Using the Software DeepWings© to Classify Honey Bees across Europe through Wing Geometric Morphometrics
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
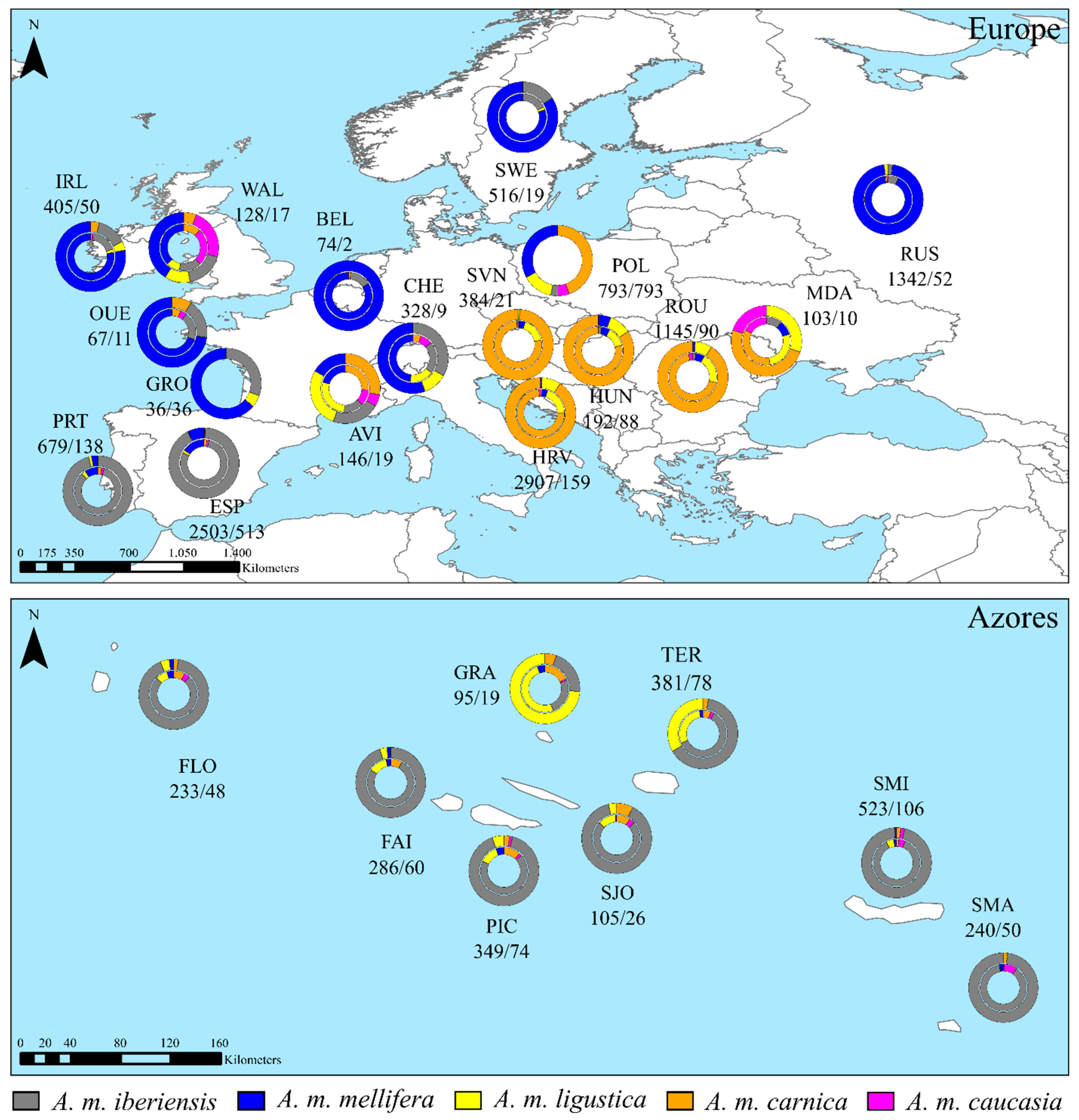
2.1. Wing Samples
2.2. DeepWings© Analysis
2.3. Association between Wing Data and Molecular Data
2.4. Statistical Analysis
3. Results
3.1. Classification of the Total Wings Dataset
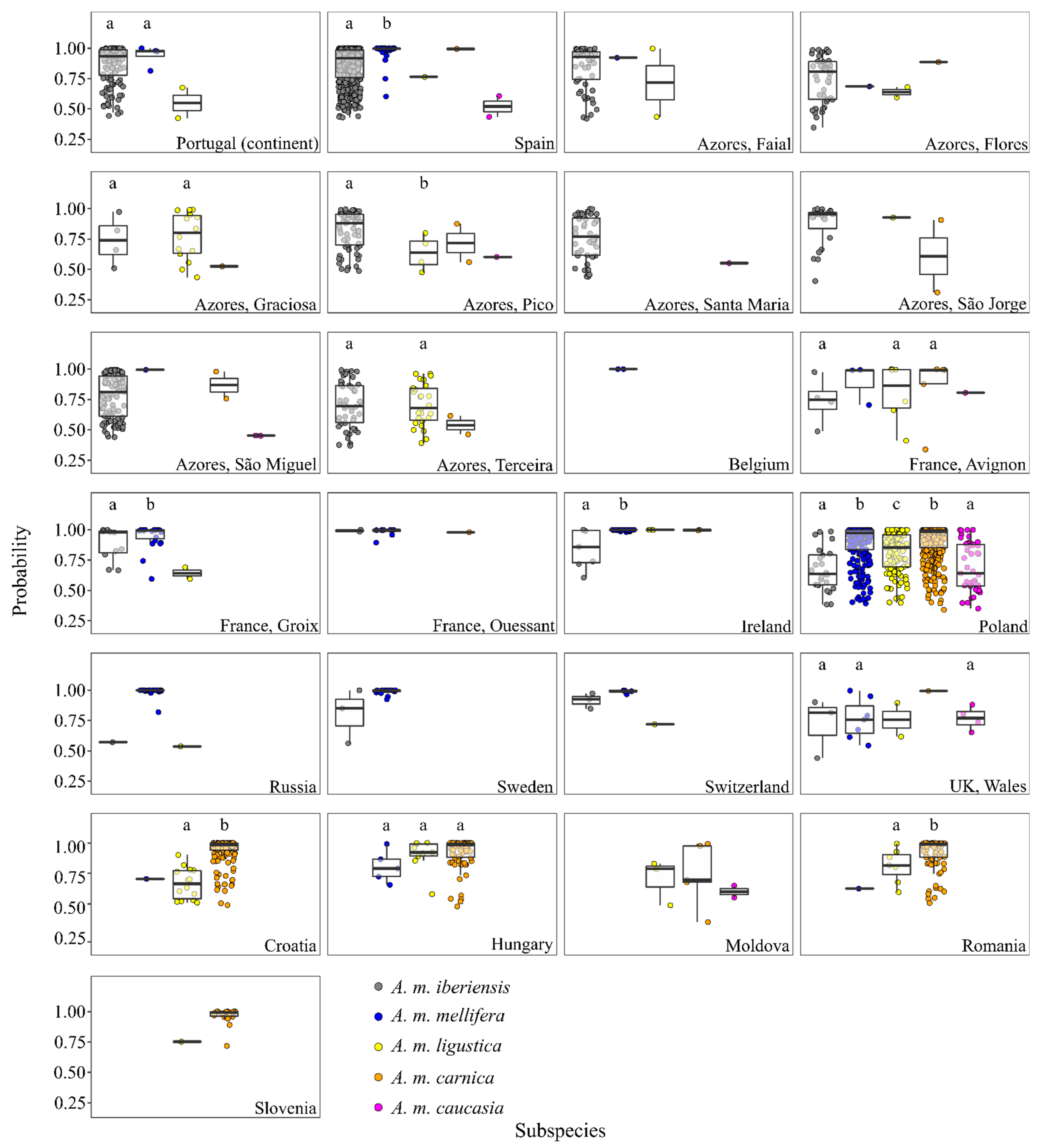
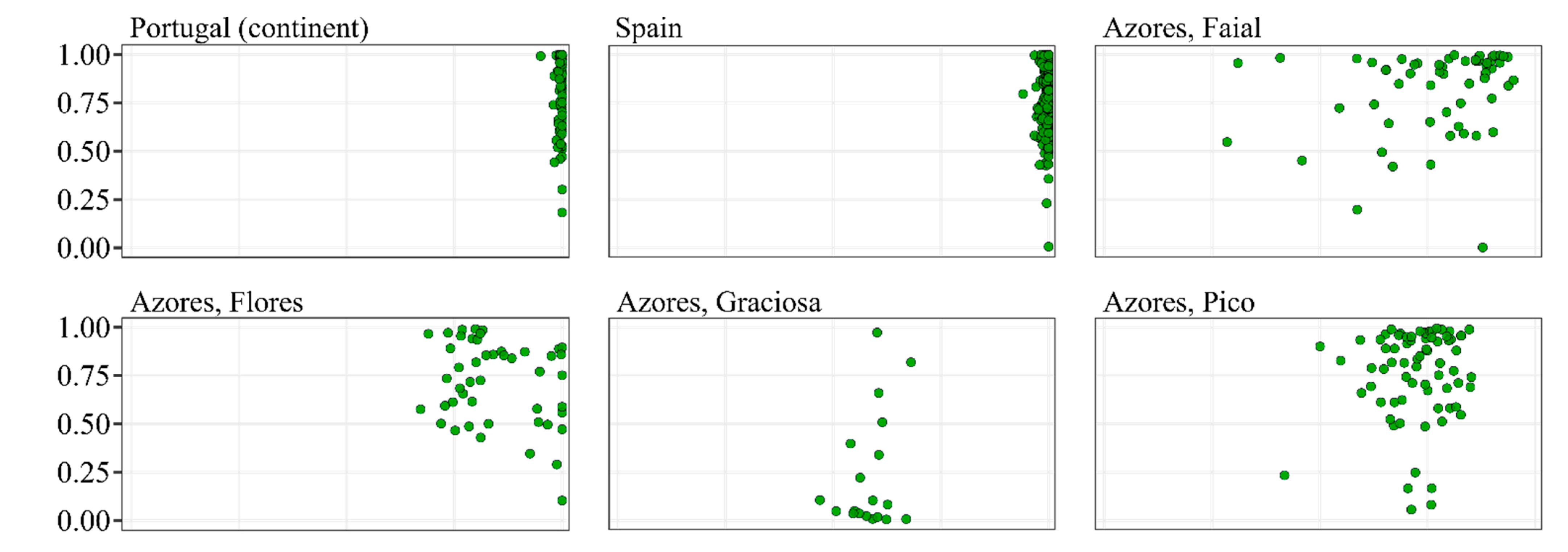
3.2. Classification of Colonies Sampled in the Native Range of A. m. iberiensis
3.3. Classification of Colonies Sampled in the Native Range of A. m. mellifera
3.4. Classification of Colonies Sampled in the Native Range of A. m. carnica
3.5. Association between Wing Data and Molecular Data
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Engel, M.S. The taxonomy of recent and fossil honey bees (Hymenoptera: Apidae; Apis). J. Hymenopt. Res. 1999, 8, 165–196. [Google Scholar]
- Meixner, M.D.; Leta, M.A.; Koeniger, N.; Fuchs, S. The honey bees of Ethiopia represent a new subspecies of Apis mellifera—Apis mellifera simensis n. ssp. Apidologie 2011, 42, 425–437. [Google Scholar] [CrossRef]
- Sheppard, W.S.; Meixner, M.D. Apis mellifera pomonella, a new honey bee subspecies from Central Asia. Apidologie 2003, 34, 367–375. [Google Scholar] [CrossRef]
- Chen, C.; Liu, Z.; Pan, Q.; Chen, X.; Wang, H.; Guo, H.; Liu, S.; Lu, H.; Tian, S.; Li, R. Genomic analyses reveal demographic history and temperate adaptation of the newly discovered honey bee subspecies Apis mellifera sinisxinyuan n. ssp. Mol. Biol. Evol. 2016, 33, 1337–1348. [Google Scholar] [CrossRef]
- Ruttner, F. Biogeography and Taxonomy of Honeybees; Springer Verlag: Berlin, Germany, 1988. [Google Scholar]
- Sheppard, W. A history of the introduction of honey bee races into the United States. Part 1. Am. Bee J. 1989, 129, 617–619. [Google Scholar]
- Sheppard, W. A history of the introduction of honey bee races into the United States. Part 2. Am. Bee J. 1989, 129, 664–667. [Google Scholar]
- De La Rúa, P.; Galián, J.; Serrano, J.; Moritz, R.F. Genetic structure and distinctness of Apis mellifera L. populations from the Canary Islands. Mol. Ecol. 2001, 10, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.B.; Palmer, K.A.; Boomsma, J.J.; Pedersen, B.V. Varying degrees of Apis mellifera ligustica introgression in protected populations of the black honeybee, Apis mellifera mellifera, in northwest Europe. Mol. Ecol. 2005, 14, 93–106. [Google Scholar] [CrossRef]
- Soland-Reckeweg, G.; Heckel, G.; Neumann, P.; Fluri, P.; Excoffier, L. Gene flow in admixed populations and implications for the conservation of the Western honeybee, Apis mellifera. J. Insect Conserv. 2009, 13, 317–328. [Google Scholar] [CrossRef]
