Simple Summary
The red spider mite, Tetranychus merganser is one of the most economically important pests in papaya and prickle pear cactus cultivars, causing major damage to fruit and defoliation. In recent years, T. merganser has increased the number of its host plants. The mechanisms of resistance of a plant to herbivorous arthropod include antixenosis and antibiosis. Antixenosis refers to the plant mechanism to affect feeding and oviposition of arthropods; antibiosis refers to the plant capacity to affect the biology of the arthropod. The aim of this research is to assess antibiosis and antixenosis as resistance mechanisms in seven host plants (Thevetia ahouai, Carica papaya, Phaseolus vulgaris, Moringa oleifera, Pittosporum tobira, Helietta parvifolia, Capsicum annuum var. glabriusculum) to red spider mites. Oviposition and damage by feeding of T. merganser were greater on C. papaya than on the other host plants. The population growth of the spider mite was lower in P. tobira and T. ahouai than in the other host plants. Results based on the analysis of demographic parameters, food intake, survival and oviposition of T. merganser females suggest that P. tobira and T. ahouai were the most resistant to red spider mites, whereas C. papaya was the most susceptible of the seven host plants. The resistant plants can be studied as alternatives in the management of red spider mites.
Abstract
Red spider mites, Tetranychus merganser Boudreaux (Acari: Tetranychidae), is an agricultural pest that causes economic losses in papaya and nopal crops in Mexico. The aim of this research was to assess antibiosis and antixenosis as resistance mechanisms in seven host plants (Thevetia ahouai, Carica papaya, Phaseolus vulgaris, Moringa oleifera, Pittosporum tobira, Helietta parvifolia, Capsicum annuum var. glabriusculum) to red spider mites. Antixenosis was evaluated by non-preference for oviposition and feeding, antibiosis by infinitesimal rate of increase, finite rate of increase and doubling time, and the percentage of spider mites mortality. Oviposition and damage by feeding of T. merganser were significantly greater on C. papaya than on the other host plants. The growth rate of the spider mite was significantly lower in P. tobira and T. ahouai than in the other host plants. The percentage of hatched eggs of T. merganser was significantly higher in P. vulgaris than in the other plant species. Based on the demographic parameters, survival, food intake, and oviposition, these results indicated that compared with C. papaya, P. tobira and T. ahouai were more resistant. These results may be due to the fact that they were plants species of different families. The resistant plants can be studied as alternatives in the management of T. merganser.
1. Introduction
Tetranychidae family include more than 1300 species of phytophagous mites, of which one hundred can be considered pests, ten of them being of great importance [1]. The mites that cause serious damage to crops and ornamental plants are found in the genera Tetranychus Dufour, Panonychus Yokoyama, Oligonychus Berlese, and Eutetranychus Banks [1,2]. The red spider mite, Tetranychus merganser Boudreaux (Acari: Tetranychidae), causes severe damage by its feeding in different species of plants of the family Aquifoliaceae, Apocynaceae, Cactaceae, Caricaceae, Cucurbitaceae, Euphorbiaceae, Fabaceae, Moringaceae, Oleaceae, Pittosporaceae, Rosaceae, Ranunculaceae Rutaceae and Solanaceae [1,3,4,5,6,7,8]. The red spider mite is distributed in the United States, China, Mexico, and Thailand [1]. Moreover, it is considered a potential pest for Mexican agriculture [3,8], e.g., causing losses of 586 ± 234 dollars per hectare in prickly pear cactus, (Opuntia ficus-indica L.) Miller (Cactaceae) crops [3]. The red spider mite can develop and reproduce in a wide range of climatic factors [9,10]. Ullah et al. [9] evaluated the behavior of T. merganser at different temperatures, 15 to 37.5 °C and 60–70% relative humidity, on bean disc, Phaseolus vulgaris L. (Fabaceae). They documented that T. merganser has better performance at 30 °C. Furthermore, Reyes-Pérez [10] found that optimal development for spider mites was at 27 °C on Carica papaya L. (Caricaceae), when evaluated between 19 and 35 °C and 60 ± 2% relative humidity. Chacón-Hernández et al. [11] reported that the performance of spider mites was better on beans when compared to wild chili peppers, Capsicum annuum L. var. glabriusculum (Solanaceae). The population growth parameters of T. merganser, such as daily egg production, survival, food intake, and rate of development, may vary in response to changes in temperature, host plant species, and nutrition quality of plants [9,10,11].
