Wheat-Bran-Based Artificial Diet for Mass Culturing of the Fall Armyworm, Spodoptera frugiperda Smith (Lepidoptera: Noctuidae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Diet Formula and Preparation Method
2.3. Experimental Assays
2.3.1. Effects of Different Diets on Development, Survival, and Reproduction of FAWs (Spodoptera frugiperda)
2.3.2. Effects of Diet on Flight Ability of FAWs (Spodoptera frugiperda)
2.3.3. Comparison of Cost of Different Diets
2.4. Data Analyses
3. Results
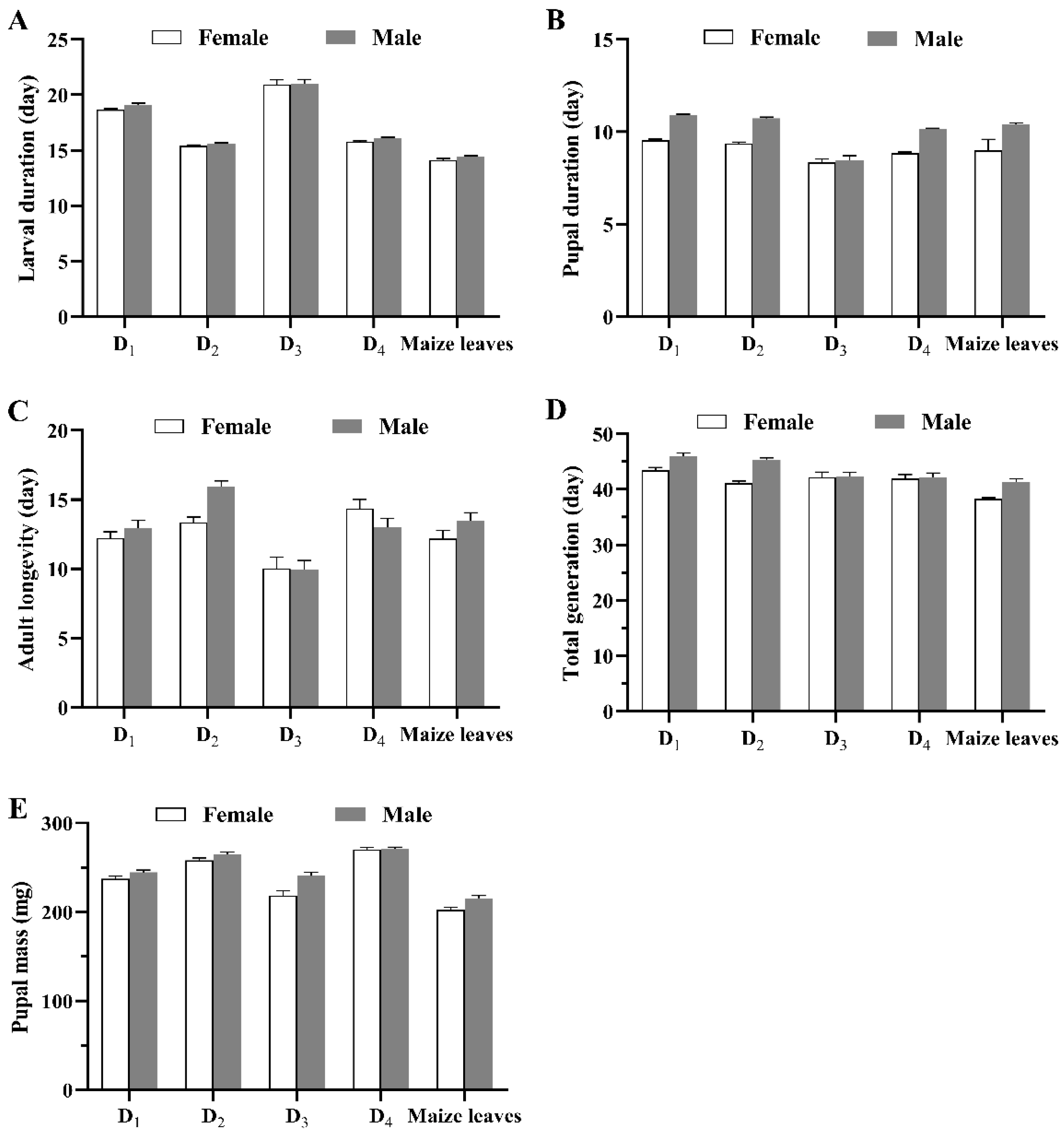
3.1. Developmental Period and Reproductive Parameters
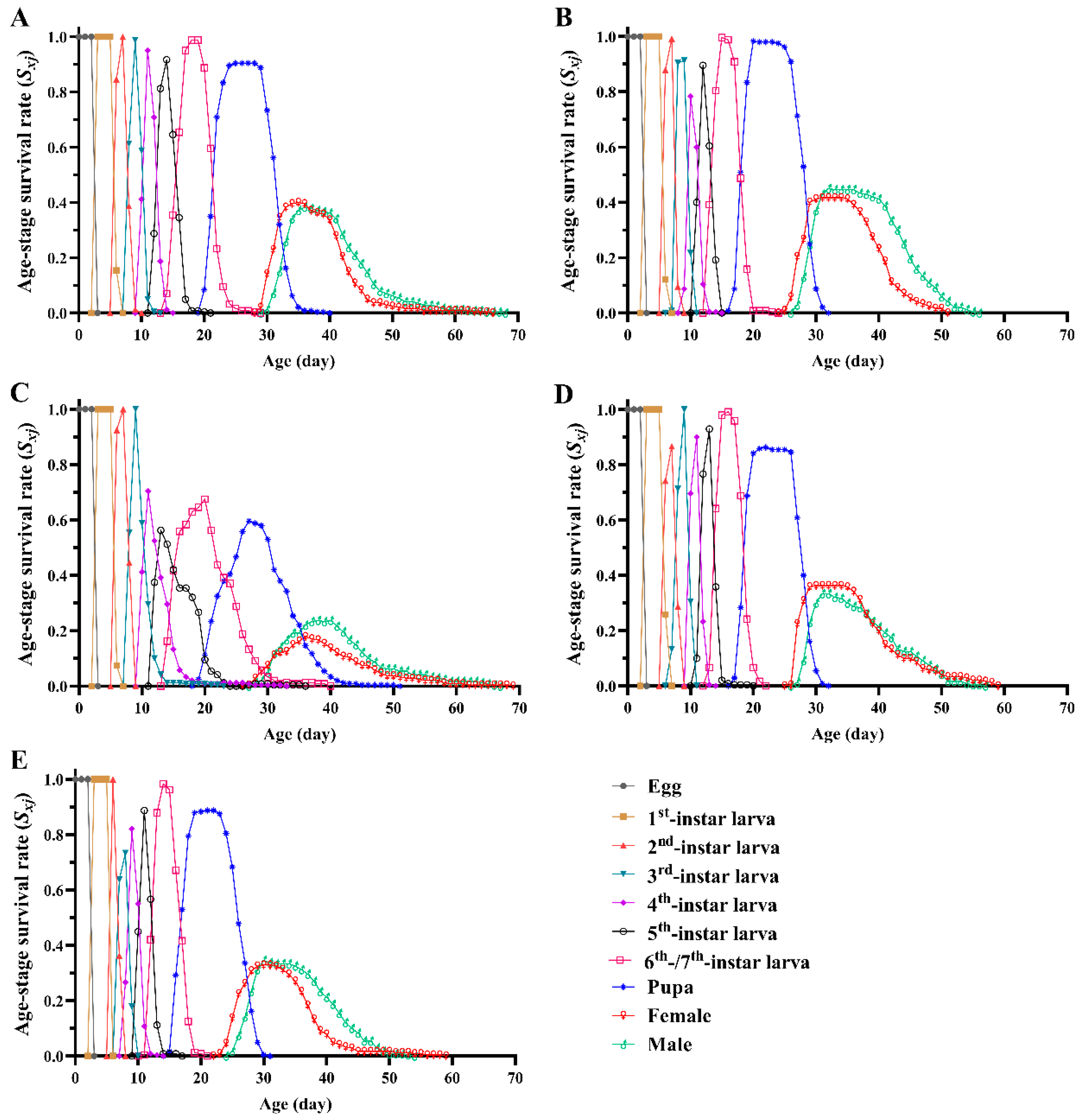
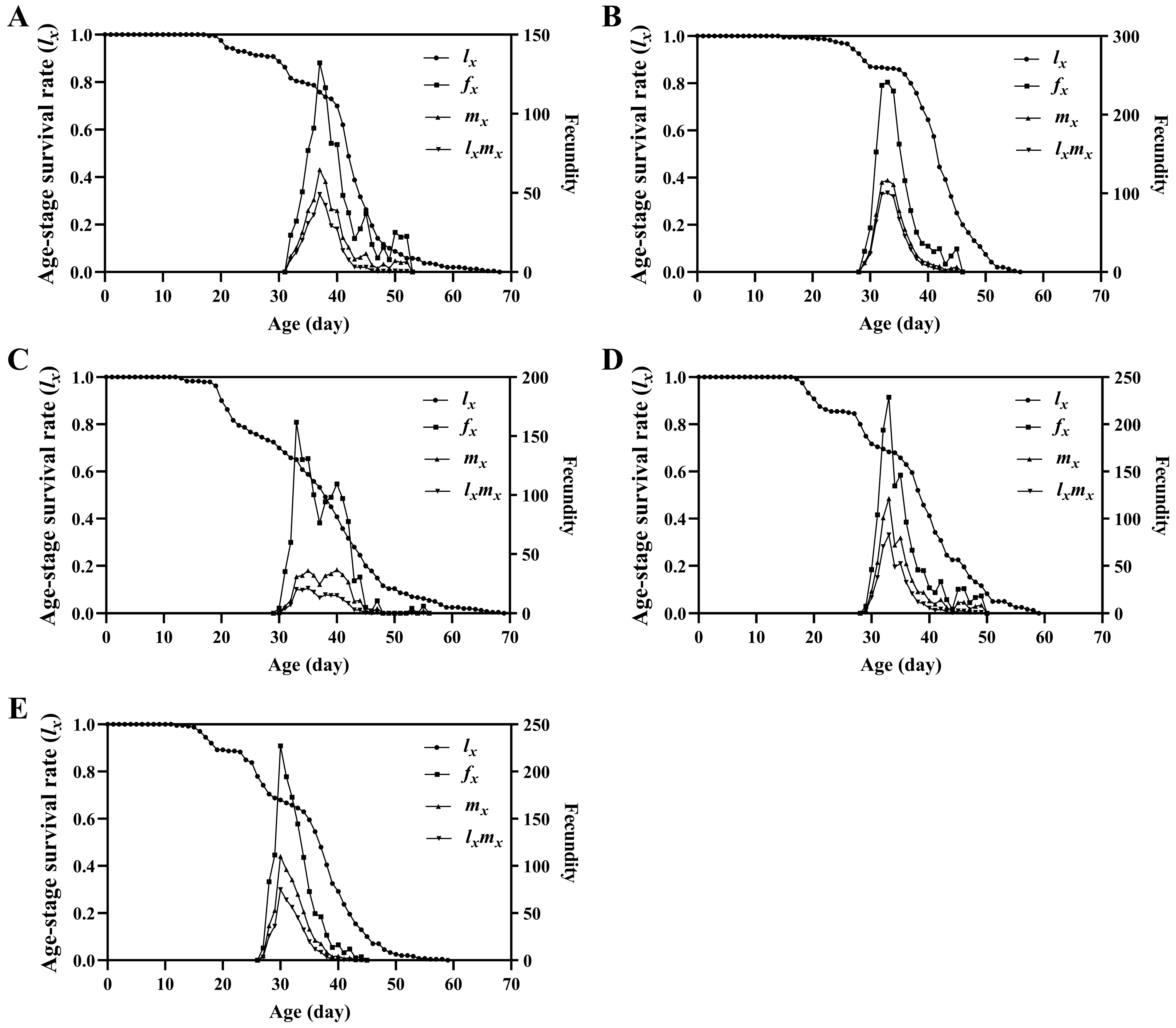
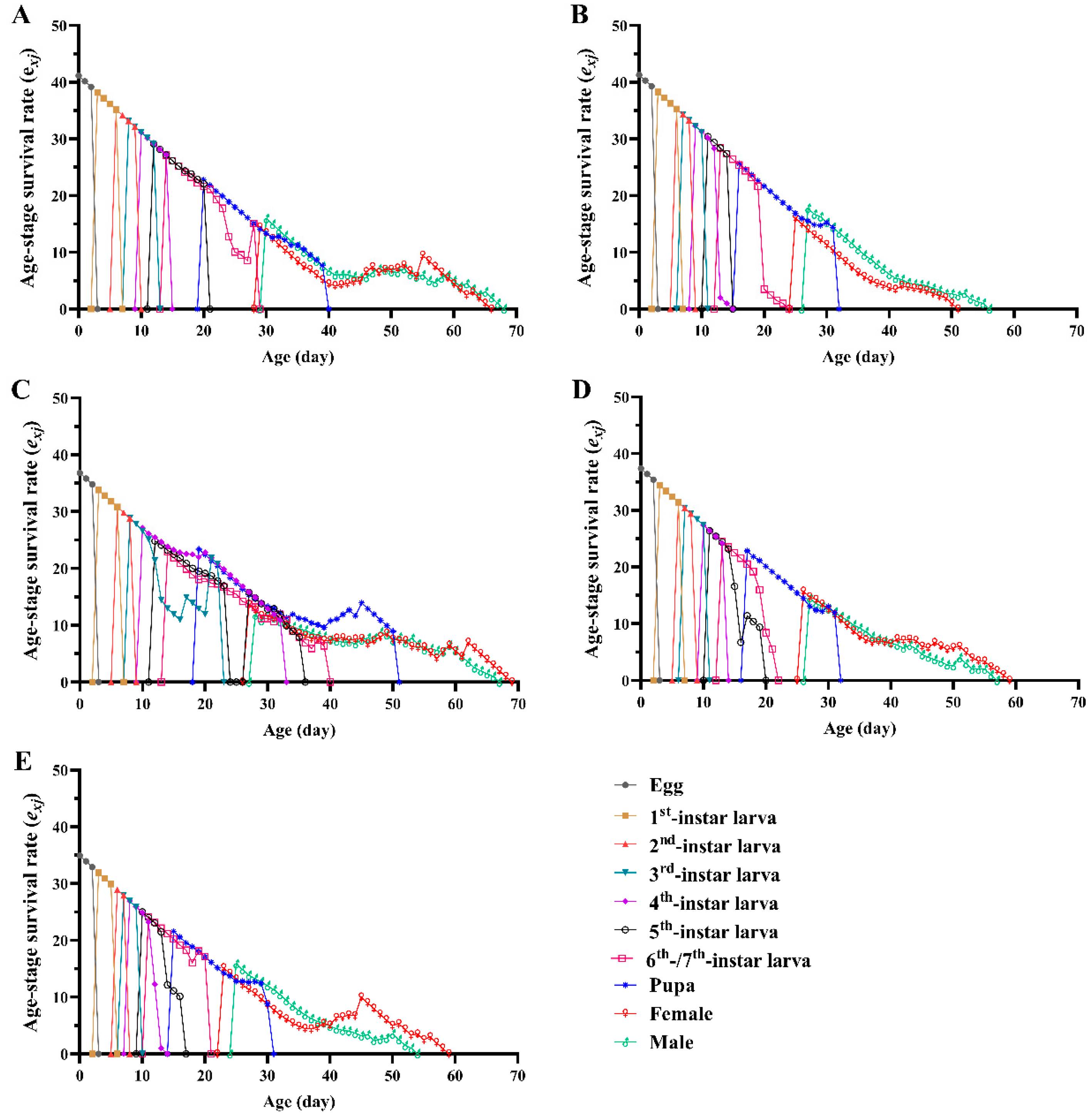
3.2. Survival Rate, Fecundity and Life Expectancy
3.3. Life Table Parameters
3.4. Flight Ability
3.5. Evaluation of Cost of Four Artificial Diets
4. Discussion
5. Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sparks, A.N. A review of the biology of the fall Armyworm. Fla. Entomol. 1979, 62, 82–87. [Google Scholar] [CrossRef]
- Goergen, G.; Kumar, P.L.; Sankung, S.B.; Togola, A.; Tamò, M. First report of outbreaks of the fall armyworm Spodoptera frugiperda (J E Smith) (Lepidoptera, Noctuidae), a new alien invasive pest in West and Central Africa. PLoS ONE 2016, 11, e165632. [Google Scholar] [CrossRef] [PubMed]
- Sharanabasappa, D.; Kalleshwaraswamy, C.M.; Asokan, R.; Swamy, M.; Maruthi, M.S.; Pavithra, H.B.; Hegde, K.; Navi, S.; Prabhu, S.T.; Goergen, G. First report of the fall armyworm, Spodaptera frugiperda (J E Smith) (Lepidoptera: Noctuidae), an alien invasive pest on maize in India. Pest Manag. Hortic. Ecosyst. 2018, 24, 23–29. [Google Scholar]
- IPPC (International Plant Protection Convention). First Detection Report of the Fall Armyworm Spodaptera frugiperda (Lepidoptera: Noctuidae) on Maize in Myanmar; Food and Agriculture Organization of the United Nations: Rome, Italy, 2019. [Google Scholar]
- Sun, X.X.; Hu, C.X.; Jia, H.R.; Wu, Q.L.; Shen, X.J.; Zhao, S.Y.; Jiang, Y.Y.; Wu, K.M. Case study on the first immigration of fall armyworm Spodoptera frugiperda invading into China. J. Integr. Agric. 2021, 20, 664–672. [Google Scholar] [CrossRef]
- Montezano, D. Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018, 26, 286–300. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, B.; Zheng, W.G.; Liu, C.H.; Zhang, D.D.; Zhao, S.Y.; Li, Z.Y.; Xu, P.J.; Wilson, K.; Withers, A.; et al. Genetic structure and insecticide resistance characteristics of fall armyworm populations invading China. Mol. Ecol. Resour. 2020, 20, 1682–1696. [Google Scholar] [CrossRef]
