Comparison of the Ecological Traits and Boring Densities of Aromia bungii (Faldermann, 1835) (Coleoptera: Cerambycidae) in Two Host Tree Species
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Log Collection
2.2. Collection and Rearing of A. bungii
2.3. Dissection of Sample Logs
2.4. Statistical Analyses
3. Results
3.1. Comparison of Log Size between Tree Species
3.2. The Number and Sex Ratio of Emerging Adults
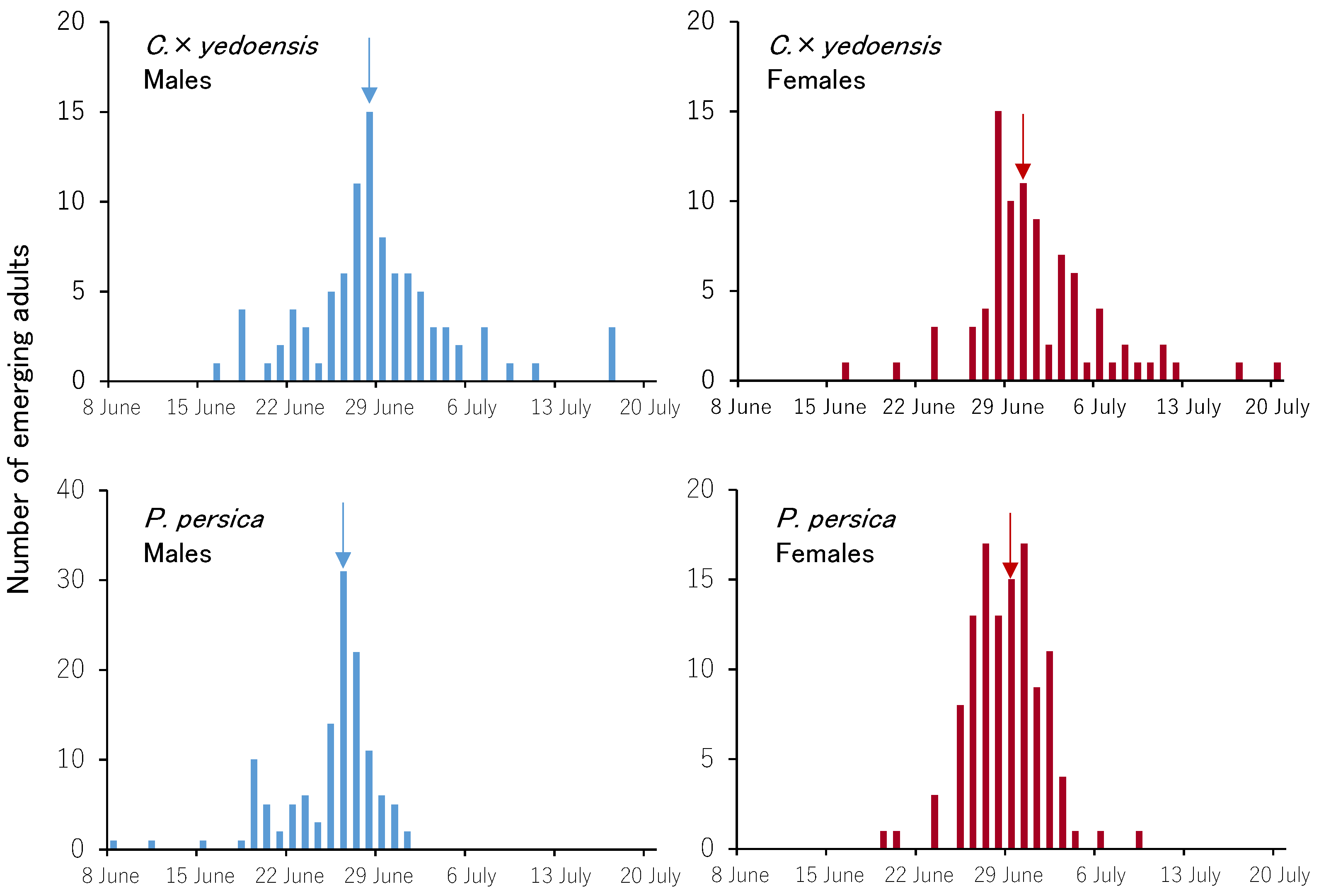
3.3. Emergence Trends of Adults
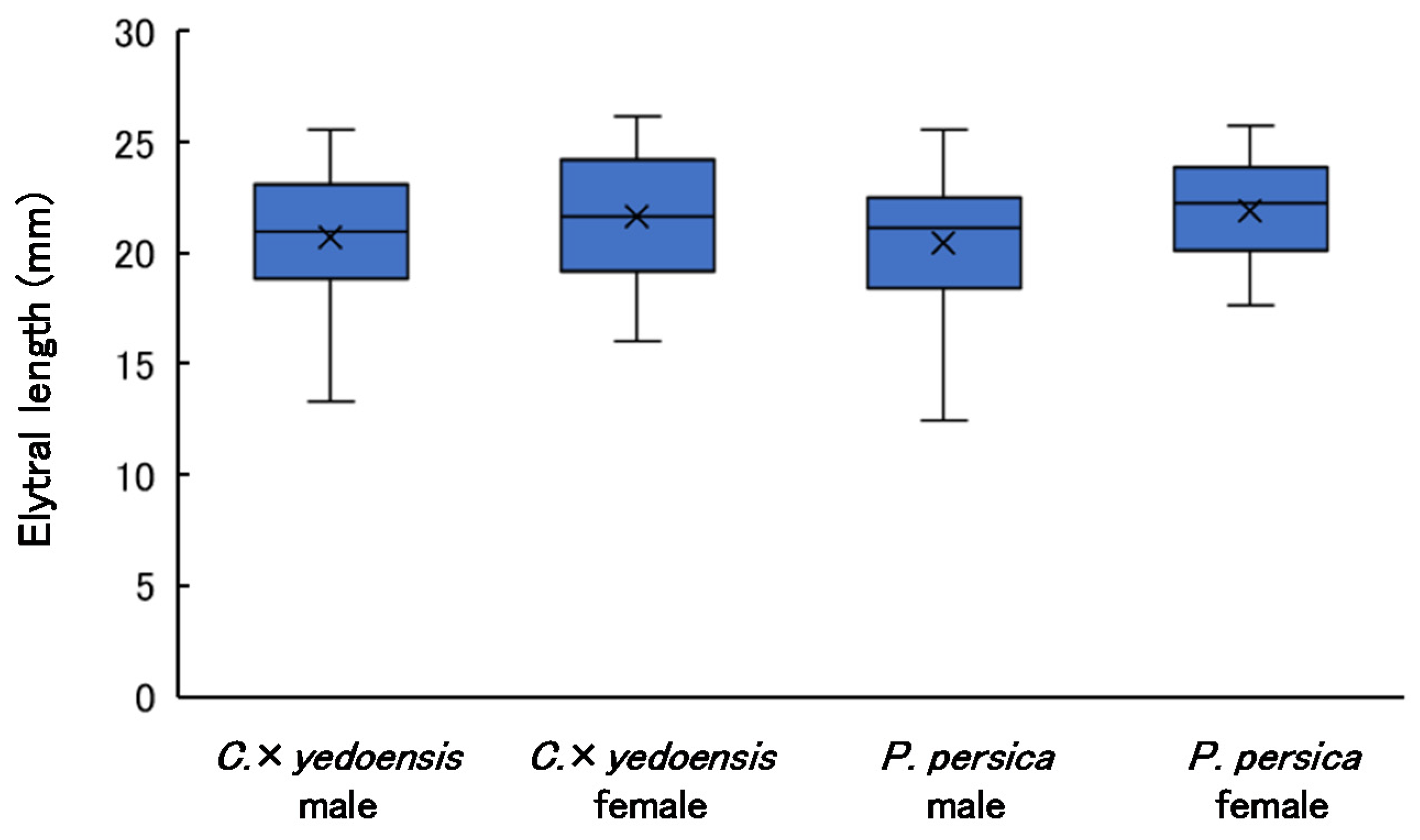
3.4. Elytral Length of Adults
3.5. Adult Lifespan
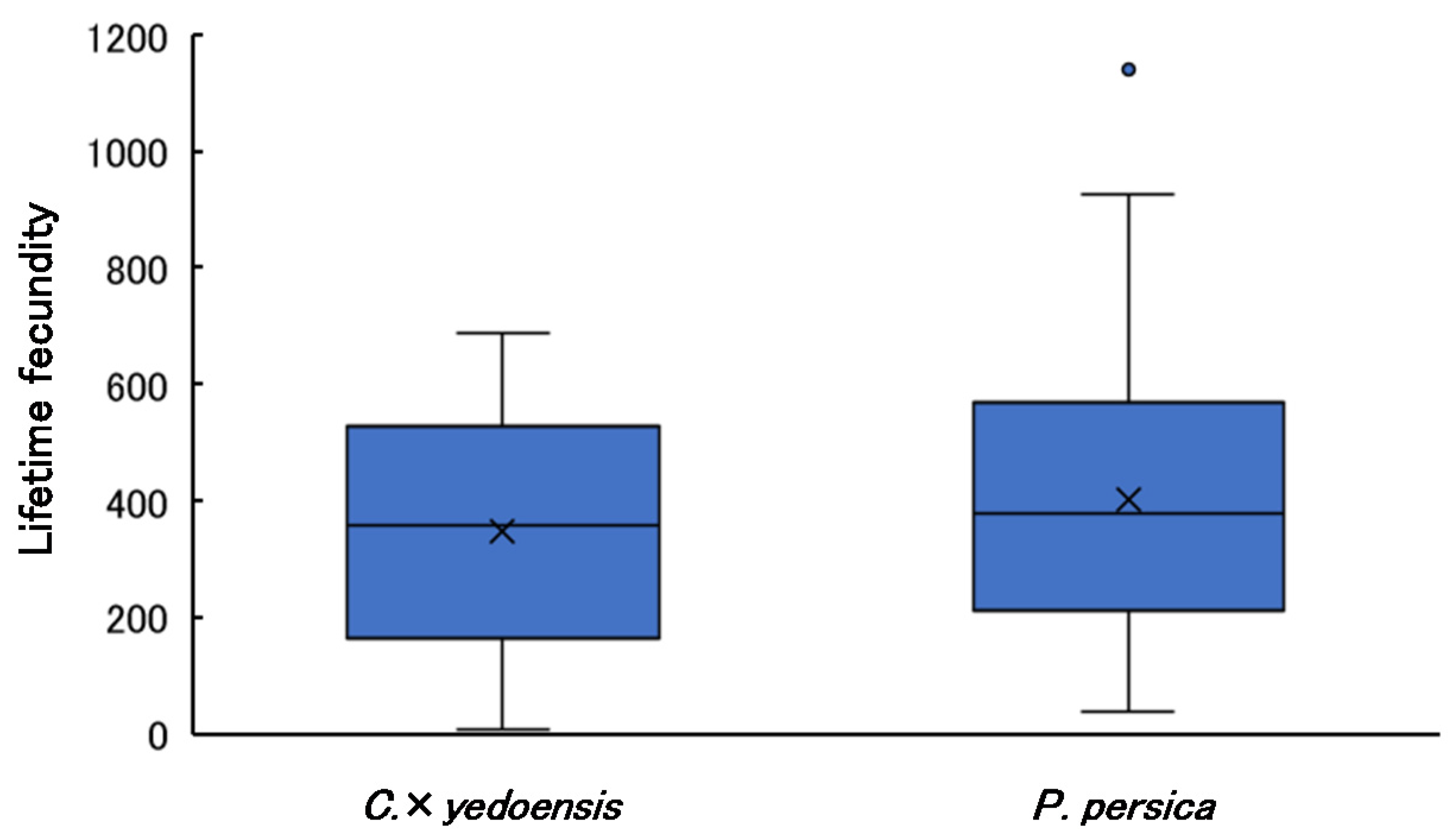
3.6. Lifetime Fecundity
3.7. Dissection of Sample Logs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iwata, R. Aromia bungii (Coleoptera: Cerambycidae): Taxonomy, distribution, biology and eradication. For. Pests 2018, 67, 189–216. (In Japanese) [Google Scholar]
- Burmeister, E.-G.; Hendrich, L.; Balke, M. Der Asiatische Moschsbock Aromia bungii (Faldermann 1835): Erstfund für Deutschland (Coleoptera: Cerambycidae). Nachr. Bayer. Entomol. 2012, 61, 29–31. (In German) [Google Scholar]