T. merganser is controlled through the use of insecticides and acaricide chemicals. However, the red spider mites’ short life cycle and high reproductive potential allows them to quickly develop resistance to these compounds [12]. The use of botanical extracts [13] and predatory mites, mainly from the family Phytoseiidae [4], are more effective and sustainable strategies to the management of red spider mites, with the added benefit of causing minimal impact on the environment. In Mexico, Chacón-Hernández et al. [11] observed that wild chili pepper (C. annuum var. glabriusculum (Dunal) Heiser & Pickersgill) was resistant to T. merganser. Plant responses to herbivorous arthropod attack are based on genetically inherited qualities, generally divided into three categories: antixenosis, antibiosis and tolerance [14,15,16]. Antibiosis occurs when a phytophagous arthropod is negatively affected, especially in its biology, by chemical and morphological presents in resistant host plants. Antixenosis or deterrence is the non-preference of a phytophagous arthropod to a resistant plant and denotes the anti-feeding and anti-oviposition caused by biophysical or allelochemical factors, resulting in the late acceptance or absolute rejection of a plant as a host. Tolerance is a polygenic trait that allows a plant to resist, repair or recover from damage caused by the phytophagous arthropod [14,15,16,17,18]. Polyphagous arthropods have the ability to tolerate or resist the defense mechanisms of host plants, which allows them to feed and reproduce [19]. The aim of this research is to assess antibiosis and antixenosis as resistance mechanisms in seven host plants species (Thevetia ahouai (L.) A. DC. (Apocynaceae), C. papaya L., P. vulgaris L., Moringa oleifera Lam. (Moringaceae), Pittosporum tobira (Thunb.) W.T. Aiton (Pittosporaceae), Helietta parvifolia (Gray) Benth. (Rutaceae), C. annuum L. var. glabriusculum) to T. merganser.
2. Materials and Methods
2.1. Red Spider Mite Colony
A red spider mite colony was started with biological material obtained from the Population Ecology Laboratory, Institute of Applied Ecology, Autonomous University of Tamaulipas (IEA-UAT). To increase the spider mite population, female and male T. merganser were placed on bean plants (P. vulgaris) under greenhouse conditions at 30 ± 2 °C and 70 ± 10% relative humidity (RH).
2.2. Collection and Preparation of Plant Material
For this study, seven plant species reported as hosts of T. merganser were selected [5,6,7,8,10] (Table 1).

Table 1.
Host plants used for the study of resistance to Tetranychus merganser.
We collected 20 mature leaves of each species of host plants in its natural habitat (Table 1). The leaves of each host plants were transported in resealable plastic bags inside a cooler with frozen gel packs at a temperature of 5 ± 2 °C to the Population Ecology Laboratory at the Ecology Institute of the Autonomous University of Tamaulipas. The transfer time of the leaves to the laboratory depended on the location of the host plants, but was between 15 to 30 min. From the 20 leaves, we selected three clean leaves, without the presence of any damage and without symptoms of the presence of fungi or bacteria. The leaves were treated with a 2 min wash with sodium hypochlorite solution with 1.5% and had 2 × 2 cm squares cut out from each leaf.
2.3. Experimental Design
We used the sand technique described by Ahmadi [19] with modifications. We cut leaf squares of each species of host plants, seven in total. The squares measured 2 × 2 cm and were cut with the help of a sterile scalpel. We placed the leaf squares on cotton soaked in water with the underside side up. We placed each leaf square inside a 5 cm diameter Petri dish, with 10 T. merganser females per leaf square of each host plant. Previously, ten adult females and five males of T. merganser were placed in each leaf square of the plant to improve reproduction and oviposition. After 24 h, the males were removed, leaving only the females (the observed eggs were removed). The experiment was carried out under laboratory conditions at 28 ± 1 °C and 70–80% relative humidity (RH), with a photoperiod of 12:12 h (light: dark). We randomly assigned three leaf squares to seven groups, one group for each host plant species. The leaf squares of each host plant species were the replicas, and had three replicas per group, 21 in total. Adult females T. merganser were two days old when transferred to the leaf square.
2.4. Antixenosis
Antixenosis was determined by non-preference (oviposition and feeding) of T. merganser for any host plant. The feeding of T. merganser was visually estimated on each leaf square through the damage index proposed by Nachman and Zemek [20], where 0 = 0% damage (no feeding damage) and 5 = 81% to 100% damage by feeding (a dense mark or wilt caused by spider mite feeding of the leaf square). Only one person registered the number of eggs laid per female and the percentage of feeding damage at 24, 48, 72 and 96 h with the help of a dissecting microscope (UNICO Stereo and Zoom Microscopes ZM180, Princenton, NJ, USA), to avoid bias during the evaluation.
2.5. Antibiosis
We used the infinitesimal rate of increase (r, day−1), the finite rate of increase (λ), and the population doubling time (DT) to determine antibiosis, and r was calculated by:
where Nt is the number of individuals at time t (surviving adult females plus the eggs laid at the end of the bioassay), and N0 is the number of individuals at time 0 (initial cohort = 10 adult females of T. merganser) and t is the number of days elapsed from the start to the end of the bioassay (equal to 3 days).
r = (1/t) × ln(Nt/N0)
The finite growth rate, i.e., the number of times the population multiplied in a unit of time, was calculated as:
λ= antiloge r
The doubling time in which the population doubled was calculated by [21]:
DT = Ln(2)/r
The demographic parameters (r, λ and DT) indicate the population growth of the red spider mite.
