Quick Spreading of Populations of an Exotic Firefly throughout Spain and Their Recent Arrival in the French Pyrenees
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection and Analysis
- −
- GusanosdeLuz http://www.gusanosdeluz.com/ (gusanosdeluz@gmail.com (GDL))
- −
- Grup Cucadellum https://cucadellum.cat/ Institució Catalana d’Historia Natural (info@cucadellum.cat) (GC)
- −
- Observatoire des Vers Luisants et des lucioles http://www.asterella.eu/ (OVL)
- −
- Biodiversidad Virtual www.biodiversidadvirtual.org/
- −
- iNaturalist www.inaturalist.org/
- −
- EcoRegistros www.ecoregistros.org
2.2. Field and Photographic Identification Characteristics of Photinus signaticollis
- Morphologically, the adults have a body plan like most Photinus species (especially nocturnal and flashing species [2,13] (see Figure 1 within), with lateral pinkish markings (curved thickened lines to oval spots) around the dark brown, almost square, central spot on the pronotum. The pinkish markings are laterally flanked by dark areas, with a similar color to the dark median spot (absent in other nocturnal Photinus species, where, usually, pale colors laterally flank the pinkish areas on the pronotum); most commonly, the pink markings show as lateral pink lines, slightly curved towards the center of the pronotum, with a faint thickening in the center on the hollow side of the pink line facing outwards.
- Greyish-brown color of elytra with clear pale margins and often a more or less apparent (sometimes absent) pale vitta (shoulder stripe) reaching from the shoulder to (almost) the pale margin at the tip of the elytrum. Shoulder vittae are uncommon in other Photinus spp. and are more typically found in Photuris spp.
- Elytra shape often taper (broadest at shoulders) and are sometimes parallel; elytra are not always touching in the middle, often slightly opening towards the apex.
2.3. Spanish Collection and Deposit of Specimens
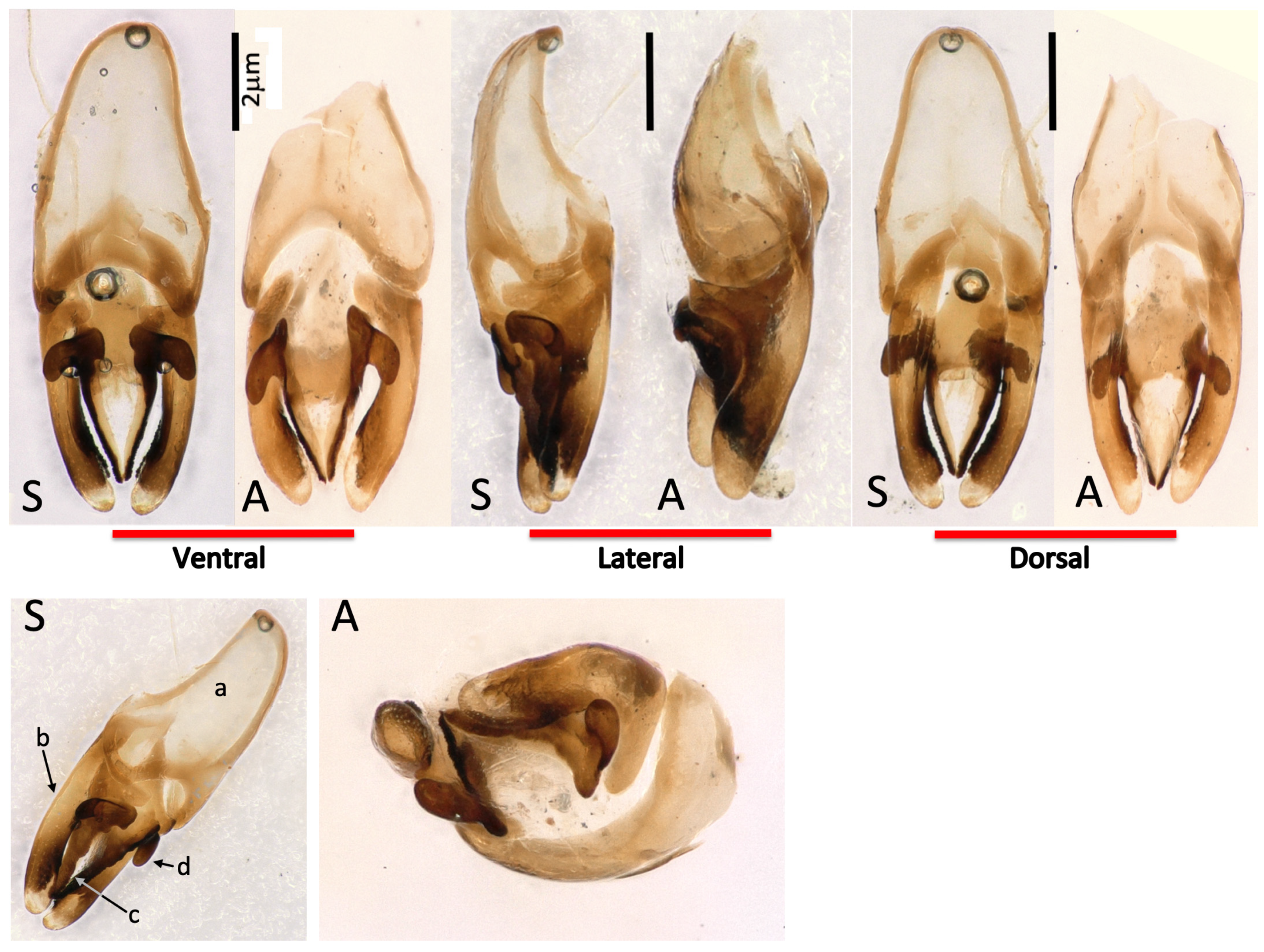
2.4. Aedeagus Preparation
2.5. Original Description of P. signaticollis by Blanchard
3. Results
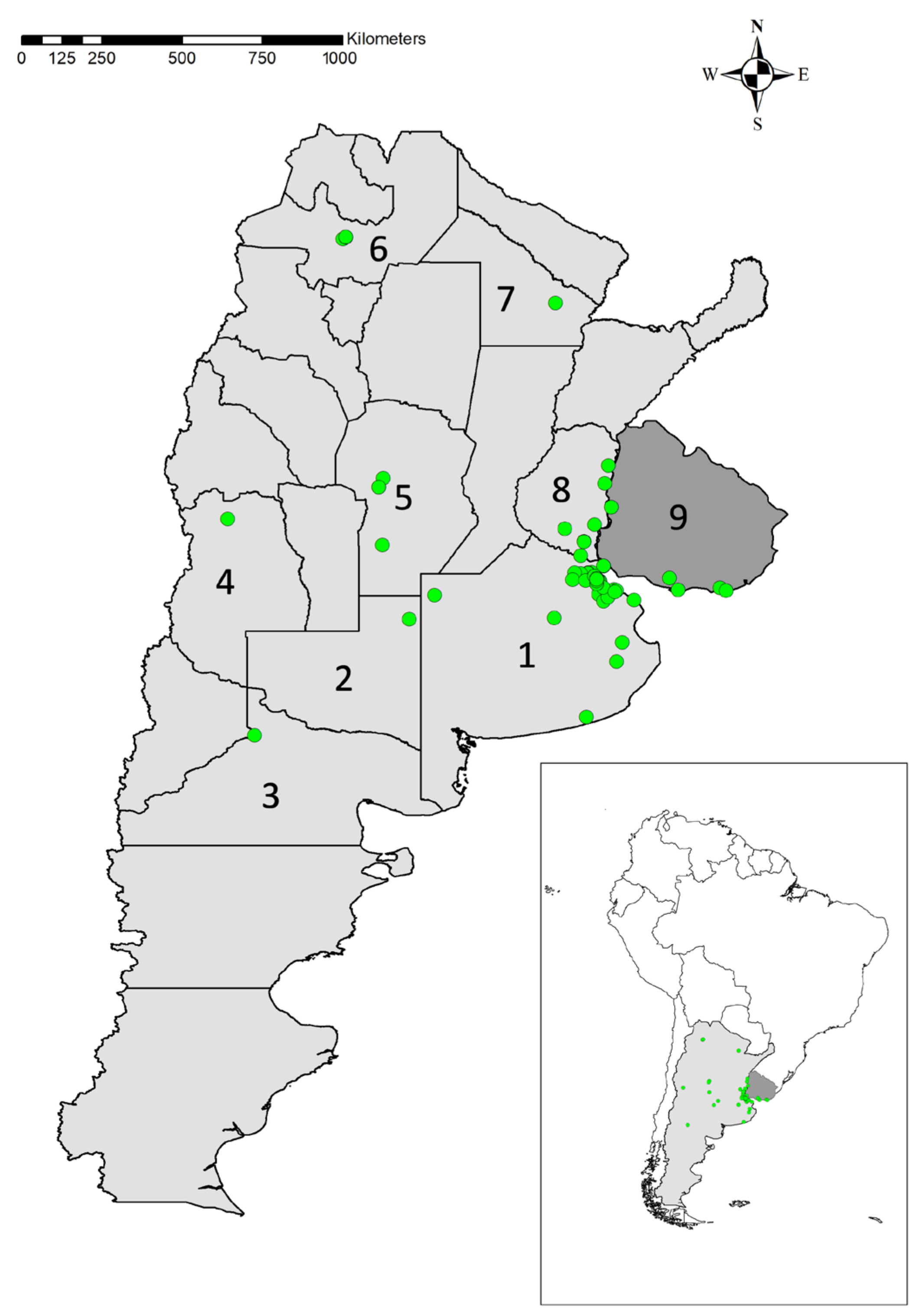
3.1. Initial Spanish Observations
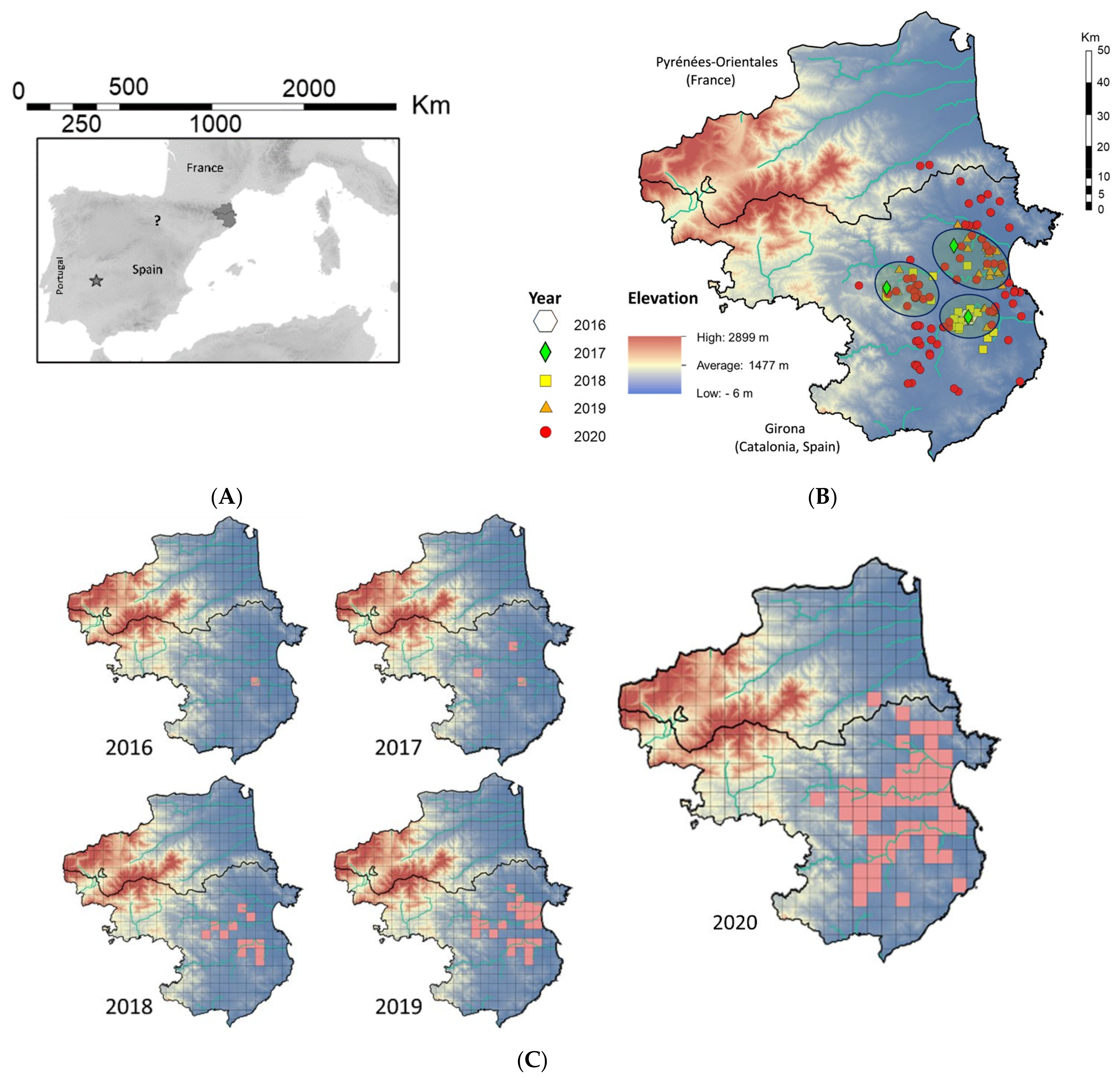
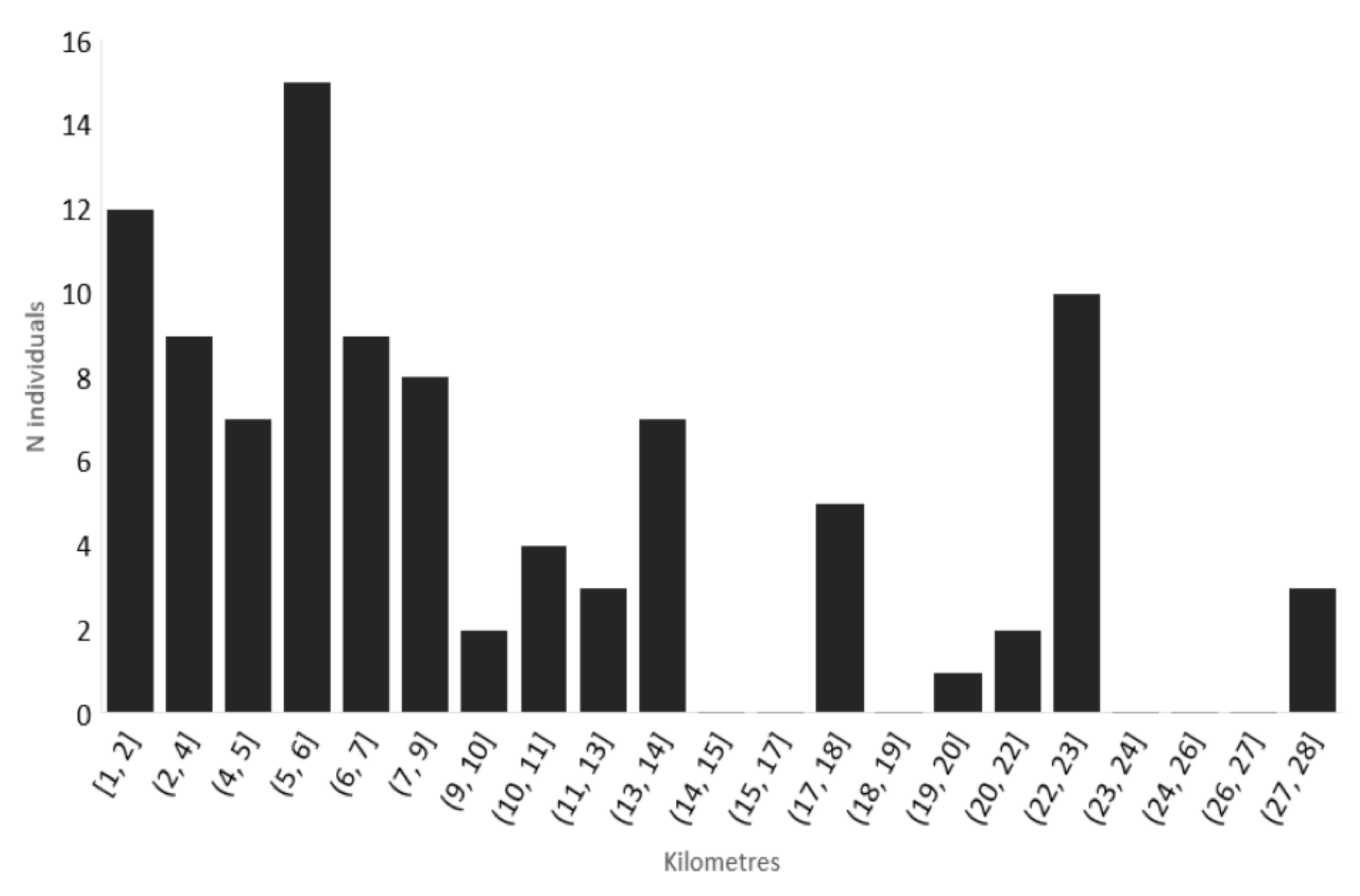
3.2. Expansion Rate
3.3. French Observations
3.4. Extremadura Observations
3.5. La Rioja Information
3.6. Synonymisation of Photinus Immigrans and Photinus signaticollis (Blanchard, 1846)
