Electroantennographic and Behavioural Responses of European Cherry Fruit Fly, Rhagoletis cerasi, to the Volatile Organic Compounds from Sour Cherry, Prunus cerasus, Fruit
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Cherry Fruit
2.3. Chemicals
2.4. Collection of Fruit Volatiles
2.5. Gas Chromatography–Electroantennogram Detection
2.6. Gas Chromatography–Mass Spectrometry
2.7. Behavioural Test
3. Results
3.1. Cherry Fruit VOCs
3.2. Olfactory Active VOCs
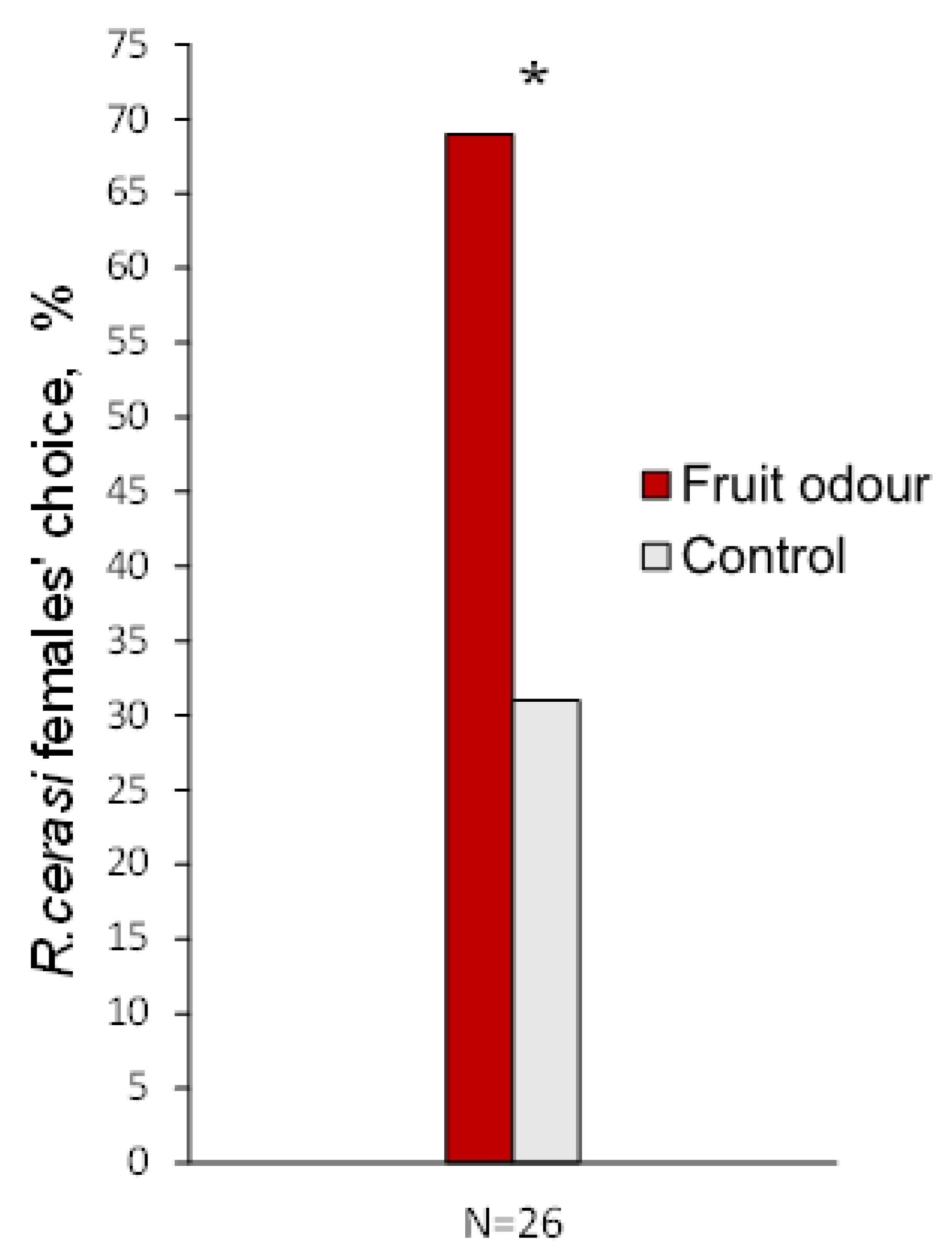
3.3. Behavioural Response to Fruit
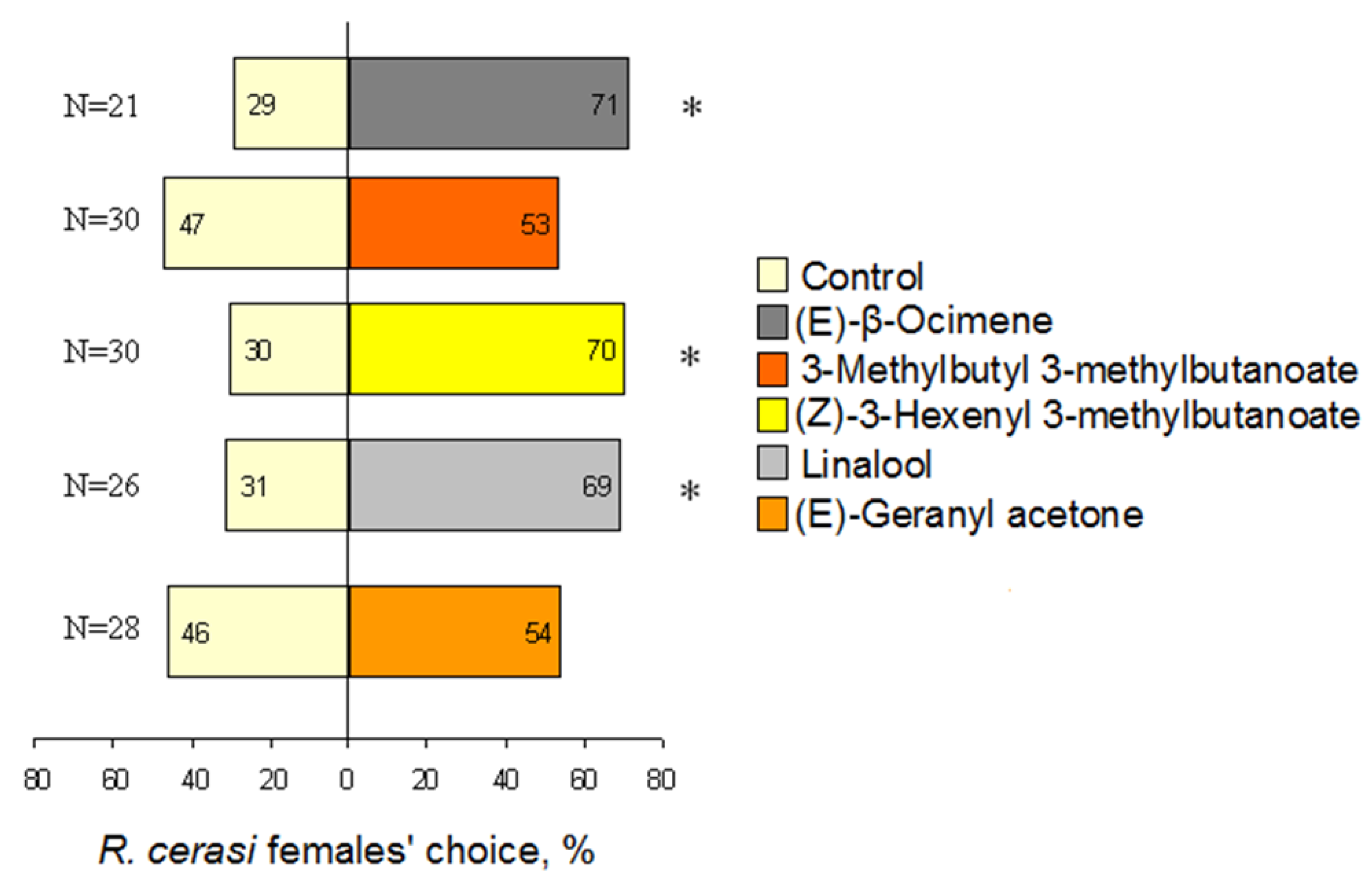
3.4. Behavioural Reaction to Single VOCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EPPO Global Database/Taxonomy Explorer/Rhagoletis cerasi (RHAGCE)/Distribution. Available online: https://gd.eppo.int/taxon/RHAGCE/distribution (accessed on 23 November 2021).