- Baudron, F.; Zaman-Allah, M.A.; Chaipa, I.; Chari, N.; Chinwada, P. Understanding the factors influencing fall armyworm (Spodoptera frugiperda JE Smith) damage in African smallholder maize fields and quantifying its impact on yield. A case study in Eastern Zimbabwe. Crop Prot. 2019, 120, 141–150. [Google Scholar] [CrossRef]
- Wyckhuys, K.A.; O’Neil, R.J. Population dynamics of Spodoptera frugiperda smith (Lepidoptera: Noctuidae) and associated arthropod natural enemies in Honduran subsistence maize. Crop Prot. 2006, 25, 1180–1190. [Google Scholar] [CrossRef]
- Mahmood, I.; Imadi, S.R.; Shazadi, K.; Gul, A.; Hakeem, K.R. Effects of pesticides on environment. In Plant, Soil and Microbes; Springer: Cham, Switzerland, 2016; pp. 253–269. [Google Scholar]
- Pitre, H.N. Chemical control of the fall armyworm (Lepidoptera: Noctuidae): An update. Fla. Entomol. 1986, 69, 570–578. [Google Scholar] [CrossRef]
- Yu, S.J. Insecticide resistance in the fall armyworm, Spodoptera frugiperda (Smith, J.E.). Pestic. Biochem. Phys. 1991, 39, 84–91. [Google Scholar] [CrossRef]
- Storer, N.P.; Babcock, J.M.; Schlenz, M.; Meade, T.; Thompson, G.D.; Bing, J.W.; Huckaba, R.M. Discovery and characterization of field resistance to Bt maize: Spodoptera frugiperda (Lepidoptera: Noctuidae) in Puerto Rico. J. Econ. Entomol. 2010, 103, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Qureshi, J.A.; Head, G.P.; Price, P.A.; Levy, R.; Yang, F.; Niu, Y. Frequency of Bacillus thuringiensis Cry1A. 105 resistance alleles in field populations of the fall armyworm, Spodoptera frugiperda, in Louisiana and Florida. Crop Prot. 2016, 83, 83–89. [Google Scholar] [CrossRef]
- Chandrasena, D.I.; Signorini, A.M.; Abratti, G.; Storer, N.P.; Olaciregui, M.L.; Alves, A.P.; Pilcher, C.D. Characterization of field-evolved resistance to Bacillus thuringiensis-derived Cry1F δ-endotoxin in Spodoptera frugiperda populations from Argentina. Pest Manag. Sci. 2018, 74, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Farias, J.R.; Andow, D.A.; Horikoshi, R.J.; Sorgatto, R.J.; Fresia, P.; dos Santos, A.C.; Omoto, C. Field-evolved resistance to Cry1F maize by Spodoptera frugiperda (Lepidoptera: Noctuidae) in Brazil. Crop Prot. 2014, 64, 150–158. [Google Scholar] [CrossRef]
- Knipling, E.F. Possibilities of insect control or eradication through the use of sexually sterile males. J. Econ. Entomol. 1955, 48, 459–462. [Google Scholar] [CrossRef]
- Klassen, W. Area-wide integrated pest management and the sterile insect technique. In Sterile Insect Technique; Springer: Dordrecht, The Netherlands, 2005; pp. 39–68. [Google Scholar]