- Garonna, A.P.; Nugnes, F.; Espinosa, B.; Griffo, R.; Benchi, D. Aromia bungii, nuovo tarlo asiatico ritrovato in Campania. Inf. Agrar. 2013, 69, 60–62. (In Italian) [Google Scholar]
- Wang, J.-T.; Sun, L.-W.; Liu, T.-Z.; Zhang, L.-Y. Research on the occurrence character and control measures of Aromia bungii. J. Hebei Agric. Sci. 2007, 11, 41–43. (In Chinese) [Google Scholar]
- Gressitt, J.L. Destructive long-horned beetle borers at Canton, China. Spec. Publ. Lingnan Nat. Hist. Surv. Mus. 1942, 1, 1–60. [Google Scholar]
- Liu, B.-S. Biology and control of Aromia bungii Faldermann. China Fruits 1982, 2, 45–49. (In Chinese) [Google Scholar]
- Lu, Y.-P. Studies on the biological character of Aromia bungii Faldermann and control techniques of the insects in various states. J. Henan Agric. Sci. 1995, 7, 25–27. (In Chinese) [Google Scholar]
- Zhao, S.-X.; Fan, Q.-H.; Den, X.-Y. A survey of insects living on nane tree from Fujian. Wuyi Sci. J. 1997, 13, 188–192. (In Chinese) [Google Scholar]