- Barringer, L. First record of the European cherry fruit fly, Rhagoletis cerasi (Linnaeus) (Diptera: Tephritidae), in North America. Insecta Mundi 2018, 0622, 1–4. [Google Scholar]
- Daniel, C.; Wyss, E. Migration and propagation of the cherry fruit fly within orchards—Possibility of biological soil treatment. Mitt. Dtsch. Ges. Allg. Angew. Ent. 2009, 17, 247–248. [Google Scholar]
- Sarles, L.; Verhaeghe, A.; Francis, F.; Verheggen, F.J. Semiochemicals of Rhagoletis fruit flies: Potential for integrated pest management. Crop Prot. 2015, 78, 114–118. [Google Scholar] [CrossRef] [Green Version]
- Kelley, D.S.; Adkins, Y.; Laugero, K.D. A review of the health benefits of cherries. Nutrients 2018, 10, 368. [Google Scholar] [CrossRef] [Green Version]
- Kepenekci, I.; Hazir, S.; Özdem, A. Evaluation of native entomopathogenic nematodes for the control of the European cherry fruit fly Rhagoletis cerasi L. (Diptera: Tephritidae) larvae in soil. Turkish J. Agricult. Forestr. 2015, 39, 74–79. [Google Scholar] [CrossRef]
- Daniel, C.; Wyss, E. Field applications of Beauveria bassiana to control the European cherry fruit fly Rhagoletis cerasi. J. Appl. Entomol. 2010, 134, 675–681. [Google Scholar] [CrossRef]
- Daniel, C.; Baker, B. Dispersal of Rhagoletis cerasi in commercial cherry orchards: Efficacy of soil covering nets for cherry fruit fly control. Insects 2013, 4, 168–176. [Google Scholar] [CrossRef] [Green Version]
- Özbek Çatal, B.; Ulusoy, M.R. Investigation into control of cherry fruit fly, Rhagoletis cerasi (L., 1758) (Diptera: Tephritidae), in organic cherry production. Türk. Entomol. Derg. 2018, 42, 33–43. [Google Scholar] [CrossRef] [Green Version]
- Özdem, A.; Kılınçer, N. The effectiveness of the trap types and lures used for mass trapping to control cherry fruit fly [Rhagoletis cerasi (L., 1758)](Diptera: Tephritidae). Mun. Ent. Zool. 2009, 4, 371–377. [Google Scholar]
- Daniel, C.; Grunder, J. Integrated management of European cherry fruit fly Rhagoletis cerasi (L.): Situation in Switzerland and Europe. Insects 2012, 3, 956–988. [Google Scholar] [CrossRef] [Green Version]
- Navarro-Llopis, V.; Vacas, S. Mass trapping for fruit fly control. In Trapping and the Detection, Control, and Regulation of Tephritid Fruit Flies: Lures, Area-Wide Programs, and Trade Implications; Shelly, T.E., Epsky, N., Jang, E.B., Reyes-Flores, J., Vargas, R.I., Eds.; Springer: Dordrecht, The Netherlands; New York, NY, USA, 2014; pp. 513–555. ISBN 978-94-017-9193-9. [Google Scholar] [CrossRef]
- Levaj, B.; Dragović-Uzelac, V.; Delonga, K.; Kovačević Ganić, K.K.; Banović, M.; Bursać Kovačević, D.B. Polyphenols and volatiles in fruits of two sour cherry cultivars, some berry fruits and their jams. Food Technol. Biotechnol. 2010, 48, 538–547. [Google Scholar]
- Sun, S.Y.; Jiang, W.G.; Zhao, Y.P. Characterization of the aroma-active compounds in five sweet cherry cultivars grown in Yantai (China). Flavour Fragr. J. 2010, 25, 206–213. [Google Scholar] [CrossRef]
- Girard, B.; Kopp, T.G. Physicochemical characteristics of selected sweet cherry cultivars. J. Agric. Food Chem. 1998, 46, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Hayaloglu, A.A.; Demir, N. Phenolic Compounds, Volatiles, and Sensory Characteristics of Twelve Sweet Cherry (Prunus avium L.) Cultivars Grown in Turkey. J. Food Sci. 2016, 81, C7–C18. [Google Scholar] [CrossRef]
- Wen, Y.-Q.; He, F.; Zhu, B.-Q.; Lan, Y.-B.; Pan, Q.-H.; Li, C.-Y.; Reeves, M.J.; Wang, J. Free and glycosidically bound aroma compounds in cherry (Prunus avium). Food Chem. 2014, 152, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Serradilla, M.J.; Martín, A.; Hernandez, A.; López-Corrales, M.; Lozano, M.; Córdoba, M.G. Effect of the Commercial Ripening Stage and Postharvest Storage on Microbial and Aroma Changes of ‘Ambrunés’ Sweet Cherries. J. Agric. Food Chem. 2010, 58, 9157–9163. [Google Scholar] [CrossRef]
- Mattheis, J.P.; Buchanan, D.A.; Fellman, J.K. Volatile compounds emitted by sweet cherries (Prunus avium Cv. Bing) during fruit development and ripening. J. Agric. Food Chem. 1992, 40, 471–474. [Google Scholar] [CrossRef]
- El-Sayed, A.M. The Pherobase: Database of Pheromones and Semiochemicals. 2019. Available online: http://www.pherobase.com (accessed on 23 November 2021).