- Baumhover, A.H.; Graham, A.J.; Bitter, B.A.; Hopkins, D.E.; New, W.D.; Dudley, F.H.; Bushland, R.C. Screw-Worm Control Through Release of Sterilized Flies. J. Econ. Entomol. 1955, 48, 462–466. [Google Scholar] [CrossRef]
- Wyss, J.H. Screwworm eradication in the Americas. Ann. N. Y. Acad. Sci. 2000, 916, 186–193. [Google Scholar] [CrossRef]
- Walters, M.L.; Staten, R.T.; Roberson, R.C. Pink bollworm integrated management using sterile insects under field trial conditions, Imperial Valley, California. In Area-Wide Control of Fruit Flies and Other Insect Pests; Penerbit Universiti Sains Malaysia: Penang, Malaysia, 2000; pp. 201–206. [Google Scholar]
- Bloem, K.A.; Bloem, S. SIT for codling moth eradication in British Columbia, Canada. In Area-Wide Control of Fruit Flies and Other Insect Pests; Penerbit Universiti Sains Malaysia: Penang, Malaysia, 2000; pp. 207–214. [Google Scholar]
- Jiang, S.; He, L.M.; He, W.; Zhao, H.Y.; Yang, X.M.; Yang, X.Q.; Wu, K.M. Effects of X-ray irradiation on the fitness of the established invasive pest fall armyworm Spodoptera frugiperda. Pest Manag. Sci. 2022, 78, 2806–2815. [Google Scholar] [CrossRef]
- Rose, A.H.; Silveriders, R.H.; Lindguist, O.H. Migration flight by an aphid, Rhopalosiphum maidis (Hemiptera: Aphididae), and a noctuid, Spodoptera frugiperda (Lepidoptera: Noctuidae). Can. Entomol. 1975, 107, 567–576. [Google Scholar] [CrossRef]
- Mitchell, E.R.; McNeil, J.N.; Westbrook, J.K.; Silvain, J.F.; Lalanne-Cassou, B.; Chalfant, R.B.; Pair, S.D.; Waddill, V.H.; Sotomayor-Rios, A.; Proshold, F.I. Seasonal periodicity of fall armyworm (Lepidoptera: Noctuidae) in the Caribbean basin and northward to Canada. J. Entomol. Sci. 1991, 26, 39–50. [Google Scholar] [CrossRef]
- Song, Y.F.; Wu, K.M. Investigation on controlling status of fall armyworm in sweet/waxy corn fields in western Yunnan province. Plant Prot. 2020, 46, 217–222. [Google Scholar]
- Gonzalez-Maldonado, M.B.; Hernandez-Zetina, D.A.; Ruiz-Cancino, E. Parasitoids (Diptera: Tachinidae) of the fall armyworm Spodoptera frugiperda (J. E. Smith) in maize in Durango, Mexico. Southwest Entomol. 2018, 43, 183–187. [Google Scholar] [CrossRef]
- López, M.A.; Martínez-Castillo, A.M.; García-Gutiérrez, C.; Cortez-Mondaca, E.; Escobedo-Bonilla, C.M. Parasitoids and entomopathogens associated with fall armyworm, Spodoptera frugiperda, in Northern Sinaloa. Southwest Entomol. 2018, 43, 867–881. [Google Scholar] [CrossRef]