- Oleksa, A.; Chybicki, I.; Tofilski, A.; Burczyk, J. Nuclear and mitochondrial patterns of introgression into native dark bees (Apis mellifera mellifera) in Poland. J. Apic. Res. 2011, 50, 116–129. [Google Scholar] [CrossRef]
- Ellis, J.S.; Soland-Reckeweg, G.; Buswell, V.G.; Huml, J.V.; Brown, A.; Knight, M.E. Introgression in native populations of Apis mellifera mellifera L: Implications for conservation. J. Insect Conserv. 2018, 22, 377–390. [Google Scholar] [CrossRef]
- Pinto, M.A.; Henriques, D.; Chávez-Galarza, J.; Kryger, P.; Garnery, L.; van der Zee, R.; Dahle, B.; Soland-Reckeweg, G.; De la Rúa, P.; Dall’Olio, R. Genetic integrity of the Dark European honey bee (Apis mellifera mellifera) from protected populations: A genome-wide assessment using SNPs and mtDNA sequence data. J. Apic. Res. 2014, 53, 269–278. [Google Scholar] [CrossRef]
- Groeneveld, L.F.; Kirkerud, L.A.; Dahle, B.; Sunding, M.; Flobakk, M.; Kjos, M.; Henriques, D.; Pinto, M.A.; Berg, P. Conservation of the dark bee (Apis mellifera mellifera): Estimating C-lineage introgression in Nordic breeding stocks. Acta Agric. Scand. Sect. A Anim. Sci. 2020, 69, 157–168. [Google Scholar] [CrossRef]
- De la Rúa, P.; Jaffé, R.; Dall’Olio, R.; Muñoz, I.; Serrano, J. Biodiversity, conservation and current threats to European honeybees. Apidologie 2009, 40, 263–284. [Google Scholar] [CrossRef]
- Garnery, L.; Solignac, M.; Celebrano, G.; Cornuet, J.-M. A simple test using restricted PCR-amplified mitochondrial DNA to study the genetic structure of Apis mellifera L. Experientia 1993, 49, 1016–1021. [Google Scholar] [CrossRef]
- Momeni, J.; Parejo, M.; Nielsen, R.O.; Langa, J.; Montes, I.; Papoutsis, L.; Farajzadeh, L.; Bendixen, C.; Căuia, E.; Charrière, J.-D. Authoritative subspecies diagnosis tool for European honey bees based on ancestry informative SNPs. BMC Genom. 2021, 22, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Henriques, D.; Browne, K.A.; Barnett, M.W.; Parejo, M.; Kryger, P.; Freeman, T.C.; Muñoz, I.; Garnery, L.; Highet, F.; Jonhston, J.S. High sample throughput genotyping for estimating C-lineage introgression in the dark honeybee: An accurate and cost-effective SNP-based tool. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef]
- Henriques, D.; Parejo, M.; Vignal, A.; Wragg, D.; Wallberg, A.; Webster, M.T.; Pinto, M.A. Developing reduced SNP assays from whole-genome sequence data to estimate introgression in an organism with complex genetic patterns, the Iberian honeybee (Apis mellifera iberiensis). Evol. Appl. 2018, 11, 1270–1282. [Google Scholar] [CrossRef]