- Quilici, S.; Atiama-Nurbel, T.; Brévault, T. Plant odours as fruit fly attractants. In Trapping and the Detection, Control, and Regulation of Tephritid Fruit Flies: Lures, Area-Wide Programs, and Trade Implications; Shelly, T.E., Epsky, N., Jang, E.B., Reyes-Flores, J., Vargas, R.I., Eds.; Springer: Dordrecht, The Netherlands; New York, NY, USA, 2014; pp. 119–144. ISBN 978-94-017-9193-9. [Google Scholar] [CrossRef]
- Epsky, N.D.; Kendra, P.E.; Schnell, E.Q. History and development of food-based attractants. In Trapping and the Detection, Control, and Regulation of Tephritid Fruit Flies: Lures, Area-Wide Programs, and Trade Implications; Shelly, T.E., Epsky, N., Jang, E.B., Reyes-Flores, J., Vargas, R.I., Eds.; Springer: Dordrecht, The Netherlands; New York, NY, USA, 2014; pp. 75–118. ISBN 978-94-017-9193-9. [Google Scholar] [CrossRef]
- Katsoyannos, B.I.; Papadopoulos, N.T.; Stavridis, D. Evaluation of trap types and food attractants for Rhagoletis cerasi (Diptera: Tephritidae). J. Econ. Entomol. 2000, 3, 1005–1010. [Google Scholar] [CrossRef]
- Grodner, J.; Świech, K.; Rozpara, E.; Danelski, W. Food attractant to control the population of Rhagoletis cerasi L. (Diptera: Tephritidae) and its use in organic sweet cherry orchard in Poland. J. Res. Appl. Agricult. Eng. 2016, 61, 167–172. [Google Scholar]
- Tóth, M.; Voigt, E.; Baric, B.; Pajac, I.; Subic, M.; Baufeld, P.; Lerche, S. Importance of application of synthetic food lures in trapping of Rhagoletis spp. and Strauzia longipennis Wiedemann. Acta Phytopathol. Entomol. Hung. 2014, 49, 25–35. [Google Scholar] [CrossRef] [Green Version]
- Raptopoulos, D.; Haniotakis, G.; Koutsaftikis, A.; Kelly, D.; Mavraganis, V. Biological activity of chemicals identified from extracts and volatiles of male Rhagoletis cerasi. J. Chem. Ecol. 1995, 21, 1287–1297. [Google Scholar] [CrossRef] [PubMed]
- Macavei, L.L.; Olten, I.; Vasian, I.; Florian, T.; Ioan, V.M.; Băeţan, R.; Viorel, M.; Maistrello, L. Potential for attractive semiochemical lures in Rhagoletis cerasi (L.) management: A field study. J. Entomol. Res. Soc. 2018, 20, 1–9. [Google Scholar]