- Hussain, A.G.; Wennmann, J.T.; Goergen, G.; Bryon, A.; Ros, V.I. Viruses of the fall armyworm Spodoptera frugiperda: A review with prospects for biological control. Viruses 2021, 13, 2220. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.C. Standards for Effective Insect Rearing Science and Technology Papers. Adv. Entomol. 2018, 6, 256–284. [Google Scholar] [CrossRef][Green Version]
- Cohen, A.C. Ecology of Insect Rearing Systems: A Mini-Review of Insect Rearing Papers from 1906–2017. Adv. Entomol. 2018, 6, 86–115. [Google Scholar] [CrossRef]
- Zeng, F.R. Research of insect artificial diet. Chin. J. Biol. Control 2018, 34, 184–197. [Google Scholar]
- Bowling, C.C. Rearing of two Lepidopterous pests of rice on a common artificial diet. Ann. Entomol. Soc. Am. 1967, 60, 1215–1216. [Google Scholar] [CrossRef]
- Pinto, J.R.L.; Torres, A.F.; Truzi, C.C.; Vieira, N.F.; Vacari, A.M.; De Bortoli, S.A. Artificial corn-based diet for rearing Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Insect Sci. 2019, 19, 2. [Google Scholar] [CrossRef]
- Wang, R.F.; Zhang, M.F.; Xu, H.H.; Zhang, Z.X. Artificial diets and rearing technique of Spodoptera frugiperda (J. E. Smith) in laboratory. J. Environ. Entomol. 2019, 41, 742–747. [Google Scholar]
- Su, X.N.; Li, C.Y.; Huang, S.H.; Liu, W.L.; Zhang, Y.P.; Pan, Z.P. Optimization of artificial diet and rearing condition of fall armyworm, Spodoptera frugiperda (J. E. Smith). J. Environ. Entomol. 2019, 41, 992–998. [Google Scholar]
- Li, C.Y.; Zhang, Y.P.; Huang, S.H.; Liu, W.L.; Su, X.N.; Pan, Z.P. A study on artificial rearing technique of Spodoptera frugiperda (J. E. Smith) in the laboratory. J. Environ. Entomol. 2019, 41, 986–991. [Google Scholar]
- Li, Z.Y.; Dai, Q.X.; Kuang, Z.L.; Liang, M.R.; Wang, L.; Lu, Y.Y.; Chen, K.W. Effects of three artificial diets on development and reproduction of the fall armyworm Spodoptera frugiperda (J. E. Smith). J. Environ. Entomol. 2019, 41, 1147–1154. [Google Scholar]
- Lekha, M.K.; Swami, H.; Vyas, A.K.; Ahir, K.C. Biology of fall armyworm, Spodoptera frugiperda (JE Smith) on different artificial diets. J. Entomol. Zool. Stud. 2020, 8, 584–586. [Google Scholar]
- Liang, G.M.; Tan, W.J.; Guo, Y.Y. Improvement of artificial rearing technique of Helicoverpa armigera. Plant Prot. 1999, 25, 15–17. [Google Scholar]
- Ge, S.S.; He, L.M.; He, W.; Yan, R.; Wyckhuys, K.A.G.; Wu, K.M. Laboratory-based flight performance of the fall armyworm Spodoptera frugiperda. J. Integr. Agric. 2021, 20, 707–714. [Google Scholar] [CrossRef]