2.6. Mortality
We used the Chacón-Hernández et al. [11] formula to measure the percentage of mortality of T. merganser. We measured mortality by the average percent of dead individuals (drowned) outside the leaf square (Σdi/n) × 100, where di is the number of drowned individuals and n the number of individuals on the leaf square [11]. We recorded the number of dead and alive mites at 24, 48, 72 and 96 h.
2.7. Hatched Eggs of Tetranychus merganser
On the fifth day, after leaving the females alone, the number of immature mites that emerged from the eggs laid by T. merganser female was recorded. The time it takes to hatch a T. merganser egg is around 3.6 ± 0.3 days, at 27.5 °C in bean discs (P. vulgaris), with a photoperiod of 16: 8 h light: dark and 60–70% HR [9]. Meanwhile, hatching time on papaya discs (C. papaya) is around 4.10 ± 0.52 days, at 27.0 °C with a photoperiod of 14:10 h L: D and 60 ± 2% RH [8]. Based on literature, eggs that did not hatch during five days were considered nonviable due to natural death or will take longer to hatch.
2.8. Statistic Analysis
The number of laid eggs, dead mites, live mites and the percentage of damage by feeding were registered daily during four days. These data was studied using analysis of variance of repeated measurements (ANOVArm). The number of immature mites was recorded on the fifth day and the demographic parameters (r, λ and DT) were calculated on the fourth day. These data was analyzed using one-way ANOVA, and in both cases, means were separated by Tukey’s multiple range comparison test (p ≤ 0.05). Finally, we correlated the mean number of eggs laid with average percent of live females, mean damage with average percent of live females and mean damage with average of eggs laid per of T. merganser female. The SAS/STAT software was used for all analyzes [22].
3. Results
3.1. Antixenosis
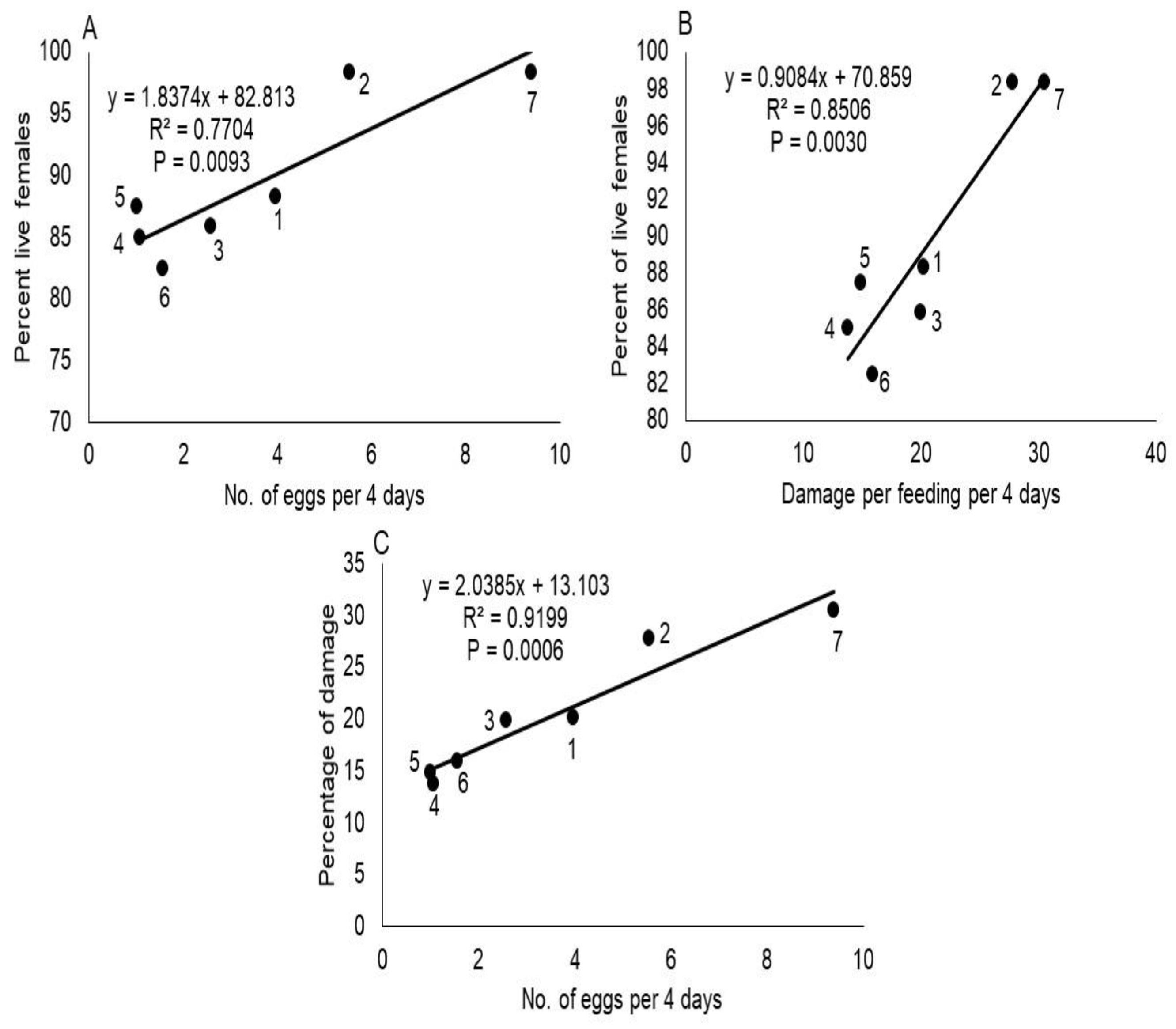
The number of T. merganser eggs laid per female differed significantly between host plants (F = 352.64; df = 6, 14; p < 0.0001), among observation time (F = 46.09; df = 3, 42; p < 0.0001) and host×time interaction (F = 19.30; df = 18, 42; p < 0.0001). The number of eggs laid on C. papaya was significantly higher while on P. tobira and T. ahouai were significantly lower (Tukey’s test, p < 0.05) (Table 2), this suggests P. tobira and T. ahouai were most resistant to T. merganser. Moreover, we found a positive correlation between fecundity and host plant species (Figure 1A).