- Nojima, S.; Lin, C., Jr.; Morris, B.; Zhang, A.; Roelofs, W.J. Identification of host fruit voletiles from hawthors (Crataegus spp.) attractive to hawthorn-origin Rhagoletis pomonela flies. J. Chem. Ecol. 2003, 29, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Cha, D.H.; Olsson, S.B.; Yee, W.L.; Goughnour, R.B.; Hood, G.R.; Mattson, M.; Schwarz, D.; Feder, J.L.; Linn, C.E., Jr. Identification of host fruit volatiles from snowberry (Symphoricarpos albus) attractive to Rhagoletis zephyria flies from Western United States. J. Chem. Ecol. 2017, 43, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, C.S.; Papadopoulos, N.T.; Kouloussis, N.A.; Tananaki, C.I.; Katsoyannos, B.I. Essential oils of citrus fruit stimulate oviposition in the Mediterranean fruit fly Ceratitis capitata (Diptera: Tephritidae). Physiol. Entomol. 2012, 34, 330–339. [Google Scholar] [CrossRef]
- Mozūraitis, R.; Aleknavičius, D.; Vepštaitė-Monstavičė, I.; Stanevičienė, R.; Noushin Emami, S.; Apšegaitė, V.; Radžiutė, S.; Blažytė-Čereškienė, L.; Servienė, E.; Būda, V. Hippophae rhamnoides berry related Pichia kudriavzevii yeast volatiles modify behaviour of Rhagoletis batava flies. J. Advanced Res. 2020, 21, 71–77. [Google Scholar] [CrossRef]
| No | Compound | RI 1 | CAS No 2 | Group 3 | ID 10 | Abundance, % |
|---|---|---|---|---|---|---|
| 1 | n-Decane | 1000 | 112-40-3 | A 4 | RC 11 | 0.71 |
| 2 | α-Pinene | 1016 | 80-56-8 | T 5 | RC | 1.15 |
| 3 | Hexanal | 1075 | 66-25-1 | AL 6 | RC | 0.65 |
| 4 | n-Undecane | 1100 | 1120-21-4 | A | RC | 0.56 |
| 5 | 3-Methylbutyl acetate | 1117 | 123-92-2 | E 7 | RC | 0.43 |
| 6 | δ-3-Carene | 1141 | 13466-78-9 | T | RC | 0.79 |
| 7 | Propyl 3-methylbutanoate | 1151 | 557-00-6 | E | RC | 0.36 |
| 8 | α-Phellandrene | 1158 | 99-83-2 | T | RC | 3.08 |
| 9 | 3-Methylbutyl propionate | 1184 | 105-69-0 | E | RC | 0.07 |
| 10 | Limonene | 1189 | 5989-27-5 | T | RC | 7.60 |
| 11 | β-Phellandrene | 1196 | 555-10-2 | T | L 12, RI | 0.36 |
| 12 | n-Dodecane | 1200 | 112-40-3 | A | RC | 0.87 |
| 13 | (E)-2-Hexenal | 1207 | 6728-28-3 | AL | RC | 3.51 |
| 14 | Ethyl hexanoate | 1224 | 123-66-0 | E | RC | 0.16 |
| 15 | (E)-β-Ocimene | 1247 | 3779-61-1 | T | RC | 4.66 |
| 16 | p-Cymene | 1261 | 99-87-6 | T | RC | 1.88 |
| 17 | Hexyl acetate | 1268 | 142-92-7 | E | RC | 0.61 |
| 18 | Octanal | 1281 | 124-13-0 | AL | RC | 0.56 |
| 19 | 3-Methylbutyl 3-methylbutanoate | 1289 | 659-70-1 | E | RC | 1.85 |
| 20 | (E)-4,8-Dimethyl-1,3,7-nonatriene | 1299 | 19945-61-0 | T | L, RI | 9.90 |
| 21 | (Z)-3-Hexenyl acetate | 1308 | 3681-71-8 | E | RC | 2.35 |