- Chi, H. Life-table analysis incorporating both sexes and variable development rate among individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Chi, H. TWOSEX-MSChart: A Computer Program for Age-Stage, Two-Sex Life Table Analysis. 2020. Available online: http://140.120.197.173/Ecology/ (accessed on 27 March 2020).
- Chi, H.; Su, H.Y. Age-stage, two-sex life tables of Aphidtus gifuensts (Ashmead) (Hymenoptera: Braconidae) and its host Myzus perstcae (Sulzer) (Homoptera: Aphididae) with mathematical proof of the relationship between female fecundity and the net reproductive rate. Environ. Entomol. 2006, 35, 10–21. [Google Scholar] [CrossRef]
- He, L.M.; Zhao, S.Y.; Ali, A.; Ge, S.S.; Wu, K.M. Ambient Humidity Affects Development, Survival, and Reproduction of the Invasive Fall Armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae), in China. J. Econ. Entomol. 2021, 114, 1145–1158. [Google Scholar] [CrossRef]
- Akca, I.; Ayvaz, T.; Yazici, E.; Smith, C.L.; Chi, H. Demography and population projection of Aphis fabae (Hemiptera: Aphididae): With additional comments on life table research criteria. J. Econ. Entomol. 2015, 108, 1466–1478. [Google Scholar] [CrossRef]
- Murúa, M.G.; Vera, M.T.; Abraham, S.; Juárez, M.L.; Prieto, S.; Head, G.P.; Willink, E. Fitness and mating compatibility of Spodoptera frugiperda (Lepidoptera: Noctuidae) populations from different host plant species and regions in Argentina. Ann. Entomol. Soc. Am. 2008, 101, 639–649. [Google Scholar] [CrossRef]
- He, L.M.; Wu, Q.L.; Gao, X.W.; Wu, K.M. Population life tables for the invasive fall armyworm, Spodoptera frugiperda fed on major oil crops planted in China. J. Integr. Agric. 2021, 20, 745–754. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chen, D.F.; Yang, M.F.; Liu, J.F. The Effect of Temperatures and Hosts on the Life Cycle of Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects 2022, 13, 211. [Google Scholar] [CrossRef]
- Meunier, J.; Kölliker, M. Inbreeding depression in an insect with maternal care: Influences of family interactions, life stage and offspring sex. J. Evol. Biol. 2013, 26, 2209–2220. [Google Scholar] [CrossRef] [PubMed]
- Mattey, S.N.; Strutt, L.; Smiseth, P.T. Intergenerational effects of inbreeding in Nicrophorus vespilloides: Offspring suffer fitness costs when either they or their parents are inbred. J. Evol. Biol. 2013, 26, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Scriber, J.M.; Slansky Jr, F. The nutritional ecology of immature insects. Annu. Rev. Entomol. 1981, 26, 183–211. [Google Scholar] [CrossRef]