Table 2.
Number mean of eggs laid per female of Tetranychus merganser on different species of host plants.
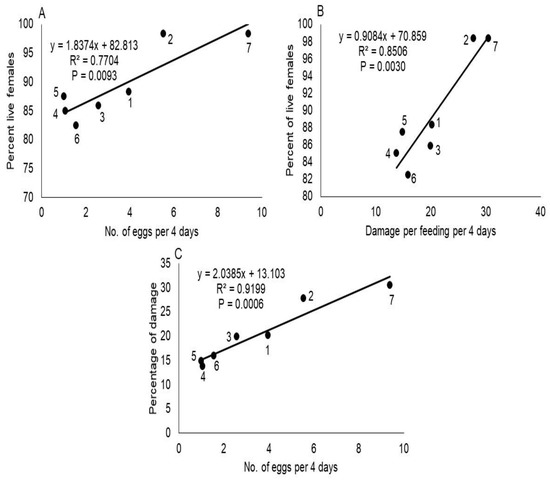
Figure 1.
The correlation between mean number of eggs laid with percent of live females (A), damage with percent of live females (B) and damage with mean number of eggs produced by these mites in 4 days (C), on different hosts plants. The plots in the figure represent different plant species: (1) Moringa oleifera, (2) Phaseolus vulgaris, (3) Capsicum annuum var. glabriusculum, (4) Thevetia ahouai, (5) Pittosporum tobira, (6) Helietta parvifolia, and (7) Carica papaya.
The feeding damage of T. merganser differed significantly between host plants (F = 26.19; df = 6, 14; p < 0.0001), both in relation to time (F = 473.13; df = 3, 42; p < 0.0001) and host×time interaction (F = 2.31; df = 18, 42; p = 0.0129). Damage was significantly greater in C. papaya and P. vulgaris, while in H. parvifolia, P. tobira and T. ahouai, it was significantly less (Table 3), which indicates H. parvifolia, P. tobira and T. ahouai were more resistant to T. merganser. Furthermore, we found a positive correlation between feeding damage and host plant species of red spider mites (Figure 1B), and among feeding damage and average of eggs laid per each T. merganser female (Figure 1C).

Table 3.
Percentage of feeding damage of Tetranychus merganser in different host plants.
3.2. Antibiosis
Infinitesimal rate of increase (r), finite rate of increase (λ), and doubling time (DT) of T. merganser were significantly different between host plants (F = 350.35; df = 6,14; p < 0.0001; F = 380.61; df = 6, 14; p < 0.0001; F = 222.09; df = 6,14; p < 0.0001), respectively. The highest average (±SE) of the r of T. merganser was observed in C. papaya (0.8482 ± 0.01) and the lowest r in P. tobira (0.3379 ± 0.00) and T. ahouai (0.3421 ± 0.01). The average number of mites added to the population per female per day (λ) was higher in the leaves of C. papaya (2.3356 ± 0.01) and lower in P. tobira (1.4021 ± 0.01) and T. ahouai (1.4080 ± 0.01). The average time in which the spider mite population doubled its population (DT) were greater in P. tobira (5.9212 ± 0.10) and T. ahouai (5.8532 ± 0.14), and less pronounced in C. papaya (2.3577 ± 0.01) (Table 4), which indicated P. tobira and T. ahouai more resistant to the development of T. merganser.