- Yu, G.-P.; Gao, B.-N. Bionomics of Aromia bungii. For. Pest Dis. 2006, 24, 15–16. (In Chinese) [Google Scholar]
- Kano, M.; Nonaka, T.; Kiriyama, S.; Iwata, R. Aromia bungii (Coleoptera: Cerambycidae), an invasive cerambycid, found at Soka, Saitama Pref., Japan, infesting cherry trees, Cerasus × yedoensis ‘Somei-yoshino’. For. Pests 2014, 63, 101–105. (In Japanese) [Google Scholar]
- Kobayashi, A. The actual damage situation of cherry trees caused by Aromia bungii and Scolytidae. Tree For. Health 2018, 22, 31–35. (In Japanese) [Google Scholar] [CrossRef]
- Shoda-Kagaya, E. Invasion of the red-necked longicorn beetle, Aromia bungii: Damage of Rosaceae trees and practical control methods. Tree For. Health 2018, 22, 68–72. (In Japanese) [Google Scholar] [CrossRef]
- Nakano, A.; Watanabe, T. Damage caused by the red-necked longhorn beetle, Aromia bungii in peach orchards in Tokushima Prefecture and a trial control method. Plant Prot. 2017, 71, 723–728. (In Japanese) [Google Scholar]
- Haruyama, N.; Yaita, S.; Fukuda, T. Larvae location and head width distribution of red-necked longhorn beetle Aromia bungii (Faldermann) inside peach tree. Ann. Rep. Kanto-Tosan Plant Prot. Soc. 2020, 67, 79–83. (In Japanese) [Google Scholar]
- Love, C.N.; Hill, M.P.; Moore, S.D. Thaumatotibia leucotreta and the Navel orange: Ovipositional preferences and host susceptibility. J. Appl. Entomol. 2014, 138, 600–611. [Google Scholar] [CrossRef]
- Shibata, E. Effects of Japanese cedar inner bark nutritional quality on development of Semanotus japonicus (Coleoptera: Cerambycidae). Environ. Entomol. 1998, 27, 1431–1436. [Google Scholar] [CrossRef]
- Haruyama, N.; Yaita, S.; Fukuda, T.; Yamazaki, K.; Watanabe, H.; Handa, M. Peach orchard damage caused by the invasive red-necked longhorn beetle Aromia bungii (Faldermann) in Tochigi Prefecture. Ann. Rep. Kanto-Tosan Plant Prot. Soc. 2019, 66, 106–109. (In Japanese) [Google Scholar] [CrossRef]
- The R Project for Statistical Computing. Available online: http://www.r-project.org/index.html (accessed on 5 May 2021).