- Solignac, M.; Vautrin, D.; Loiseau, A.; Mougel, F.; Baudry, E.; Estoup, A.; Garnery, L.; Haberl, M.; Cornuet, J.M. Five hundred and fifty microsatellite markers for the study of the honeybee (Apis mellifera L.) genome. Mol. Ecol. Notes 2003, 3, 307–311. [Google Scholar] [CrossRef]
- Muñoz, I.; Henriques, D.; Johnston, J.S.; Chávez-Galarza, J.; Kryger, P.; Pinto, M.A. Reduced SNP panels for genetic identification and introgression analysis in the dark honey bee (Apis mellifera mellifera). PLoS ONE 2015, 10, e0124365. [Google Scholar] [CrossRef]
- Chapman, N.C.; Harpur, B.A.; Lim, J.; Rinderer, T.E.; Allsopp, M.H.; Zayed, A.; Oldroyd, B.P. A SNP test to identify Africanized honeybees via proportion of ‘African’ancestry. Mol. Ecol. Resour. 2015, 15, 1346–1355. [Google Scholar] [CrossRef]
- Meixner, M.D.; Pinto, M.A.; Bouga, M.; Kryger, P.; Ivanova, E.; Fuchs, S. Standard methods for characterising subspecies and ecotypes of Apis mellifera. J. Apic. Res. 2013, 52, 1–28. [Google Scholar] [CrossRef]
- Muñoz, I.; Henriques, D.; Jara, L.; Johnston, J.S.; Chávez-Galarza, J.; De La Rúa, P.; Pinto, M.A. SNPs selected by information content outperform randomly selected microsatellite loci for delineating genetic identification and introgression in the endangered dark European honeybee (Apis mellifera mellifera). Mol. Ecol. Resour. 2017, 17, 783–795. [Google Scholar] [CrossRef] [PubMed]
- Parejo, M.; Wragg, D.; Gauthier, L.; Vignal, A.; Neumann, P.; Neuditschko, M. Using whole-genome sequence information to foster conservation efforts for the European Dark Honey Bee, Apis mellifera mellifera. Front. Ecol. Evol. 2016, 4, 140. [Google Scholar] [CrossRef]
- Whitfield, C.W.; Behura, S.K.; Berlocher, S.H.; Clark, A.G.; Johnston, J.S.; Sheppard, W.S.; Smith, D.R.; Suarez, A.V.; Weaver, D.; Tsutsui, N.D. Thrice out of Africa: Ancient and recent expansions of the honey bee, Apis mellifera. Science 2006, 314, 642–645. [Google Scholar] [CrossRef]
- Chávez-Galarza, J.; Garnery, L.; Henriques, D.; Neves, C.J.; Loucif-Ayad, W.; Jonhston, J.S.; Pinto, M.A. Mitochondrial DNA variation of Apis mellifera iberiensis: Further insights from a large-scale study using sequence data of the tRNAleu-cox2 intergenic region. Apidologie 2017, 48, 533–544. [Google Scholar] [CrossRef]
- Chávez-Galarza, J.; Henriques, D.; Johnston, J.S.; Carneiro, M.; Rufino, J.; Patton, J.C.; Pinto, M.A. Revisiting the Iberian honey bee (Apis mellifera iberiensis) contact zone: Maternal and genome-wide nuclear variations provide support for secondary contact from historical refugia. Mol. Ecol. 2015, 24, 2973–2992. [Google Scholar] [CrossRef]