Table 4.
Demographic parameters of Tetranychus merganser on different host plants.
3.3. Mortality
The number of dead red spider mites differed significantly between host plants (F = 9.53; df = 6, 14; p < 0.0003), among observation time (F = 47.00; df = 3, 42; p < 0.0001) and host × time interaction (F = 2.58; df = 18, 42; p = 0.0058). In general, the largest percentage of dead mites was observed on H. parvifolia and T. ahouai, which suggests these plant species are more resistant to T. merganser (Table 5).

Table 5.
Percentage of mortality and survival of Tetranychus merganser on leaf squares of different host plants at different times.
3.4. Hatched Eggs of Tetranychus merganser
The number of eggs hatched on the fifth day differed significantly amongst host plants (F = 123.03; df = 6, 14; p < 0.0001). The percentage of hatched eggs was significantly higher on P. vulgaris than on H. parvifolia (Table 6). This indicates that the host plant species has an effect on the eggs laid during the first 24 h, which are supposed to hatch on the fifth day after laying.

Table 6.
Average number (±SE) of hatched eggs laid by Tetranychus merganser during the first 24 h.
4. Discussion
Oviposition is the first stage in the arthropod life cycle; it becomes exposed to the environment, and it is the most important event in the chain of interactions between herbivores and plants [23,24,25]. The number of eggs laid by T. merganser females differed amongst host plants, suggesting that plants influence red spider mite biology. In this study, the oviposition trend of T. merganser in relation to the host plants was: C. papaya > P. vulgaris > M. oleifera > C. annuum var. glabriusculum > H. parvifolia ≥ P. tobira ≥ T. ahouai. This indicates that the mite prefers to oviposit on caricaceous plants than on fabaceous, moringaceous, solanaceous, rutaceous, pittosporaceous and apocynaceous plants. Several studies have shown that Tetranychus spp. fecundity is related to the host plant species. Yano et al. [26] found a positive correlation between the mean number of eggs laid in 5 days and the host plant acceptance of T. urticae. They evaluated seven herbaceous plants (Houttuynia cordata (Saururaceae), Rumex crispus (Polygonaceae), Solidago altissima (Asteraceae), Cayratia japonica (Vitaceae), Desmodium sp. (Fabaceae), Rorippa indica (Brassicaceae) and Taraxacum officinale (Asteraceae)) and four cultivated ones (Fragaria (strawberry) sp. (Rosaceae), Chrysanthemum sp. (Asteraceae), P. lunatus (Fabaceae) and P. vulgaris (Fabaceae)), and found that T. urticae Koch lays more eggs on fabaceous plants. Further, De Lima et al. [27] documented that the total fecundity and daily oviposition rate of T. bastosi Tuttle, Baker & Sales were higher in C. papaya (50.6 ± 4.4), than on P. vulgaris (36.1 ± 2.5) and Manihot esculenta (Euphorbiaceae) (26.5 ± 2.2). Chacón-Hernández et al. [11] reported that T. merganser oviposited more on fabaceous plants than on solonaceous plants. Islam et al. [28] found that the fecundity of T. truncatus Ehara was greater on malvaceous plants (Corchorus capsularis L. (Malvaceae) (129.6 ± 3.95)) followed by fabaceous plants (Lablab purpureus L. (86.5 ± 3.08)) and caricaceous plants (C. papaya, 84.2 ± 3.59). Puspitarini et al. [29] documented the highest number of eggs laid per day and a higher total fecundity rate of T. urticae on both caricaceous and roseaceous plants compared to asteraceous plants. Greco et al. [30] found the mean of eggs per female per 5 days of T. urticae was lower on amaryllidaceous (Allium cepa L. and A. porrum L.) and apiaceous plants (Petroselinum sativum (Mill.) FUSS) than on rosaceous plants (Fragaria ananassa var. Selva Duch.), while Ullah et al. [9] reported a higher number of eggs per female red spider mite (75.91 ± 1.24 ≈ 15.18 eggs/female/day) on bean leaf discs during the first five days oviposition at 30 °C, RH 60–70% and photoperiod of L16: D8 h. Reyes-Pérez et al. [10] reported lower oviposition per T. merganser female (6.95 eggs/day) on C. papaya discs at 23 °C, photoperiod of 14:10 h light: dark and relative humidity of 60 ± 2%. It is likely that T. merganser females lay a higher numbers of eggs on C. papaya than on other plant species, this is caused by differences in nutrient contents, morphological characteristics and different secondary metabolites found in different plant families. [26,27,28,29,30].