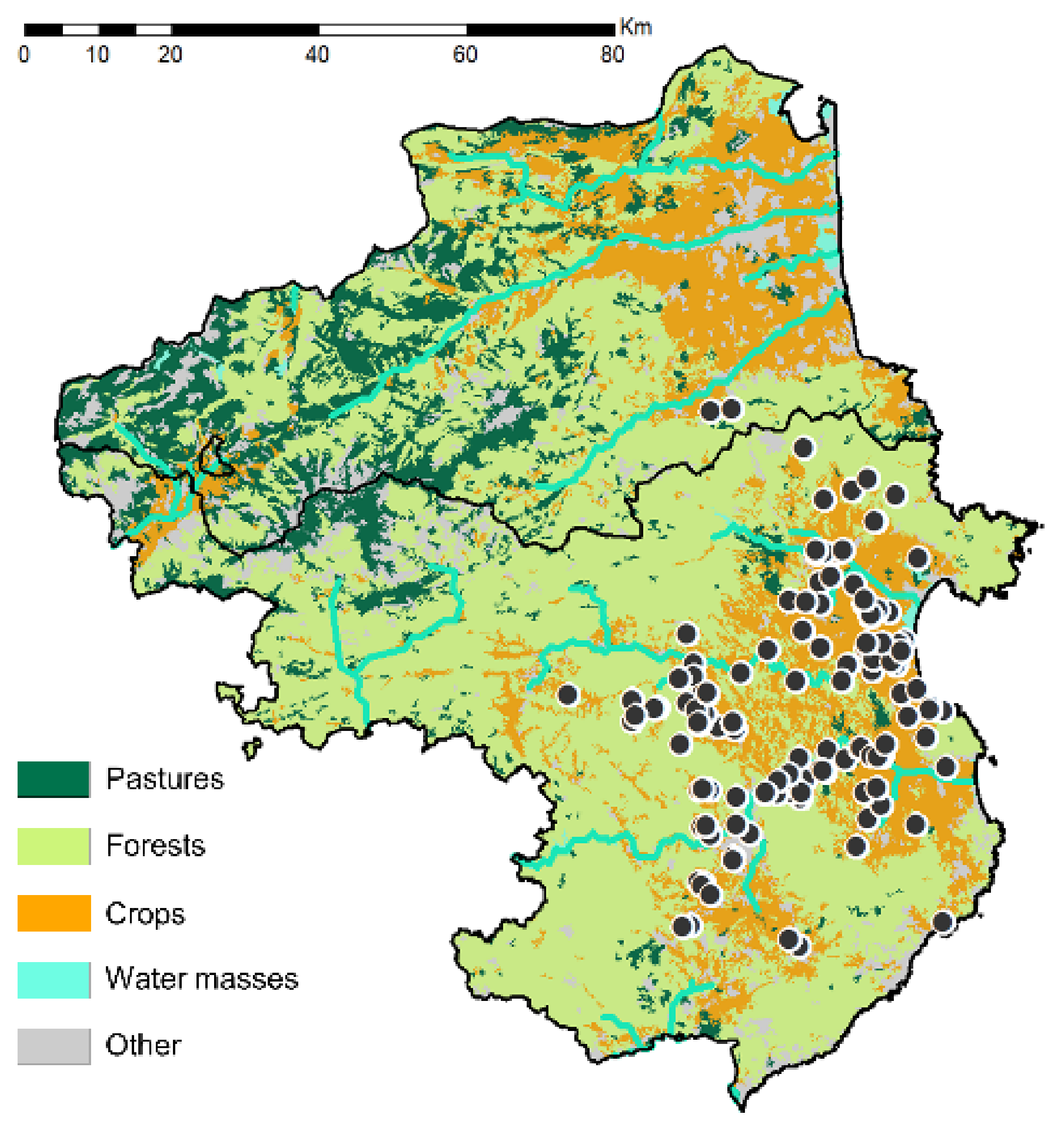
3.7. Land Use Coverage
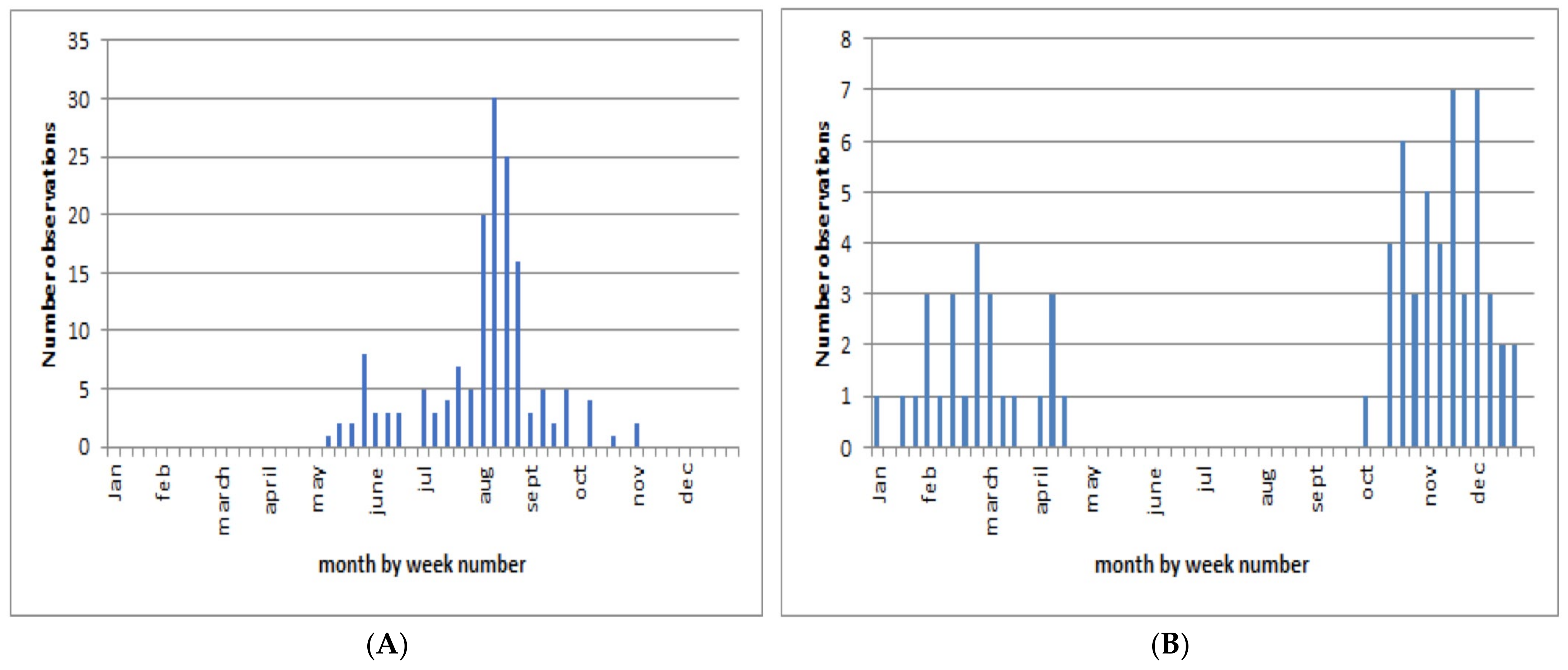
3.8. Observation Phenology
3.9. Identification Help
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Martin, G.J.; Stanger-Hall, K.F.; Branham, M.A.; Da Silveira, L.F.L.; Lower, S.E.; Hall, D.W.; Li, X.; Lemmon, A.R.; Lemmon, E.M.; Bybee, S.M. Higher-level phylogeny and reclassification of Lampyridae (Coleoptera: Elateroidea). Insect Syst. Divers. 2019, 3, 1–15. [Google Scholar] [CrossRef]
- Faust, L.F. Fireflies, Glow-Worms, and Lightning Bugs: Identification and Natural History of the Fireflies of the E. and C. U.S. & Canada; University of Georgia Press: Athens, GA, USA, 2017; p. 400. ISBN 9780820348728/9780820348724. [Google Scholar]
- Lewis, S.M.; Faust, L.; De Cock, R. The dark side of the light show: Predators of fireflies in the great smoky mountains. Psyche 2012, 2012, 7. [Google Scholar] [CrossRef]
- Lewis, S.M. Silent Sparks: The Wondrous World of Fireflies; Princeton University Press: Princeton, NJ, USA, 2016; p. 240. ISBN 9780691162683. [Google Scholar]