- Brodbeck, B.V.; Peter, C.A.; Russell, F.M. Effects of total dietary nitrogen and nitrogen form on the development of xylophagous leafhoppers. Arch. Insect Biochem. 1999, 42, 37–50. [Google Scholar] [CrossRef]
- Opit, G.P.; Throne, J.E. Effects of diet on population growth of the psocids Lepinotus reticulatus and Liposcelis entomophila. J. Econ. Entomol. 2014, 101, 616–622. [Google Scholar] [CrossRef]
- Wu, K.J.; Li, M.H. Nutritional ecology of the cotton bollworm, Heliothis armigera (Hübner): Life tables of the population on the artificial diets with different protein levels. Acta Entomol. Sin. 1993, 36, 21–28. [Google Scholar]
- Cohen, A.C.; Zeng, F.; Crittenden, P. Adverse effects of raw soybean extract on survival and growth of Lygus hesperus. J. Entomol. Sci. 2005, 40, 390–400. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chen, L.; Shang, H.W.; Zhu, C.Y. Research advances and nutrition analysis of the artificial diets for Tephritid fruit flies. Plant Quarant. 2013, 27, 13–19. [Google Scholar]
- Pascacio-Villafán, C.; Williams, T.; Sivinski, J.; Birke, A.; Aluja, M. Costly nutritious diets do not necessarily translate into better performance of artificially reared fruit flies (Diptera: Tephritidae). J. Econ. Entomol. 2015, 108, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Pascacio-Villafán, C.; Birke, A.; Williams, T.; Aluja, M. Modeling the cost-effectiveness of insect rearing on artificial diets: A test with a tephritid fly used in the sterile insect technique. PLoS ONE 2017, 12, e0173205. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.B. Comparison of two gelling agents for screwworm (Diptera: Calliphoridae) larval diets. J. Econ. Entomol. 1988, 81, 1414–1419. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.B.; Bruce, J.C.; Garcia, R. Gelled diet for screwworm (Diptera: Calliphoridae) mass production. J. Econ. Entomol. 1991, 84, 927–935. [Google Scholar] [CrossRef]
- Chaudhury, M.F.; Alvarez, L.A. A new starch-grafted gelling agent for screwworm (Diptera: Calliphoridae) larval diet. J. Econ. Entomol. 1999, 92, 1138–1141. [Google Scholar] [CrossRef][Green Version]
- Ge, S.S.; Zhang, H.W.; Liu, D.Z.; Lv, C.Y.; Cang, X.Z.; Sun, X.X.; Song, Y.F.; He, W.; Chu, B.; Zhao, S.Y.; et al. Seasonal migratory activity of Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae) across China and Myanmar. Pest Manag. Sci. 2022, 78, 4975–4982. [Google Scholar] [CrossRef] [PubMed]
| Material | D1 | D2 | D3 | D4 |
|---|---|---|---|---|
| Wheat bran (g) | 50 | 150 | - | 260 |
| Soybean powder (g) | 40 | 80 | 260 | - |
| Maize powder (g) | 100 | - | - | - |
| Yeast powder (g) | 30 | 30 | - | - |