- Muñoz, I.; Dall’Olio, R.; Lodesani, M.; De la Rúa, P. Population genetic structure of coastal Croatian honeybees (Apis mellifera carnica). Apidologie 2009, 40, 617–626. [Google Scholar] [CrossRef]
- Franck, P.; Garnery, L.; Celebrano, G.; Solignac, M.; Cornuet, J.M. Hybrid origins of honeybees from Italy (Apis mellifera ligustica) and Sicily (A. m. sicula). Mol. Ecol. 2000, 9, 907–921. [Google Scholar] [CrossRef]
- Franck, P.; Garnery, L.; Solignac, M.; Cornuet, J.M. The origin of west European subspecies of honeybees (Apis mellifera): New insights from microsatellite and mitochondrial data. Evolution 1998, 52, 1119–1134. [Google Scholar]
- Uzunov, A.; Meixner, M.D.; Kiprijanovska, H.; Andonov, S.; Gregorc, A.; Ivanova, E.; Bouga, M.; Dobi, P.; Büchler, R.; Francis, R. Genetic structure of Apis mellifera macedonica in the Balkan Peninsula based on microsatellite DNA polymorphism. J. Apic. Res. 2014, 53, 288–295. [Google Scholar] [CrossRef]
- Péntek-Zakar, E.; Oleksa, A.; Borowik, T.; Kusza, S. Population structure of honey bees in the Carpathian Basin (Hungary) confirms introgression from surrounding subspecies. Ecol. Evol. 2015, 5, 5456–5467. [Google Scholar] [CrossRef] [PubMed]
- Bouga, M.; Alaux, C.; Bienkowska, M.; Büchler, R.; Carreck, N.L.; Cauia, E.; Chlebo, R.; Dahle, B.; Dall’Olio, R.; De la Rúa, P. A review of methods for discrimination of honey bee populations as applied to European beekeeping. J. Apic. Res. 2011, 50, 51–84. [Google Scholar] [CrossRef]
- Tofilski, A. Automatic Measurement of Honeybee Wings. In Automated Taxon Identification in Systematics; MacLeod, N., Ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 289–298. [Google Scholar]
- Nawrocka, A.; Kandemir, İ.; Fuchs, S.; Tofilski, A. Computer software for identification of honey bee subspecies and evolutionary lineages. Apidologie 2018, 49, 172–184. [Google Scholar] [CrossRef]
- Barour, C.; Baylac, M. Geometric morphometric discrimination of the three African honeybee subspecies Apis mellifera intermissa, A. m. sahariensis and A. m. capensis (Hymenoptera, Apidae): Fore wing and hind wing landmark configurations. J. Hymenopt. Res. 2016, 52, 61–70. [Google Scholar] [CrossRef]
- Henriques, D.; Chávez-Galarza, J.; Teixeira, J.S.; Ferreira, H.; Neves, C.J.; Francoy, T.M.; Pinto, M.A. Wing geometric morphometrics of workers and drones and single nucleotide polymorphisms provide similar genetic structure in the Iberian honey bee (Apis mellifera iberiensis). Insects 2020, 11, 89. [Google Scholar] [CrossRef]
- Oleksa, A.; Tofilski, A. Wing geometric morphometrics and microsatellite analysis provide similar discrimination of honey bee subspecies. Apidologie 2015, 46, 49–60. [Google Scholar] [CrossRef]