| 22 | (Z)-2-Hexenyl acetate | 1327 | 56922-75-9 | E | L, RI | 0.98 |
| 23 | 6-Methyl-5-hepten-2-one | 1328 | 110-93-0 | K | RC | 1.10 |
| 24 | 1-Hexanol | 1349 | 111-27-3 | OH 8 | RC | 0.53 |
| 25 | Unknown | 1368 | 0.93 | |||
| 26 | (Z)-3-Hexen-1-ol | 1378 | 928-96-1 | OH | RC | 0.82 |
| 27 | Nonanal | 1385 | 124-19-6 | AL | RC | 2.83 |
| 28 | n-Tetradecane | 1400 | 629-59-4 | A | RC | 3.47 |
| 29 | Ethyl octanoate | 1429 | 106-32-1 | E | RC | 17.82 |
| 30 | Unknown | 1441 | 2.12 | |||
| 31 | 1-Octen-3-ol | 1446 | 3391-86-4 | OH | RC | 0.18 |
| 32 | (Z)-3-Hexenyl 3-methylbutanoate | 1480 | 35154-45-1 | E | RC | 0.53 |
| 33 | 2-Ethylhexan-1-ol | 1484 | 104-76-7 | OH | RC | 3.03 |
| 34 | Decanal | 1490 | 112-31-2 | AL | RC | 2.44 |
| 35 | n-Pentadecane | 1500 | 629-62-9 | A | RC | 3.08 |
| 36 | Benzaldehyde | 1502 | 100-52-7 | AL | RC | 0.87 |
| 37 | (E)-2-Nonenal | 1517 | 78-70-6 | AL | RC | 0.70 |
| 38 | Linalool | 1541 | 78-70-6 | T | RC | 1.89 |
| 39 | n-Hexadecane | 1600 | 544-76-3 | A | RC | 2.61 |
| 40 | 6-Methylheptan-1-ol | 1609 | 1653-40-3 | OH | L, RI | 0.74 |
| 41 | Unknown | 1623 | 1.02 | |||
| 42 | Acetophenone | 1630 | 98-86-2 | K 9 | L, RI | 0.57 |
| 43 | 1-Nonan-1-ol | 1661 | 143-08-08 | OH | L, RI | 0.39 |
| 44 | Unknown | 1664 | 0.05 | |||
| 45 | α-Terpinyl acetate | 1683 | 80-26-2 | T | RC | 0.24 |
| 46 | 3-Ethylbenzaldehyde | 1688 | 34246-54-3 | AL | L, RI | 1.10 |
| 47 | Germacrene D | 1693 | 23986-74-5 | T | L, RI | 0.39 |
| 48 | n-Heptadecane | 1700 | 629-78-7 | A | RC | 1.94 |
| 49 | α-Muurolene | 1711 | 17627-24-6 | T | L, RI | 0.15 |
| 50 | 4-Ethylbenzaldehyde | 1717 | 4748-78-1 | AL | L, RI | 0.41 |
| 51 | α-Farnesene | 1738 | 502-61-4 | T | RC | 0.22 |
| 52 | δ-Cadinene | 1745 | 483-76-1 | T | L, RI | 0.32 |
| 53 | 2-Phenylethyl acetate | 1798 | 103-45-7 | E | RC | 2.78 |
| 54 | Methylethyl dodecanoate | 1812 | 10233-13-3 | E | L, RI | 0.87 |
| 55 | (E)-Geranylacetone | 1819 | 37-96-70-1 | T | RC | 0.75 |
| No 1 | Compound | RT 2 | Amount | EAG 4 Activity | ||
|---|---|---|---|---|---|---|
| Mean ± SE 3 | % | Mean ± SE 5 | Number | |||
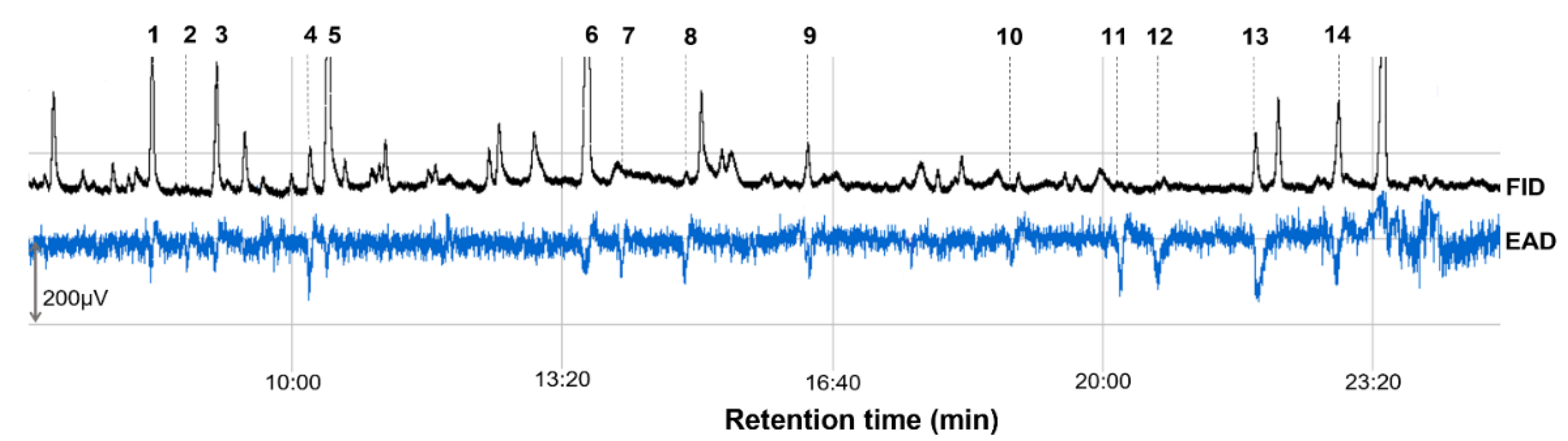
| 1 | (E)-2-Hexenal | 8.3 | 12.95 ± 0.56 | 12.52 | 48.33 ± 14.03 | 7 6 (12) 7 |
| 2 | Ethyl hexanoate | 8.74 | 0.06 ± 0.01 | 0.06 | 11.67 ± 6.26 | 3 (12) |
| 3 | (E)-β-Ocimene | 9.09 | 10.60 ± 0.28 | 10.25 | 36.37 ± 13.22 | 6 (12) |