| Agar (g) | 24 | 20 | 20 | 20 |
| Casein (g) | 40 | 40 | 40 | 40 |
| Sorbic acid (g) | 3 | 3 | 3 | 3 |
| Ascorbic acid (g) | 3.5 | 3 | 3 | 3 |
| Vitamins (g) | 0.15 | 0.1 | 0.1 | 0.1 |
| Formaldehyde (mL) | 4 | 2 | 2 | 2 |
| Glacial acetic acid (mL) | 4 | 4 | 4 | 4 |
| Distilled water (mL) | 1200 | 1500 | 1500 | 1500 |
| Parameter | D1 | D2 | D3 | D4 | Maize Leaves |
|---|---|---|---|---|---|
| Egg (day) | 3.00 ± 0.00 | 3.00 ± 0.00 | 3.00 ± 0.00 | 3.00 ± 0.00 | 3.00 ± 0.00 |
| 1st-instar larva (day) | 3.15 ± 0.02 b | 3.12 ± 0.02 bc | 3.08 ± 0.02 cd | 3.26 ± 0.03 a | 3.00 ± 0.00 d |
| 2nd-instar larva (day) | 2.25 ± 0.03 b | 1.97 ± 0.02 c | 2.37 ± 0.03 a | 1.90 ± 0.03 c | 1.36 ± 0.03 d |
| 3rd-instar larva (day) | 2.24 ± 0.03 b | 2.04 ± 0.03 c | 2.56 ± 0.07 a | 2.15 ± 0.03 bc | 1.55 ± 0.03 d |
| 4th-instar larva (day) | 2.27 ± 0.03 b | 1.57 ± 0.03 d | 2.75 ± 0.10 a | 1.83 ± 0.03 c | 1.74 ± 0.03 cd |
| 5th-instar larva (day) | 3.08 ± 0.05 b | 2.10 ± 0.02 c | 3.50 ± 0.09 c | 2.18 ± 0.03 c | 2.04 ± 0.02 a |
| 6th-/7th-instar larva (day) | 5.91 ± 0.08 b | 4.73 ± 0.04 c | 6.47 ± 0.11 a | 4.62 ± 0.04 c | 4.50 ± 0.06 c |
| Larval stage (day) | 18.90 ± 0.08 b | 15.54 ± 0.06 c | 20.94 ± 0.27 a | 15.88 ± 0.06 c | 14.22 ± 0.08 d |
| Pupa (day) | 10.22 ± 0.07 a | 10.08 ± 0.06 ab | 9.82 ± 0.08 bc | 9.48 ± 0.06 d | 9.74 ± 0.07 cd |
| Egg–pupa (day) | 32.10 ± 0.12 b | 28.59 ± 0.10 c | 33.80 ± 0.33 a | 28.40 ± 0.10 c | 27.01 ± 0.13 d |
| Adult longevity (day) | 12.60 ± 0.36 b | 14.63 ± 0.31 a | 9.96 ± 0.50 c | 13.70 ± 0.47 ab | 12.85 ± 0.42 b |
| Total generation (day) | 44.70 ± 0.39 a | 43.28 ± 0.33 ab | 42.29 ± 0.55 b | 42.10 ± 0.49 b | 39.86 ± 0.42 c |
| Pupal mass (mg) | 241.00 ± 1.98 c | 261.65 ± 1.90 b | 231.48 ± 3.35 d | 270.45 ± 1.64 a | 209.17 ± 2.22 e |
| Pre-oviposition period (day) | 4.85 ± 0.21 a | 4.58 ± 0.21 ab | 4.21 ± 0.24 ab | 4.71 ± 0.18 ab | 3.96 ± 0.16 b |
| Oviposition period (day) | 5.22 ± 0.23 b | 6.84 ± 0.21 a | 4.90 ± 0.29 b | 6.26 ± 0.27 a | 6.32 ± 0.23 a |
| Eggs deposited per female (n) | 859.69 ± 43.40 b | 1439.16 ± 53.71 a | 1005.38 ± 77.77 b | 1364.78 ± 70.51 a | 1261.41 ± 62.72 a |
| Mating rate (%) | 86.57 ± 2.11 ab | 93.93 ± 1.92 ab | 83.89 ± 2.98 b | 89.53 ± 2.13 ab | 94.86 ± 1.43 a |
| Parameters | D1 | D2 | D3 | D4 | Maize Leaves |
|---|---|---|---|---|---|
| Larval survival rate % | 92.08 ± 2.08 ab | 98.33 ± 1.10 a | 71.25 ± 8.20 b | 87.08 ± 4.81 ab | 90.83 ± 2.08 ab |
| Pupal survival rate % | 86.44 ± 2.83 a | 91.55 ± 2.89 a | 89.29 ± 3.63 a | 85.15 ± 5.40 a | 78.87 ± 2.15 a |
