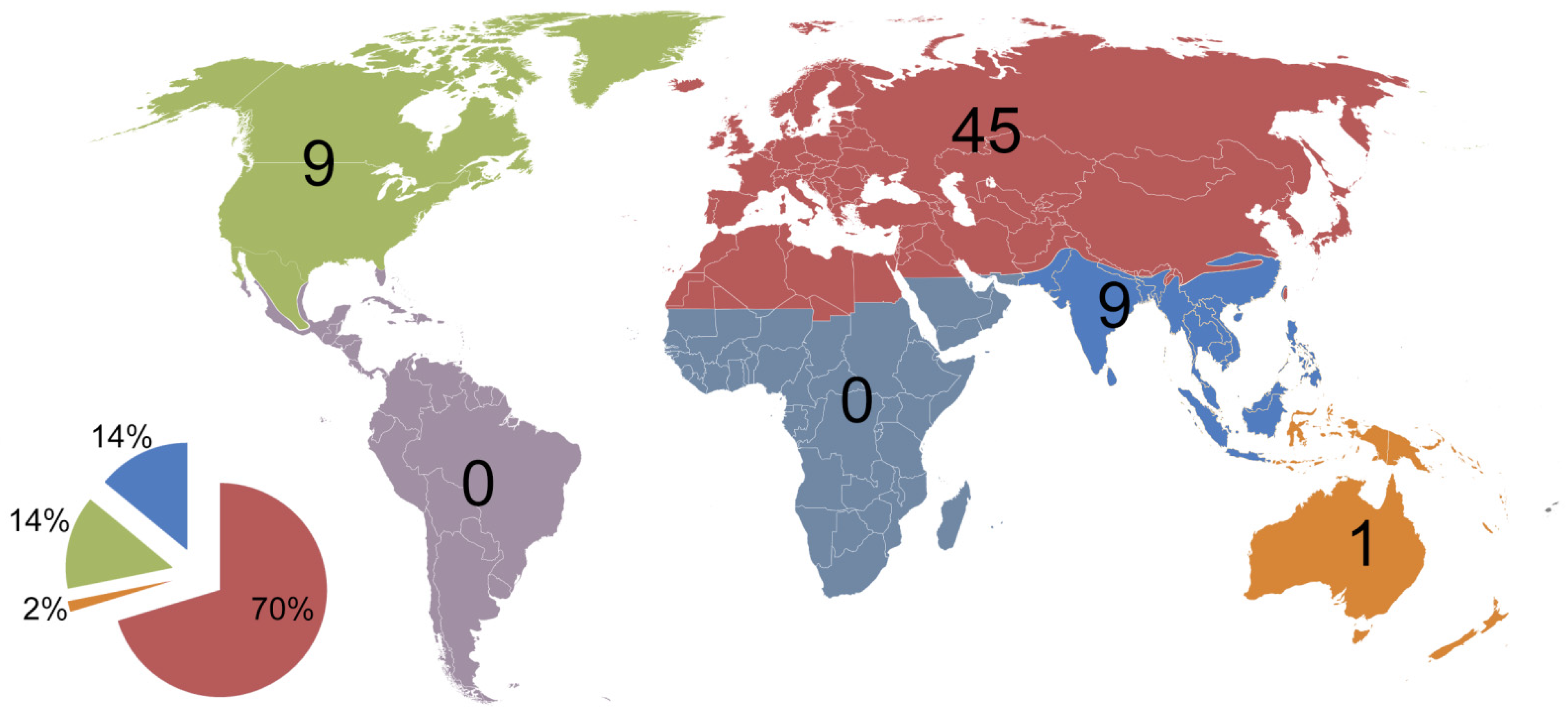
| Country/Area | Number of Species | References | Area (km2) |
|---|---|---|---|
| America north of Mexico (Nearctic) | ~130 | [47,57,58,72,73] | 19,782,990 |
| Mexico (North and Central America) | 33 | [74] | 1,972,550 |
| Costa Rica (Central America) | 10 | [75] | 51,100 |
| Chile (Neotropics) | 23 | [76] | 756,096.3 |
| Brazil (Neotropics) | 19 | [77] | 8,515,767 |
| Australia (Australasia) | 23 | [78] | 7,692,024 |
| New Zealand (Australasia) | 15 | [79] | 268,021 |
| Subsaharan Africa (Afrotropics) | 22 | [80,81,82,83,84] | 23,290,000 |
| Madagascar | 7 | [84] | 592,800 |
| Russia (Palaearctic) | 198 | [54,61,85] | 17,098,246 |
| Middle East and North Africa | 108 | [86] | 11,695,164 |
| China (Asia) | 130 | [16] | 9,596,961 |
| India (South Asia) | 127 | [29,34] | 3,287,263 |
| Japan (Far East) | ≈80 | [42,55,64,87] | 377,975 |
| Kyrgyzstan (Central Asia) | 35 | [60] | 199,951 |
| Norway (Northern Europe) | 26 | [58,88,89] | 385,207 |
| Czech Republic (Central Europe) | ≈135 | [21] | 78,871 |
| Germany (Central and Western Europe) | 109 | [88,90] | 357,022 |
| Great Britain and Ireland (British Isles) | 96 | [88,91] | 293,752 |
| Serbia (Southern Europe) | 121 | [69] | 88,361 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrović, A. Sizing the Knowledge Gap in Taxonomy: The Last Dozen Years of Aphidiinae Research. Insects 2022, 13, 170. https://doi.org/10.3390/insects13020170
Petrović A. Sizing the Knowledge Gap in Taxonomy: The Last Dozen Years of Aphidiinae Research. Insects. 2022; 13(2):170. https://doi.org/10.3390/insects13020170
Chicago/Turabian StylePetrović, Andjeljko. 2022. "Sizing the Knowledge Gap in Taxonomy: The Last Dozen Years of Aphidiinae Research" Insects 13, no. 2: 170. https://doi.org/10.3390/insects13020170
APA StylePetrović, A. (2022). Sizing the Knowledge Gap in Taxonomy: The Last Dozen Years of Aphidiinae Research. Insects, 13(2), 170. https://doi.org/10.3390/insects13020170