The food intake was evaluated by the percentage of damage, which was significantly different between host plants. H. parvifolia, P. tobira and T. ahouai were the host plants that suffered less damage from the feeding of the red spider mite, which suggests that those three plants species possibly have different morphological characteristics or secondary metabolites that deter the feeding of T. merganser. Secondary metabolites like alkaloids, flavonoids, terpenes and phenols are produced by many plants species and are stored in the cell walls of leaves to deter feeding and oviposition of arthropods [15]. The diversity and quantity of these metabolites in plants affect herbivory species (e.g., oviposition, mate selection, and feeding); and are generally due to both genetic and environmental influences [16,31,32]. In this study, the plant species differ greatly in their suitability as hosts for T. merganser when measured in terms of fecundity and as resources for feeding. Studies suggest that a protein present in caricaceous, roseaceous, and asteraceous plants can increase the fecundity of T. urticae [29]. In this regard, Islam et al. [28] mention that this occurs because adult females need feeding resources (e.g., nitrogen and carbohydrate) to develop mature ovaries and eggs and obtain energy.
The demographic parameters measured in this experiment (r, λ and DT) indicate that T. merganser population growth differ between host plants species. These parameters demonstrated that T. merganser had the best performance on C. papaya. This is mainly due to higher egg production (9.39 eggs per 4 days) and low mortality of mites. Chacón-Hernández et al. [11] reported values of r of T. merganser on fabaceous (0.4237) and solanaceous (0.6014) plants, lower than those obtained in this present study (0.7133 and 0.5568, respectively). De Lima et al. [27] found that T. bastosi had the best reproduction rates on both caricaceous and fabaceous plants than on euphorbiaceous plants. There are a variety of factors influencing the population growth parameters of tetraniquids, e.g., host plant species, quality of the host plant, strain of mite, environmental factors, plant breeding method and data analysis method [14,26,27,29,33].
In this research, the percentage of eggs hatched on the fifth day after laying differed between host plants, although more in-depth research is required to understand these differences. Plants initiate the attack on arthropods when they lay their eggs on the leaves [34,35]. Hilker and Fatouros [24] and Beyaert et al. [36] mention that the laying of eggs by herbivorous arthropods induces secondary metabolites in the plant that can prevent the hatching of the eggs or can generate a detachment of the part of the leaf where the egg was laid by the herbivore. Hilker and Meiners [37] mentioned that high concentrations of volatile terpenes could enter the egg via the aeropyles in the outermost layer, i.e., the protein chorion and expand through the wax layer, thus reaching the embryo behind the vitelline envelope and serosa which would cause the death of the egg.
5. Conclusions
To conclude, the results show that the host plants H. parvifolia, P. tobira and T. ahouai present antibiosis and antixenosis on the red spider mite, causing a lower oviposition and feeding of T. merganser, a low growth rate in its population and a higher period of time to double the population of red spider mites. Therefore, these plant species can be studied as alternatives in the management of T. merganser.
Author Contributions
Conceptualization, J.C.C.H. and G.T.-B.; methodology, J.C.C.H., A.H.-J. and S.G.M.-R.; validation, J.C.C.H.; formal analysis, J.C.C.H. and S.O.-S.; resources, G.G.-G.; data curation, J.C.C.H. and G.T.-B.; writing—original draft preparation, J.C.C.H. and G.T.-B.; writing—review and editing, J.C.C.H., G.T.-B., and S.G.M.-R.; visualization, G.G.-G., A.H.-J., and S.O.-S.; supervision, J.C.C.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Migeon, A.; Dorkeld, F. Spider Mites Web: A Comprehensive Data Base for the Tetranychidae. Available online: http://www1.montpellier.inra.fr/CBGP/spmweb (accessed on 6 September 2021).