- Eisner, T.; Goetz, M.; Hill, D.; Smedley, S.; Meinwald, J. Firefly “femmes fatales” acquire defensive steroids (lucibufagins) from their firefly prey. Proc. Natl. Acad. Sci. USA 1997, 94, 9723–9728. [Google Scholar] [CrossRef]
- Lloyd, J.E. Studies on the flash communication system in photinus fireflies. In Miscellaneous Publications Museum of Zoology; Arbor, A., Ed.; University of Michigan: Ann Arbor, MI, USA, 1966; Volume 130, pp. 1–95. [Google Scholar]
- Díaz, S.; Settele, J.; Brondízio, E.S.; Ngo, H.T.; Guèze, M.; Agard, J.; Arneth, A.; Balvanera, P.; Brauman, K.A.; Butchart, S.H.M.; et al. Summary for Policymakers of the Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; da Cunha, M.C., Mace, G.M., Mooney, H., Eds.; Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services (IPBES) Secretariat: Bonn, Germany, 2019; p. 56. ISBN 978-3-947851-13-3. [Google Scholar]
- Gil-Tapetado, D.; Castedo-Dorado, F.; Lombardero, M.J.; Martel, J.; Álvarez-Álvarez, P. Spatial propagation and patterns of abundance of Dryocosmus kuriphilus throughout an invaded region. J. Appl. Entomol. 2020, 145, 10–25. [Google Scholar] [CrossRef]
- Sesma, J.M.; Gil-Tapetado, D. La expansión de Harmonia axyridis (Pallas, 1773) en la Península Ibérica (Coleoptera: Coccinellidae). BV News Publ. Cient. 2020, 9, 1–7. [Google Scholar]