- Aglagane, A.; Tofilski, A.; Er-Rguibi, O.; Laghzaoui, E.-M.; Kimdil, L.; El Mouden, E.H.; Fuchs, S.; Oleksa, A.; Aamiri, A.; Aourir, M. Geographical Variation of Honey Bee (Apis mellifera L. 1758) Populations in South-Eastern Morocco: A Geometric Morphometric Analysis. Insects 2022, 13, 288. [Google Scholar] [CrossRef]
- Tofilski, A.; Căuia, E.; Siceanu, A.; Vișan, G.O.; Căuia, D. Historical Changes in Honey Bee Wing Venation in Romania. Insects 2021, 12, 542. [Google Scholar] [CrossRef]
- Tofilski, A. Using geometric morphometrics and standard morphometry to discriminate three honeybee subspecies. Apidologie 2008, 39, 558–563. [Google Scholar] [CrossRef]
- Bookstein, F.L. Morphometric Tools for Landmark Data: Geometry and Biology; Cambridge University Press: Cambridge, UK, 1997; p. 435. [Google Scholar]
- Rodrigues, P.J.; Gomes, W.; Pinto, M.A. DeepWings©: Automatic Wing Geometric Morphometrics Classification of Honey Bee (Apis mellifera) Subspecies Using Deep Learning for Detecting Landmarks. Big Data Cogn. Comput. 2022, 6, 70. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.H.; Novembre, J.; Lange, K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009, 19, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, H.; Henriques, D.; Neves, C.J.; Machado, C.A.; Azevedo, J.C.; Francoy, T.M.; Pinto, M.A. Historical and contemporaneous human-mediated processes left a strong genetic signature on honey bee populations from the Macaronesian archipelago of the Azores. Apidologie 2020, 51, 316–328. [Google Scholar] [CrossRef]
- Wallberg, A.; Han, F.; Wellhagen, G.; Dahle, B.; Kawata, M.; Haddad, N.; Simões, Z.L.P.; Allsopp, M.H.; Kandemir, I.; De la Rúa, P. A worldwide survey of genome sequence variation provides insight into the evolutionary history of the honeybee Apis mellifera. Nat. Genet. 2014, 46, 1081–1088. [Google Scholar] [CrossRef]
- Meixner, M.D.; Worobik, M.; Wilde, J.; Fuchs, S.; Koeniger, N. Apis mellifera mellifera in eastern Europe–morphometric variation and determination of its range limits. Apidologie 2007, 38, 191–197. [Google Scholar] [CrossRef]
- Requier, F.; Garnery, L.; Kohl, P.L.; Njovu, H.K.; Pirk, C.W.; Crewe, R.M.; Steffan-Dewenter, I. The conservation of native honey bees is crucial. Trends Ecol. Evol. 2019, 34, 789–798. [Google Scholar] [CrossRef]
- Bouga, M.; Harizanis, P.C.; Kilias, G.; Alahiotis, S. Genetic divergence and phylogenetic relationships of honey bee Apis mellifera (Hymenoptera: Apidae) populations from Greece and Cyprus using PCR–RFLP analysis of three mtDNA segments. Apidologie 2005, 36, 335–344. [Google Scholar] [CrossRef]