| 4 | 3-Methylbutyl 3-methylbutanoate | 10.24 | 3.55 ± 0.08 | 3.43 | 36.67 ± 17.20 | 5 (12) |
| 5 | (E)-4,8-Dimethyl-1,3,7-nonatriene | 10.46 | 24.11 ± 0.50 | 23.32 | 31.67 ± 12.42 | 10 (12) |
| 6 | Ethyl octanoate | 13.66 | 35.09 ± 0.90 | 33.94 | 61.67 ± 16.60 | 7 (12) |
| 7 | 1-Octen-3-ol | 14.03 | 0.17 ± 0.04 | 0.16 | 48.33 ± 15.85 | 8 (12) |
| 8 | (Z)-3-Hexenyl 3-methylbutanoate | 14.87 | 0.73 ± 0.01 | 0.71 | 65.00 ± 18.44 | 9 (12) |
| 9 | Linalool | 16.36 | 3.47 ± 0.09 | 3.36 | 53.33 ± 8.99 | 9 (12) |
| 10 | Unknown | 18.85 | 0.05 ± 0.01 | 0.05 | 11.67 ± 6.26 | 3 (12) |
| 11 | α-Muurolene | 20.23 | 0.10 ± 0.01 | 0.10 | 30.00 ± 13.37 | 4 (12) |
| 12 | α-Farnesene | 20.67 | 0.30 ± 0.01 | 0.29 | 28.33 ± 12.18 | 4 (12) |
| 13 | 2-Phenylethyl acetate | 21.88 | 4.81 ± 0.10 | 4.65 | 80.00 ± 21.46 | 8 (12) |
| 14 | (E)-Geranyl acetone | 22.91 | 7.41 ± 0.30 | 7.17 | 76.67 ± 16.67 | 11 (12) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Būda, V.; Radžiutė, S.; Apšegaitė, V.; Blažytė-Čereškienė, L.; Čepulytė, R.; Bumbulytė, G.; Mozūraitis, R. Electroantennographic and Behavioural Responses of European Cherry Fruit Fly, Rhagoletis cerasi, to the Volatile Organic Compounds from Sour Cherry, Prunus cerasus, Fruit. Insects 2022, 13, 114. https://doi.org/10.3390/insects13020114
Būda V, Radžiutė S, Apšegaitė V, Blažytė-Čereškienė L, Čepulytė R, Bumbulytė G, Mozūraitis R. Electroantennographic and Behavioural Responses of European Cherry Fruit Fly, Rhagoletis cerasi, to the Volatile Organic Compounds from Sour Cherry, Prunus cerasus, Fruit. Insects. 2022; 13(2):114. https://doi.org/10.3390/insects13020114
Chicago/Turabian StyleBūda, Vincas, Sandra Radžiutė, Violeta Apšegaitė, Laima Blažytė-Čereškienė, Rasa Čepulytė, Gabrielė Bumbulytė, and Raimondas Mozūraitis. 2022. "Electroantennographic and Behavioural Responses of European Cherry Fruit Fly, Rhagoletis cerasi, to the Volatile Organic Compounds from Sour Cherry, Prunus cerasus, Fruit" Insects 13, no. 2: 114. https://doi.org/10.3390/insects13020114
APA StyleBūda, V., Radžiutė, S., Apšegaitė, V., Blažytė-Čereškienė, L., Čepulytė, R., Bumbulytė, G., & Mozūraitis, R. (2022). Electroantennographic and Behavioural Responses of European Cherry Fruit Fly, Rhagoletis cerasi, to the Volatile Organic Compounds from Sour Cherry, Prunus cerasus, Fruit. Insects, 13(2), 114. https://doi.org/10.3390/insects13020114