- Polidori, C.; Garcia-Gara, J.; Blasco-Aróstegui, J.; Gil-Tapetado, D. Urban areas are favouring the spread of an alien mud-dauber wasp into climatically non-optimal latitudes. Acta Oecol. 2021, 110, 103678. [Google Scholar] [CrossRef]
- Viñolas, A.; Vicens, N.; Muñoz-Batet, J. Sobre la presència del gènere Photinus Laporte, 1833 in Catalunya (Coleoptera: Lampyridae: Lampyrinae: Photinini). Butlletí Inst. Catalana D’Història Nat. 2018, 82, 133–135. [Google Scholar]
- Zaragoza-Caballero, S.; Viñolas, A. Photinus immigrans sp. nov. (Coleoptera: Lampyridae: Photinini): Primer registro del género Photinus en Cataluña, España. Rev. Gaditana Entomol. IX 2018, 1, 273–286. [Google Scholar]
- Zaragoza-Caballero, S.; López-Pérez, S.; Vega-Badillo, V.; Domínguez-León, D.E.; Rodríguez-Mirón, G.M.; González-Ramírez, M.; Gutiérrez-Carranza, I.G.; Cifuentes-Ruiz, P.; Zurita-García, M.L. Luciérnagas del centro de México (Coleoptera: Lampyridae): Descripción de 37 especies nuevas. Rev. Mex. Biodivers. 2020, 91, 1–70. [Google Scholar] [CrossRef]
- Ohba, N.; Meyer-Rochow, V.B. Insect species co-existing with the Papua New Guinea firefly Pteroptyx effulgens share aspects of appearance and behavior. Lampyrid 2012, 2, 127–137. [Google Scholar]