- Ivanova, E.; Staykova, T.; Bouga, M. Allozyme variability in honey bee populations from some mountainous regions in the southwest of Bulgaria. J. Apic. Res. 2007, 46, 3–7. [Google Scholar] [CrossRef]
- Nedić, N.; Francis, R.M.; Stanisavljević, L.; Pihler, I.; Kezić, N.; Bendixen, C.; Kryger, P. Detecting population admixture in honey bees of Serbia. J. Apic. Res. 2014, 53, 303–313. [Google Scholar] [CrossRef]
- Henriques, D.; Lopes, A.R.; Chejanovsky, N.; Dalmon, A.; Higes, M.; Jabal-Uriel, C.; Le Conte, Y.; Reyes-Carreño, M.; Soroker, V.; Martín-Hernández, R. Mitochondrial and nuclear diversity of colonies of varying origins: Contrasting patterns inferred from the intergenic tRNAleu-cox2 region and immune SNPs. J. Apic. Res. 2021, 61, 305–308. [Google Scholar] [CrossRef]
- Chen, C.; Parejo, M.; Momeni, J.; Langa, J.; Nielsen, R.O.; Shi, W.; CONTRIBUTORS, S.W.D.; Vingborg, R.; Kryger, P.; Bouga, M. Population Structure and Diversity in European Honey Bees (Apis mellifera L.)—An Empirical Comparison of Pool and Individual Whole-Genome Sequencing. Genes 2022, 13, 182. [Google Scholar] [CrossRef]
- Keller, E.M.; Harris, I.; Cross, P. Identifying suitable queen rearing sites of Apis mellifera mellifera at a regional scale using morphometrics. J. Apic. Res. 2014, 53, 279–287. [Google Scholar] [CrossRef]
- Skonieczna, Ł. Conservation of Apis mellifera mellifera in Poland. Биoмика 2016, 8, 61–64. [Google Scholar]
- Oleksa, A.; Kusza, S.; Tofilski, A. Mitochondrial DNA suggests the introduction of honeybees of African ancestry to East-Central Europe. Insects 2021, 12, 410. [Google Scholar] [CrossRef]
- Kaskinova, M.D.; Gaifullina, L.R.; Saltyoka, E.S.; Poskryakov, A.V.; Nikolenko, A.G. Dynamics of the Genetic Structure of Apis mellifera Populations in the Southern Urals. Russ. J. Genet. 2022, 58, 36–41. [Google Scholar] [CrossRef]
- Miguel, I.; Iriondo, M.; Garnery, L.; Sheppard, W.S.; Estonba, A. Gene flow within the M evolutionary lineage of Apis mellifera: Role of the Pyrenees, isolation by distance and post-glacial re-colonization routes in the western Europe. Apidologie 2007, 38, 141–155. [Google Scholar] [CrossRef]
- Cánovas, F.; De la Rúa, P.; Serrano, J.; Galián, J. Geographical patterns of mitochondrial DNA variation in Apis mellifera iberiensis (Hymenoptera: Apidae). J. Zool. Syst. Evol. Res. 2008, 46, 24–30. [Google Scholar] [CrossRef]
- Canto, E. Arquivo dos Açores—Volume 1. 1878, Volume 1. Available online: https://portulanclarin.net/repository/browse/archivo-dos-acores-dir-ernesto-do-canto-1a-serie-ponta-delgada-vol-1-12/e87bcf2e2b2c11ea843902420a000004f9e866e2ae1347fea56119669130a535/ (accessed on 2 November 2022).