- Ma, W.-H.; Sun, L.-Y.; Yu, L.-G.; Wang, J.-T.; Chen, J.-Y. Study on the occurrence and life history of Aromia bungii (Faldermann). Acta Agric. Boreali-Sin. 2007, 22, 247–249. (In Chinese) [Google Scholar]
- Hu, C.-X.; Ding, Y.-H.; Sun, K. Research advances of Aromia bungii in China. Agric. Technol. 2007, 27, 63–67. (In Chinese) [Google Scholar]
- Japan Meteorological Agency. Available online: https://www.data.jma.go.jp/obd/stats/data/en/smp/index.htm (accessed on 6 October 2021).
- Urano, T.; Shoda-Kagaya, E. Longevity and lifetime fecundity of rearing adults of Aromia bungii (Coleoptera: Cerambycidae). Kanto J. For. Res. 2017, 68, 25–28. (In Japanese) [Google Scholar]
- Russo, E.; Nugnes, F.; Vicinanza, F.; Garonna, A.P.; Bernardo, U. Biological and molecular characterization of Aromia bungii (Faldermann, 1835) (Coleoptera: Cerambycidae), an emerging pest of stone fruits in Europe. Sci. Rep. 2020, 10, 7112. [Google Scholar] [CrossRef]
- Adachi, I. Reproductive biology of the white-spotted longicorn beetle, Anoplophora malasiaca Thomson (Coleoptera: Cerambycidae), in citrus trees. Appl. Entomol. Zool. 1988, 23, 256–264. [Google Scholar] [CrossRef]
- Iba, M. Effect of adult feeding on the sexual maturation and the oviposition of the yellow-spotted longicorn beetle, Psacothea hilaris Pascoe. J. Sericul. Sci. Jpn. 1982, 51, 223–227. (In Japanese) [Google Scholar] [CrossRef]
- Jikumaru, S.; Togashi, K.; Taketsune, A.; Takahashi, F. Oviposition biology of Monochamus saltuarius (Coleoptera: Cerambycidae) at a constant temperature. Appl. Entomol. Zool. 1994, 29, 555–561. [Google Scholar] [CrossRef]
- Kato, K.; Yamada, H.; Shibata, E. Role of female adult size in reproductive fitness of Semanotus japonicas (Coleoptera: Cerambycidae). Appl. Entomol. Zool. 2000, 35, 327–331. [Google Scholar] [CrossRef][Green Version]
- Togashi, K. Lifetime fecundity and body size of Monochamus alternatus (Coleoptera: Cerambycidae) at a constant temperature. Jpn. J. Entomol. 1997, 65, 458–470. [Google Scholar]
- Togashi, K. Lifetime fecundity and female body size in Paraglenea fortunei (Coleoptera: Cerambycidae). Appl. Entomol. Zool. 2007, 42, 549–556. [Google Scholar] [CrossRef]
- Togashi, K.; Yamashita, H. Effects of female body size on lifetime fecundity of Monochamus urussovii (Coleoptera: Cerambycidae). Appl. Entomol. Zool. 2017, 52, 79–87. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Ishikawa, Y. Occurrence of Aromia bungii in Osaka Prefecture. Annu. Rep. Kansai Plant Prot. Soc. 2018, 60, 17–21. (In Japanese) [Google Scholar] [CrossRef]
- Lu, Z.; Yu, Z.; Heong, K.; Cui, H.U. Effect of nitrogen fertilizer on herbivores and its stimulation to major insect pests in rice. Rice Sci. 2007, 141, 56–66. [Google Scholar] [CrossRef]