- Balogh, A.C.V.; Gamberale-Stille, G.; Leimar, L. Learning and the mimicry spectrum: From quasi-Bates to super-Müller. Anim. Behav. 2008, 76, 1591–1599. [Google Scholar] [CrossRef]
- Baxter, S.W.; Papa, R.; Chamberlain, N.; Humphray, S.J.; Joron, M.; Morrison, C.; Ffrench-Constant, R.H.; McMillan, W.O.; Jiggins, C.D. Convergent evolution in the genetic basis of Müllerian mimicry in heliconius butterflies. Genetics 2008, 180, 1567–1577. [Google Scholar] [CrossRef]
- Guzmán-Álvarez, J.R.; De Cock, R. Final Report on the Authorization of the Capture of Specimens of the Lampyridae Family for Identification in the Municipality of Corça, Girona. EPI-28—2018; Report Presented to the General Directorate of Environmental Policies and Natural Environment, Generalitat of Catalonia, 10/9/2018 [Informe Final Autoritzación de la Captura de Ejemplares de la Familia de los Lampíridos para su Identificación en el Municipio de Corça, Girona. EPI-28—2018. Informe presentado a la Dirección General de Polítiques Ambientals i Medi Natural, Generalitat de Cataluña, 10/9/2018]; Unpublished Report, Barcelona, Spain. A Copy Can Be Obtained from the Authors upon Request.