- Bieńkowska, M.; Splitt, A.; Węgrzynowicz, P.; Maciorowski, R. The Buzz Changes within Time: Native Apis mellifera mellifera Honeybee Subspecies Less and Less Popular among Polish Beekeepers Since 1980. Agriculture 2021, 11, 652. [Google Scholar] [CrossRef]
- Coroian, C.O.; Muñoz, I.; Schlüns, E.A.; Paniti-Teleky, O.R.; Erler, S.; Furdui, E.M.; Mărghitaş, L.A.; Dezmirean, D.S.; Schlüns, H.; De La Rua, P. Climate rather than geography separates two European honeybee subspecies. Mol. Ecol. 2014, 23, 2353–2361. [Google Scholar] [CrossRef]
- Muñoz, I.; De la Rúa, P. Wide genetic diversity in Old World honey bees threaten by introgression. Apidologie 2021, 52, 200–217. [Google Scholar] [CrossRef]
- Božič, J.; Kordiš, D.; Križaj, I.; Leonardi, A.; Močnik, R.; Nakrst, M.; Podgoršek, P.; Prešern, J.; BAJEC, S.S.; ZORC, M. Novel aspects in characterisation of Carniolan honey bee (Apis mellifera carnica, Pollmann 1879). Acta Agric. Slov 2016, 5, 18–27. [Google Scholar]
- Mărghitaş, L.A.; Coroian, C.; Dezmirean, D.; Stan, L.; Furdui, E. Genetic Diversity of Honeybees from Moldova (Romania) Based on mtDNA Analysis. Bull. UASVM Anim. Sci. Biotechnol. 2010, 67, 396–402. [Google Scholar]
- Buescu, E.; Gurau, M.R.; Danes, D. Identification Of The Honeybee Subspecies From Some Romanian Counties Using A Semiautomatic System For Analyzing Wings. In Proceedings of the CBU International Conference Proceedings, Prague, Czech Republic, 21–23 March 2018; pp. 1124–1128. [Google Scholar]
- Browne, K.A.; Hassett, J.; Geary, M.; Moore, E.; Henriques, D.; Soland-Reckeweg, G.; Ferrari, R.; Mac Loughlin, E.; O’Brien, E.; O’Driscoll, S. Investigation of free-living honey bee colonies in Ireland. J. Apic. Res. 2020, 60, 229–240. [Google Scholar] [CrossRef]
- Hassett, J.; Browne, K.A.; McCormack, G.P.; Moore, E.; Society, N.I.H.B.; Soland, G.; Geary, M. A significant pure population of the dark European honey bee (Apis mellifera mellifera) remains in Ireland. J. Apic. Res. 2018, 57, 337–350. [Google Scholar] [CrossRef]
- Garnery, L. Rapport d´ Expertise 2018. Analyses Génétiques de la Population d´Abeilles Melliféres de I´Ile de Groix; Laboratoires Evolution Génomes Comportement et Ecologie: Gif-sur-Yvette, France, 2018. [Google Scholar]
- Ilyasov, R.; Poskryakov, A.; Petukhov, A.; Nikolenko, A. Molecular genetic analysis of five extant reserves of black honeybee Apis melifera melifera in the Urals and the Volga region. Russ. J. Genet. 2016, 52, 828–839. [Google Scholar] [CrossRef]
- Ilyasov, R.A.; Lee, M.-L.; Yunusbaev, U.; Nikolenko, A.; Kwon, H.-W. Estimation of C-derived introgression into A. m. mellifera colonies in the Russian Urals using microsatellite genotyping. Genes Genom. 2020, 42, 987–996. [Google Scholar] [CrossRef]
- Garnery, L.; Franck, P.; Baudry, E.; Vautrin, D.; Cornuet, J.-M.; Solignac, M. Genetic diversity of the west European honey bee (Apis mellifera mellifera and A. m. iberica) I. Mitochondrial DNA. Genet. Sel. Evol. 1998, 30, S31–S47. [Google Scholar] [CrossRef]
- De la Rúa Tarín, P.; Radloff, S.; Hepburn, R.; Serrano, J. Do molecular markers support morphometric and pheromone analyses? A preliminary case study in Apis Mellifera populations of Morocco. Arch. De Zootec. 2007, 56, 33–42. [Google Scholar]
- Irati, M.; Baylac, M.; Iriondo, M.; Manzano, C.; Garnery, L.; Estonba, A. Both geometric morphometric and microsatellite data consistently support the differentiation of the Apis mellifera M evolutionary branch. Apidologie 2011, 42, 150–161. [Google Scholar]