| adult deformity rate % | 0.57 ± 0.56 c | 2.37 ± 0.49 bc | 14.36 ± 3.48 a | 6.05 ± 2.32 ab | 5.67 ± 1.91 abc |
| Parameter | D1 | D2 | D3 | D4 | Maize Leaves |
|---|---|---|---|---|---|
| Net reproductive rate R0 (offspring/individual) | 288.59 ± 29.55 c | 580.37 ± 50.13 a | 176.53 ± 28.10 d | 410.23 ± 44.28 b | 384.64 ± 41.97 bc |
| Mean generation time T (d) | 37.89 ± 0.27 a | 34.25 ± 0.17 b | 37.11 ± 0.49 a | 34.52 ± 0.22 b | 32.16 ± 0.20 c |
| Intrinsic rate of natural increase r (d−1) | 0.1495 ± 0.0031 c | 0.1858 ± 0.0028 a | 0.1394 ± 0.0050 c | 0.1743 ± 0.0036 b | 0.1851 ± 0.0037a |
| Finite rate of increase λ (d−1) | 1.161 ± 0.0035 c | 1.204 ± 0.0033 a | 1.150 ± 0.0057 c | 1.1904 ± 0.0043 b | 1.203 ± 0.0044 a |
| Parameter | D1 | D2 | D3 | D4 | Maize Leaves |
|---|---|---|---|---|---|
| Flight distance (km) | 24.14 ± 1.80 a | 22.97 ± 2.19 a | 18.88 ± 2.95 b | 19.73 ± 1.58 ab | 18.71 ± 1.27 ab |
| Flight duration (h) | 7.11 ± 0.39 a | 7.12 ± 0.54 a | 6.20 ± 0.71 a | 6.91 ± 0.46 a | 5.65 ± 0.35 a |
| Flight velocity (km/h) | 3.34 ± 0.14 a | 3.30 ± 0.18 a | 2.67 ± 0.21 b | 2.90 ± 0.13 ab | 3.44 ± 0.16 a |
| Diet | Cost Per Ton (RMB) | Diet Amount of Culturing 1 Million FAWs (ton) | Saving Cost by Culturing 1 Million FAWs (%) |
|---|---|---|---|
| D1 | 6890.00 | 6.62 | 43.08 |
| D2 | 5670.00 | 6.00 | 23.68 |
| D3 | 5900.00 | 5.03 | 12.51 |
| D4 | 3970.00 | 6.54 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, S.; Chu, B.; He, W.; Jiang, S.; Lv, C.; Gao, L.; Sun, X.; Yang, X.; Wu, K. Wheat-Bran-Based Artificial Diet for Mass Culturing of the Fall Armyworm, Spodoptera frugiperda Smith (Lepidoptera: Noctuidae). Insects 2022, 13, 1177. https://doi.org/10.3390/insects13121177
Ge S, Chu B, He W, Jiang S, Lv C, Gao L, Sun X, Yang X, Wu K. Wheat-Bran-Based Artificial Diet for Mass Culturing of the Fall Armyworm, Spodoptera frugiperda Smith (Lepidoptera: Noctuidae). Insects. 2022; 13(12):1177. https://doi.org/10.3390/insects13121177
Chicago/Turabian StyleGe, Shishuai, Bo Chu, Wei He, Shan Jiang, Chunyang Lv, Lingyun Gao, Xiaoting Sun, Xianming Yang, and Kongming Wu. 2022. "Wheat-Bran-Based Artificial Diet for Mass Culturing of the Fall Armyworm, Spodoptera frugiperda Smith (Lepidoptera: Noctuidae)" Insects 13, no. 12: 1177. https://doi.org/10.3390/insects13121177
APA StyleGe, S., Chu, B., He, W., Jiang, S., Lv, C., Gao, L., Sun, X., Yang, X., & Wu, K. (2022). Wheat-Bran-Based Artificial Diet for Mass Culturing of the Fall Armyworm, Spodoptera frugiperda Smith (Lepidoptera: Noctuidae). Insects, 13(12), 1177. https://doi.org/10.3390/insects13121177