- Blanchard, C.E. Insectes de l’Amèrique méridionale recueillis par Alcide D’Orbigny (Tribu des Malacodermes. Famille de Lampyriens). In Voyage dans l’Amérique Méridionale: (le Brésil, la République Orientale de L’Uruguay, la République Argentine, la Patagonie, la République du Chili, la République de Bolivia, la République du Pérou), Exécuté Pendant les Années 1826, 1827, 1828, 1829, 1830, 1831, 1832, et 1833 par Alcide D’Orbigny; Bertrand: Paris, France, 1846; Volume 6, Part 2; pp. 101–126. [Google Scholar]
- Papi, F. Light emission, sex attraction and male flash dialogues in firefly Luciola lusitanica. Monit. Zool. Ital. 1969, 3, 135–184. [Google Scholar]
- Mikšić, R. Die Lampyriden Europas (Coleoptera, Malacodermata). Acta Entomol. Jugosl. 1982, 17, 19–26. [Google Scholar]
- De Cock, R. Biology and behaviour of European Lampyrids. In Bioluminescence in Focus—A collection of Illuminating Essays; Meyer-Rochow, V.B., Ed.; Research Signpost: Kerala, India, 2009; pp. 161–200. [Google Scholar]
- Europa Press Madrid. Una Luciérnaga Voladora Vista Por Última Vez Hace Casi un Siglo en España, Objetivo de Los Expertos este Verano [English: A Flying Firefly Last Seen Almost a Century Ago in Spain, Targeted by Experts This Summer]. Available online: https://www.europapress.es/sociedad/medio-ambiente-00647/noticia-luciernaga-voladora-vista-ultima-vez-hace-casi-siglo-espana-objetivo-expertos-verano-20170817134641.html (accessed on 20 October 2020).
- De la Fuente, J.M. Catálogo sistemático-geográfico de los coleópteros observados en la Península Ibérica, Pirineos propiamente dichos y Baleares. Bol. Soc. Entomol. Esp. 1931, 14, 33–36. [Google Scholar]
- Blackwelder, R.E. Checklist of the coleopterous insects of Mexico, Central America, the West Indies, and South America. U. S. Natl. Mus. Bull. 1944, 185, 927–1492. [Google Scholar]
- McDermott, F.A. Illustrations of the aedeagi of the Lampyridae (Coleoptera). Coleopt. Bull. 1962, 16, 21–27. [Google Scholar]
- Ballantyne, L.A.; Lambkin, C.L.; Ho, J.-Z.; Jusoh, W.F.A.; Nada, B.; Nak-Eiam, S.; Thancharoen, A.; Wattanachaiyingcharoen, W.; Yiu, V. The Luciolinae of S. E. Asia and the Australopacific region: A revisionary checklist (Coleoptera: Lampyridae) including description of three new genera and 13 new species. Zootaxa 2019, 4687, 1–174. [Google Scholar] [CrossRef]
- Rodríguez-Flores, P.C.; Recuero, E. On the larva of the Iberian allochthonous endemic firefly Photinus immigrans Zaragoza-Caballero & Viñolas, 2018 (Coleoptera, Lampyridae). Graellsia 2021, 77, 1–4. [Google Scholar] [CrossRef]
- Brown, P.M.; Thomas, C.E.; Lombaert, E.; Jeffries, D.L.; Estoup, A.; Handley, L.J.L. The global spread of Harmonia axyridis (Coleoptera: Coccinellidae): Distribution, dispersal and routes of invasion. BioControl 2011, 56, 623–641. [Google Scholar] [CrossRef]
- Lewis, S.M.; Wong, C.H.; Owens, A.; Fallon, C.; Jepsen, S.; Thancharoen, A.; Wu, C.; De Cock, R.; Novák, M.; López-Palafox, T. A global perspective on firefly extinction threats. BioScience 2020, 70, 157–167. [Google Scholar] [CrossRef]
- Gurgel, K.; Chittaro, Y.; Sanchez, A.; Rieger, I. Contribution à la connaissance des lucioles et lampyres de Suisse et observation de Luciola lusitanica Charpentier, 1825 á Genève (Coleoptera, Lampyridae). Entomo Helv. 2020, 13, 81–96. [Google Scholar]
- Kaufmann, T. Ecological and biological studies on the West African firefly Luciola discicollis (Coleoptera: Lampyridae). Ann. Entomol. Soc. Am. 1965, 58, 414–426. [Google Scholar] [CrossRef]
- Faust, L.; Faust, H. The Occurrence and Behaviors of North American Fireflies (Coleoptera: Lampyridae) on Milkweed, Asclepias syriaca L. Coleopt. Bull. 2014, 68, 283–291. [Google Scholar] [CrossRef]
- Cratsley, C. Flash Signals, Nuptial gifts and female preference in Photinus fireflies. Integr. Comp. Biol. 2004, 44, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.M.; Cratsley, C.K.; Rooney, J.A. Nuptial gifts and sexual selection in Photinus fireflies. Integr. Comp. Biol. 2004, 44, 234–237. [Google Scholar] [CrossRef]
- Horne, J. A sexual difference in the timing of adult emergence in the glow-worm Lampyris noctiluca L. (Coleoptera: Lampyridae). Lampyrid 2011, 1, 9–13. [Google Scholar]
- Tyler, J. A study of the male flight season in the glow-worm Lampyris noctiluca (L.) (Coleoptera: Lampyridae). Lampyrid 2011, 1, 32–38. [Google Scholar]
- Hickmott, W.; Tyler, J. Seasonal variation in the female display period of the glow-worm Lampyris noctiluca L. (Coleoptera: Lampyridae). Lampyrid 2011, 1, 14–21. [Google Scholar]