- Moritz, R.F. The limitations of biometric control on pure race breeding in Apis mellifera. J. Apic. Res. 1991, 30, 54–59. [Google Scholar] [CrossRef]
- Weinstock, G.M.; Robinson, G.E.; Gibbs, R.A.; Weinstock, G.M.; Weinstock, G.M.; Robinson, G.E.; Worley, K.C.; Evans, J.D.; Maleszka, R.; Robertson, H.M.; et al. Insights into social insects from the genome of the honeybee Apis mellifera. Nature 2006, 443, 931–949. [Google Scholar] [CrossRef]
- Chávez-Galarza, J.; Henriques, D.; Johnston, J.S.; Azevedo, J.C.; Patton, J.C.; Muñoz, I.; De la Rúa, P.; Pinto, M.A. Signatures of selection in the Iberian honey bee (Apis mellifera iberiensis) revealed by a genome scan analysis of single nucleotide polymorphisms. Mol. Ecol. 2013, 22, 5890–5907. [Google Scholar]
- Henriques, D.; Lopes, A.R.; Pinto, M.A. Centro de Investigaçao de Montanha-Instituto Politécnico de Bragança, Bragança, Portugal. Manuscript in preparation.

| A. m. iberiensis Native Range | A. m. mellifera Native Range | A. m. carnica Native Range | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Subspecies | 1st Highest Probability | 2nd Highest Probability | Probability > 0.950 | 1st Highest Probability | 2nd Highest Probability | Probability > 0.950 | 1st Highest Probability | 2nd Highest Probability | Probability > 0.950 |
| A. m. iberiensis | 77.2/89.7 | 11.2/5.8 | 75.0/88.4 | 11.3/5.8 | 45.0/31.8 | 6.7/3.0 | 1.6/0.0 | 9.8/3.0 | 0.5/0.0 |
| A. m. mellifera | 9.8/4.0 | 58.8/76.3 | 19.2/9.6 | 67.1/41.1 | 12.8/12.2 | 80.0/51.4 | 4.5/1,9 | 15.1/5.4 | 1.9/0.4 |
| A. m. ligustica | 6.8/4.7 | 10.98.3 | 3.5/1.5 | 6.5/12.5 | 16.5/28.1 | 3.1/6.0 | 20.0/9.2 | 48.9/77.2 | 10.6/2.2 |
| A. m. carnica | 3.8/1.0 | 7.7/3.8 | 1.9/0.5 | 11.5/35.9 | 7.0/12.9 | 9.3/38.7 | 72.0/88.3 | 17.9/9.8 | 86.7/97.3 |
| A. m. caucasia | 2.4/0.5 | 11.4/5.9 | 0.4/0.0 | 3.7/4.8 | 18.7/15.0 | 0.8/0.9 | 1.8/0.5 | 8.3/4.6 | 0.3/0.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García, C.A.Y.; Rodrigues, P.J.; Tofilski, A.; Elen, D.; McCormak, G.P.; Oleksa, A.; Henriques, D.; Ilyasov, R.; Kartashev, A.; Bargain, C.; et al. Using the Software DeepWings© to Classify Honey Bees across Europe through Wing Geometric Morphometrics. Insects 2022, 13, 1132. https://doi.org/10.3390/insects13121132
García CAY, Rodrigues PJ, Tofilski A, Elen D, McCormak GP, Oleksa A, Henriques D, Ilyasov R, Kartashev A, Bargain C, et al. Using the Software DeepWings© to Classify Honey Bees across Europe through Wing Geometric Morphometrics. Insects. 2022; 13(12):1132. https://doi.org/10.3390/insects13121132
Chicago/Turabian StyleGarcía, Carlos Ariel Yadró, Pedro João Rodrigues, Adam Tofilski, Dylan Elen, Grace P. McCormak, Andrzej Oleksa, Dora Henriques, Rustem Ilyasov, Anatoly Kartashev, Christian Bargain, and et al. 2022. "Using the Software DeepWings© to Classify Honey Bees across Europe through Wing Geometric Morphometrics" Insects 13, no. 12: 1132. https://doi.org/10.3390/insects13121132
APA StyleGarcía, C. A. Y., Rodrigues, P. J., Tofilski, A., Elen, D., McCormak, G. P., Oleksa, A., Henriques, D., Ilyasov, R., Kartashev, A., Bargain, C., Fried, B., & Pinto, M. A. (2022). Using the Software DeepWings© to Classify Honey Bees across Europe through Wing Geometric Morphometrics. Insects, 13(12), 1132. https://doi.org/10.3390/insects13121132