- Rashid, M.M.; Jahan, M.; Islam, K.S. Impact of nitrogen, phosphorus and potassium on brown planthopper and tolerance of its host rice plants. Rice Sci. 2016, 23, 119–131. [Google Scholar] [CrossRef]
- Aqueel, M.A.; Leather, S.R. Effect of nitrogen fertilizer on the growth and survival of Rhopalosiphum padi (L.) and Sitobion avenae (F.) (Homoptera: Aphididae) on different wheat cultivars. Crop Prot. 2011, 30, 216–221. [Google Scholar] [CrossRef]
- Yardim, E.N.; Edwards, C.A. Effects of organic and synthetic fertilizer sources on pest and predatory insects associated with tomatoes. Phytoparasitica 2003, 31, 324–329. [Google Scholar] [CrossRef]
- Jansson, R.K.; Leibee, G.L.; Sanchez, C.A.; Lecrone, S.H. Effects of nitrogen and foliar biomass on population parameters of cabbage insects. Entomol. Exp. Appl. 1991, 61, 7–16. [Google Scholar] [CrossRef]
- Weinbaum, S.A.; Johnson, R.S.; DeJong, T.M. Causes and consequences of overfertilization in orchards. HortTechnology 1992, 2, 112–121. [Google Scholar] [CrossRef]
- Heijari, J.; Nerg, A.M.; Kainulainen, P.; Noldt, U.; Levula, T.; Raitio, H.; Holopainen, J.K. Effect of long-term forest fertilization on Scots pine xylem quality and wood borer performance. J. Chem. Ecol. 2008, 34, 26–31. [Google Scholar] [CrossRef]
- Shoda-Kagaya, E. Why Aromia bungii became a threat in Japan? Shinrin Kagaku 2020, 89, 2–5. (In Japanese) [Google Scholar]



| C. × yedoensis | P. persica | |
|---|---|---|
| Total tree volume (m3) | 3.32 | 0.60 |
| Number of males | 70 | 126 |
| Number of females | 64 | 115 |
| Number of males/volume | 21.07 | 208.34 |
| Number of females/volume | 19.26 | 190.15 |
| Proportion of males | 0.52 | 0.52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urano, T.; Taki, H.; Shoda-Kagaya, E. Comparison of the Ecological Traits and Boring Densities of Aromia bungii (Faldermann, 1835) (Coleoptera: Cerambycidae) in Two Host Tree Species. Insects 2022, 13, 151. https://doi.org/10.3390/insects13020151
Urano T, Taki H, Shoda-Kagaya E. Comparison of the Ecological Traits and Boring Densities of Aromia bungii (Faldermann, 1835) (Coleoptera: Cerambycidae) in Two Host Tree Species. Insects. 2022; 13(2):151. https://doi.org/10.3390/insects13020151
Chicago/Turabian StyleUrano, Tadahisa, Hisatomo Taki, and Etsuko Shoda-Kagaya. 2022. "Comparison of the Ecological Traits and Boring Densities of Aromia bungii (Faldermann, 1835) (Coleoptera: Cerambycidae) in Two Host Tree Species" Insects 13, no. 2: 151. https://doi.org/10.3390/insects13020151
APA StyleUrano, T., Taki, H., & Shoda-Kagaya, E. (2022). Comparison of the Ecological Traits and Boring Densities of Aromia bungii (Faldermann, 1835) (Coleoptera: Cerambycidae) in Two Host Tree Species. Insects, 13(2), 151. https://doi.org/10.3390/insects13020151