- Fathipour, Y.; Maleknia, B. Mite Predators. In Ecofriendly Pest Management for Food Security; Omkar, Ed.; Elsevier, Academic Press: London, UK, 2016; pp. 329–366. [Google Scholar] [CrossRef]
- Lima-Espíndola, J.; Vanegas-Rico, J.M. Damage caused by Tetranychus merganser Boudreaux (Acari: Tetranychidae) on nopal verdura Opuntia ficus-indica (L.) Miller during winter. JEAR 2017, 49, 121–122. [Google Scholar] [CrossRef][Green Version]
- Valencia-Domínguez, H.M.; Otero-Colina, G.; Santillán-Galicia, M.T.; Hernández-Castro, E. Acarofauna en papaya var. Maradol (Carica papaya L.) en el estado de Yucatán, México. Entomotropica 2011, 26, 17–30. [Google Scholar]
- Monjarás-Barrera, J.I.; Lara-Villalón, M.; Juárez-Aragón, M.C.; Torres-Castillo, J.A. New Report of Tetranychus merganser Boudreaux and Oligonychus punicae Hirst on Moringa oleifera Lam. Southwest. Entomol. 2015, 40, 1103–1105. [Google Scholar] [CrossRef]
- Monjarás-Barrera, J.I.; Chacón-Hernández, J.C.; Mora-Olivo, A.; Guerra-Pérez, A.; de Jesús Yáñez-Pacheco, M. New Record of Tetranychus merganser Boudreaux on Thevetia ahouai L. and Acacia farnesiana (L.) Willd at Ciudad Victoria, Tamaulipas, Mexico. Southwest. Entomol. 2017, 42, 847–849. [Google Scholar] [CrossRef]
- Monjarás-Barrera, J.I.; Ordaz-Silva, S.; Heinz-Castro, R.T.Q.; López-Sánchez, I.V.; Pedro-Méndez, J.G.; Chacón-Hernández, J.C. Two New Hosts of Tetranychus merganser Boudreaux in Northeastern Mexico: Pittosporum tobira (Pittosporaceae) and Helietta parvifolia (Rutaceae). Southwest. Entomol. 2020, 3, 819–822. [Google Scholar] [CrossRef]
- Monjarás-Barrera, J.I.; Chacón-Hernández, J.C.; Silva, G.L.; Johann, L.; Santos-Silva, O.; Landeros-Flores, J.; Vanoye-Eligio, V.; Reyes-Zepeda, F.; Juarez-Ferla, N. Mites associated to chile piquín (Capsicum annuum L. var. glabriusculum) in two Protect Natural Areas in Northeastern México. Syst. Appl. Acarol. 2019, 24, 2537–2551. [Google Scholar] [CrossRef]
- Ullah, M.S.; Moriya, D.; Badii, M.H.; Nachman, G.; Gotoh, T. A comparative study of development and demographic parameters of Tetranychus merganser and Tetranychus kanzawai (Acari: Tetranychidae) at different temperatures. Exp. Appl. Acarol. 2011, 54, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Pérez, N.; Villanueva-Jiménez, J.A.; de la Cruz Vargas-Mendoza, M.; Cabrera-Mireles, H.; Otero-Colina, G. Parámetros poblacionales de Tetranychus merganser Boudreaux (Acari: Tetranychidae) en papayo (Carica papaya L.) a diferentes temperaturas. Agrociencia 2013, 47, 147–157. [Google Scholar]
- Chacón-Hernández, J.C.; Ordaz-Silva SMireles-Rodriguez, E.; Rocandio- Rodríguez, M.; López-Sánchez, I.V.; Heinz-Castro, R.T.Q.; Reyes-Zepeda, F.; Castro-Nava, S. Resistance of wild chili (Capsicum annuum L. var. glabriusculum) to Tetranychus merganser1 Boudreaux. Southwest. Entomol. 2020, 45, 89–98. [Google Scholar] [CrossRef]
- Ullah, M.S.; Moriya, D.; Kongchuensin, M.; Konvipasruang, P.; Gotoh, T. Comparative toxicity of acaricides to Tetranychus merganser Boudreaux and Tetranychus kanzawai Kishida (Acari: Tetranychidae). Int. J. Acarol. 2011, 37, 535543. [Google Scholar] [CrossRef]
- Heinz-Castro, R.T.Q.; Arredondo-Valdés, R.; Ordaz-Silva, S.; Méndez-Cortés, H.; Hernández-Juárez, A.; Chacón-Hernández, J.C. Bioacaricidal potential of Moringa oleifera ethanol extract for Tetranychus merganser Boudreaux (Acari: Tetranychidae) control. Plants 2021, 10, 1034. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, M.E.; Arnaiz, A.; Rosa-Diaz, I.; González-Melendi, P.; Romero-Hernandez, G.; Ojeda-Martinez, D.A.; Garcia, A.; Contreras, E.; Martinez, M.; Diaz, I. Plant defenses against Tetranychus urticae: Mind the gaps. Plants 2020, 9, 464. [Google Scholar] [CrossRef]
- Smith, C.M. Plant Resistance to Arthropods: Molecular and Conventional Approaches; Springer: Dordretcht, The Netherlands, 2005; p. 423. [Google Scholar]
- Smith, C.M.; Clement, S.L. Molecular bases of plant resistance to arthropods. Annu. Rev. Entomol. 2012, 57, 309–328. [Google Scholar] [CrossRef] [PubMed]
- Agut, B.; Pastor, V.; Jaques, J.A.; Flors, V. Can plant defence mechanisms provide new approaches for the sustainable control of the two-spotted spider mite Tetranychus urticae? Int. J. Mol. Sci. 2018, 19, 614. [Google Scholar] [CrossRef]
