Domestic Triatoma spp. Infections with Trypanosoma cruzi, Household Infestations, and Molecular Identification in Oaxaca, México
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Morphological Identification and Molecular Analysis
2.3. Parasitological Examination
2.4. Geographical Distribution
2.5. Statistical Analysis
3. Results
3.1. Morphological and Molecular Identification
3.2. Endophilic Behavior
3.3. Entomological Indices
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (OMS) Enfermedad de Chagas. 2022. Available online: https://www.paho.org/es/temas/enfermedad-chagas (accessed on 29 November 2022).
- Cruz-Reyes, A.; Pickering-López, J.M. Chagas disease in Mexico: An analysis of geographical distribution during the past 76 years-A review. Mem. Do Inst. Oswaldo Cruz 2006, 101, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Schettino, P.M.; Rojas-Wastavino, G.E.; Cabrera-Bravo, M.; Bucio-Torres, M.I.; Martínez-Ibarra, J.A.; Monroy-Escobar, M.C.; Torres-Gutiérrez, E. A revision of thirteen species of Triatominae (Hemiptera: Reduviidae) vectors of Chagas disease in Mexico. J. Selva Andin. Res. Soc. 2010, 1, 57–80. [Google Scholar] [CrossRef]
- Ramos-Ligonio, A.; Torres-Montero, J.; López-Monteon, A.; Dumonteil, E. Extensive diversity of Trypanosoma cruzi discrete typing units circulating in Triatoma dimidiata from central Veracruz, Mexico. Infect. Genet. Evol. 2012, 12, 1341–1343. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Acosta, V.; Ibáñez-Bernal, S.; Martínez-Campos, C. Infección natural de chinches Triatominae con Trypanosoma cruzi asociadas a la vivienda humana en México. Salud Públ. Méx. 2000, 42, 496–503. [Google Scholar] [CrossRef]
- Leitner, W.W.; Wali, T.; Kincaid, R.; Costero-Saint Denis, A. Arthropod vectors and disease transmission: Translational aspects. PLoS Negl. Trop. Dis. 2015, 9, e0004107. [Google Scholar] [CrossRef]
- Cenaprece. Programa de Acción Especifico Prevención y Control de la Enfermedad de Chagas 2013–2018. 2 de enero del 2021, de Secretaría de Salud, México. 2014. Available online: http://www.cenaprece.salud.gob.mx/descargas/pdf/PAE_PrevencionControlEnfermedadChagas2013_2018.pdf (accessed on 29 November 2022).
- Coura, J.R. The main sceneries of Chagas disease transmission. The vectors, blood and oral transmissions-A comprehensive review. Memórias Do Inst. Oswaldo Cruz 2014, 110, 277–282. [Google Scholar] [CrossRef]
- Freitas-Lidani, K.C.; Andrade, F.A.; Bavia, L.; Damasceno, F.S.; Beltrame, M.H.; Messias-Reason, I.J.; Sandri, T.L. Chagas disease: From discovery to a worldwide health problem. Front. Public Health 2019, 7, 166. [Google Scholar] [CrossRef]
- World Health Organization. Chagas disease in Latin America: An epidemiological update based on 2010 estimates. Wkly. Epidemiol. Rec. 2015, 6, 33–43. [Google Scholar]
- Dirección General de Epidemiología. (DGE). Histórico Boletín Epidemiológico 1 de enero 2021, de Secretaría de Salud, México. Available online: https://www.gob.mx/salud/acciones-y-programas/historico-boletin-epidemiologico. (accessed on 29 November 2022).