- Wing, S. Female monogamy and male competition in Photinus collustrans (Coleoptera: Lampyridae). Psyche A J. Entomol. 1984, 91, 153–160. [Google Scholar] [CrossRef]
- Tyler, J. What do glow-worms do on their day off? The diurnal behaviour of adult Lampyris noctiluca (L.) (Coleoptera: Lampyridae). Lampyrid 2013, 3, 23–35. [Google Scholar]
- Companyo, L. Histoire Naturelle du Département des Pyrénées, Tôme III; Imprimerie de J.-B. Alzine: Perpignan, France, 1863; p. 657. [Google Scholar]
- De Cock, R. Rare, or simply overlooked? Practical notes for survey and monitoring of the small glow-worm Phosphaenus hemipterus (Coleoptera: Lampyridae). Belg. J. Zool. 2000, 130, 93–101. [Google Scholar]
- Majka, C.J.; MacIvor, J.S. The European lesser glow worm, Phosphaenus hemipterus (Goeze), in North America (Coleoptera, Lampyridae). ZooKeys 2009, 29, 35–47. [Google Scholar] [CrossRef]
- Justine, J.-L.; Winsor, L.; Gey, D.; Gros, P.; Thévenot, J. Giant worms chez moi! Hammerhead flatworms (Platyhelminthes, Geoplanidae, Bipalium spp., Diversibipalium spp.) in metropolitan France and overseas French territories. PeerJ 2018, 6, e4672. [Google Scholar] [CrossRef] [PubMed]
- Justine, J.-L.; Winsor, L.; Gey, D.; Gros, P.; Thévenot, J. Obama chez moi! The invasion of metropolitan France by the land planarian Obama nungara (Platyhelminthes, Geoplanidae). PeerJ 2020, 8, e8385. [Google Scholar] [CrossRef]
- Berger, A.; Petschenka, G.; Degenkolb, T.; Geisthardt, M.; Vilcinskas, A. Insect Collections as an Untapped Source of Bioactive Compounds-Fireflies (Coleoptera: Lampyridae) and Cardiotonic Steroids (2021) as a Proof of Concept. Insects 2021, 12, 689. [Google Scholar] [CrossRef]
- Owens, A.; Lewis, S.M. The impact of artificial light at night on nocturnal insects: A review and synthesis. Ecol. Evol. 2018, 8, 11337–11358. [Google Scholar] [CrossRef]
- Elgert, C.; Hopkins, J.; Kaitala, A.; Candolin, U. Reproduction under light pollution: Maladaptive response to spatial variation in artificial light in a glow-worm. Proc. R. Soc. B 2020, 287, 20200806. [Google Scholar] [CrossRef]
- Van den Broeck, M.; De Cock, R.; Van Dongen, S.; Matthysen, E. Blinded by the Light: Artificial Light Lowers Mate Attraction Success in Female Glow-Worms (Lampyris noctiluca L.). Insects 2021, 12, 734. [Google Scholar] [CrossRef]
- De Cock, R. Larval and adult emission spectra of bioluminescence in three European species of fireflies (Coleoptera: Lampyridae). Photochem. Photobiol. 2004, 79, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Tisi, L.C.; De Cock, R.; Stewart, A.J.A.; Booth, D.; Day, J.C. Bioluminescent leakage throughout the body of the glow-worm Lampyris noctiluca (Coleoptera: Lampyridae). Entomol. Gen. 2014, 35, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Booth, D.; Stewart, A.J.A.; Osorio, D. Colour vision in the glow-worm Lampyris noctiluca (L.) (Coleoptera: Lampyridae): Evidence for a green-blue chromatic mechanism. J. Exp. Biol. 2004, 207, 2373–2378. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koken, M.; Guzmán-Álvarez, J.R.; Gil-Tapetado, D.; Romo Bedate, M.A.; Laurent, G.; Rubio, L.E.; Rovira Comas, S.; Wolffler, N.; Verfaillie, F.; De Cock, R. Quick Spreading of Populations of an Exotic Firefly throughout Spain and Their Recent Arrival in the French Pyrenees. Insects 2022, 13, 148. https://doi.org/10.3390/insects13020148
Koken M, Guzmán-Álvarez JR, Gil-Tapetado D, Romo Bedate MA, Laurent G, Rubio LE, Rovira Comas S, Wolffler N, Verfaillie F, De Cock R. Quick Spreading of Populations of an Exotic Firefly throughout Spain and Their Recent Arrival in the French Pyrenees. Insects. 2022; 13(2):148. https://doi.org/10.3390/insects13020148
Chicago/Turabian StyleKoken, Marcel, José Ramón Guzmán-Álvarez, Diego Gil-Tapetado, Miguel Angel Romo Bedate, Geneviève Laurent, Lucas Ezequiel Rubio, Segimon Rovira Comas, Nicole Wolffler, Fabien Verfaillie, and Raphaël De Cock. 2022. "Quick Spreading of Populations of an Exotic Firefly throughout Spain and Their Recent Arrival in the French Pyrenees" Insects 13, no. 2: 148. https://doi.org/10.3390/insects13020148
APA StyleKoken, M., Guzmán-Álvarez, J. R., Gil-Tapetado, D., Romo Bedate, M. A., Laurent, G., Rubio, L. E., Rovira Comas, S., Wolffler, N., Verfaillie, F., & De Cock, R. (2022). Quick Spreading of Populations of an Exotic Firefly throughout Spain and Their Recent Arrival in the French Pyrenees. Insects, 13(2), 148. https://doi.org/10.3390/insects13020148