- Marinosci, C.; Magalhaes, S.; Macke, E.; Navajas, M.; Carbonell, D.; Devaux, C.; Olivieri, I. Effects of host plant on life-history traits in the polyphagous spider mite Tetranychus urticae. Ecol. Evol. 2015, 5, 3151–3158. [Google Scholar] [CrossRef]
- Ahmadi, A. Demographic toxicology as a method for studying the dicofol two spotted spider mite (Acari: Tetranychidae) system. J. Econ. Entomol. 1983, 76, 239242. [Google Scholar] [CrossRef]
- Nachman, G.; Zemek, R. Interactions in a tritrophic acarine predator-prey metapopulation system III: Effects of Tetranychus urticae (Acari: Tetranychidae) on host plant condition. Exp. Appl. Acarol. 2002, 26, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Birch, L.C. The intrinsic rate of increase of an insect population. J. Anim. Ecol. 1948, 17, 15–26. [Google Scholar] [CrossRef]
- SAS Institute. SAS/STAT 9.1: User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2002; pp. 3703–3796. [Google Scholar]
- Hilker, M.; Fatouros, T. Plant Responses to insect egg deposition. Annu. Rev. Entomol. 2015, 60, 493–515. [Google Scholar] [CrossRef]
- Khaliq, A.; Ahmad, M.H.; Ullah, R.; Anas, M. Behavioral ecology of oviposition in insects—A dumpy review. SJAR 2015, 4, 1–7. [Google Scholar]
- War, A.R.; Taggar, G.K.; Hussain, B.; Taggar, M.S.; Nair, R.M.; Sharma, H.H. Plant defence against herbivory and insect adaptations. AoB PLANTS 2018, 10, ply037. [Google Scholar] [CrossRef]
- Yano, S.; Wakabayashi, M.; Takabayashi, J.; Takafuji, A. Factors determining the host plant range of the phytophagous mite, Tetranychus urticae (Acari: Tetranychidae): A method for quantifying host plant acceptance. Exp. Appl. Acarol. 1998, 22, 595–601. [Google Scholar] [CrossRef]
- De lima, R.P.; Becerra, M.M.; de Moraes, G.J.; Furtado, I. Life table of the red spider mite Tetranychus bastosi (Acari: Tetranychidae) on different host plants. Acarologia 2017, 57, 601–605. [Google Scholar] [CrossRef]
- Islam, M.T.; Jahan, M.; Gotoh, T.; Ullah, M.S. Host-dependent life history and life table parameters of Tetranychus truncatus (Acari: Tetranychidae). Syst. Appl. Acarol. 2017, 22, 2068–2082. [Google Scholar] [CrossRef]
- Puspitarini, R.D.; Fernando, I.; Rachmawati, R.; Hadi, M.S.; Rizali, A. Host plant variability affects the development and reproduction of Tetranychus urticae. Int. J. Acarol. 2021, 47, 381–386. [Google Scholar] [CrossRef]
- Greco, N.M.; Pereyra, P.C.; Guillade, A. Host-plant acceptance and performance of Tetranychus urticae (Acari, Tetranychidae). J. Appl. Entomol. 2006, 130, 32–36. [Google Scholar] [CrossRef]
- Städler, E. Plant chemical cues important for egg deposition by herbivorous insects. In Chemoecology of Insect Eggs and Egg Deposition; Hilker, M., Meiners, T., Eds.; Blackwell: Oxford, UK, 2002; pp. 171–204. [Google Scholar]
- Chapman, R.F. Contact chemoreception in feeding by phytophagous insects. Annu. Rev. Entomol. 2003, 48, 455–484. [Google Scholar] [CrossRef]
- Bazazzadeh, F.; Shishehbor, P.; Esfandiari, M.; Farahi, S. Development, reproduction and life table parameters of Tetranychus turkestani (Acari: Tetranychidae) on three different host plants. Acarologia 2020, 60, 643–655. [Google Scholar] [CrossRef]
- Kim, J.; Tooker, J.F.; Luthe, D.S.; De-Moraes, C.M.; Felton, G.W. Insect eggs can enhance wound response in plants: A study system of tomato Solanum lycopersicum L. and Helicoverpa zea Boddie. PLoS ONE 2012, 7, e37420. [Google Scholar] [CrossRef] [PubMed]
- Lortzing, V.; Oberländer, J.; Lortzing, T.; Tohge, T.; Steppuhn, A.; Kunze, R.; Hilker, M. Insect egg deposition renders plant defense against hatching larvae more effective in a salicylic acid-dependent manner. Plant Cell. Environ. 2019, 42, 1019–1032. [Google Scholar] [CrossRef]
- Beyaert, I.; Köpke, D.; Stiller, J.; Hammerbacher, A.; Yoneya, K.; Schmidt, A.; Gershenzon, J.; Hilker, M. Can insect egg deposition ‘warn’ a plant of future feeding damage by herbivorous larvae? Proc. R. Soc. B. 2012, 279, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Hilker, M.; Meiners, T. Plants and insect eggs: How do they affect each other? Phytochemistry 2011, 72, 1612–1623. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).