- Ramsey, J.M.; Ordoñez, R.; Cruz-Celis, A.; Alvear, A.L.; Chavez, V.; Lopez, R.; Carrillo, S. Distribution of domestic Triatominae and stratification of Chagas disease transmission in Oaxaca, Mexico. Med. Vet. Entomol. 2000, 14, 19–30. [Google Scholar] [CrossRef]
- Mazzotti, L. Dos casos de enfermedad de Chagas en el estado de Oaxaca. Gac. Médica Mex. 1940, 70, 417–420. [Google Scholar]
- Salazar-Schettino, P.M.; Bucio-Torres, M.I.; Cabrera-Bravo, M.; Alba-Alvarado, M.C.D.; Castillo-Saldaña, D.R.; Zenteno-Galindo, E.A.; Perera-Salazar, M.G. Enfermedad de Chagas en México. Rev. Fac. Med. UNAM. 2016, 59, 6–16. [Google Scholar]
- Abras, A.; Muñoz, C.; Ballart, C.; Berenguer, P.; Llovet, T.; Herrero, M.; Gállego, M. Towards a new strategy for diagnosis of congenital Trypanosoma cruzi infection. J. Clin. Microbiol. 2017, 55, 1396–1407. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, R.S.; Kagan, I.G.; Zarate, R.J.; Reyes-González, M.A.; Cedeño-Ferreira, J. Epidemiologic studies of Chagas’ disease in Oaxaca, Mexico. Bull. Pan Am. Health Organ. 1978, 12, 236–250. [Google Scholar] [PubMed]
- Salazar-Schettino, P.M.; Bucio-Torres, M.I.; de Haro-Arteaga, I. Reservorios y transmisores de Trypanosoma cruzi en el estado de Oaxaca. Salud Públ. Méx. 1987, 29, 26–32. [Google Scholar]
- CENAPRECE. Guia Metodológica de Estudios Entomológicos Para Triatominos, Secretaría de Salud, México. 2015. Available online: https://www.gob.mx/cms/uploads/attachment/file/39201/GuiaEntomologiaChagas.pdf (accessed on 29 November 2022).
- Lent, H.; Wygodzinsky, P. Revision of the Triatominae (Hemiptera, Reduviidae), and their significance as vectors of Chagas disease. Bull. Am. Mus. Nat. His. 1979, 163, 123–520. [Google Scholar]
- Truett, G.E.; Heeger, P.; Mynatt, R.L.; Truett, A.A.; Walker, J.A.; Warman, M.L. Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and tris (HotSHOT). Biotechniques 2000, 29, 52–54. [Google Scholar] [CrossRef] [PubMed]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase sub- unit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Hernandez-Triana, L.M.; Crainey, J.L.; Hall, A.; Fatih, F.; Mackenzie-Dodds, J.; Shelley, A.J.; Hebert, P.D.N. DNA barcodes reveal cryptic genetic diversity within the blackfly subgenus Trichodagmia Enderlein (Diptera: Simuliidae: Simulium) and related taxa in the New World. Zootaxa 2012, 3514, 43–69. [Google Scholar] [CrossRef]
- Basic Local Alignment Search Tool (BLAST). 2022. Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 29 November 2022).
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Kingdoms of Life Being Barcoded (BOLDSYSTEMS). Available online: http://boldsystems.org/index.php/TaxBrowser_Home?searchMenu=taxonomy (accessed on 29 November 2022).
- Ratnasingham, S.; Hebert, P.D. BOLD: The Barcode of Life Data System. Mol. Ecol. Notes 2007, 7, 355–364. Available online: http://www.barcodinglife.org (accessed on 29 November 2022).
- Souza, W.D. Structural organization of Trypanosoma cruzi. Mem. Do Inst. Oswaldo Cruz 2009, 104, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Pérez España, V.H.; Morales Evangelista, C.L.; Vázquez Chagoyán, J.C.; Valladares Carranza, B.; Romero Cortés, T.; Cuervo Parra, J.A.; Aparicio Burgos, J.E. Caracterización molecular de aislados de Trypanosoma cruzi de triatominos recolectados en los municipios del Estado de Hidalgo, México. Nova Sci. 2019, 11, 171–185. [Google Scholar] [CrossRef]
- Martínez-Tovar, J.G.; Rodríguez-Rojas, J.J.; Arque-Chunga, W.; Lozano-Rendón, J.A.; Ibarra-Juárez, L.A.; Dávila-Barboza, J.A.; Rebollar-Téllez, E.A. Nuevos registros geográficos y notas de infección de Triatoma gerstaeckeri (Stål) y Triatoma rubida (Uhler)(Hemiptera: Reduviidae: Triatominae) en Nuevo León y Coahuila, México. Acta Zool. Mex. 2013, 29, 227–233. [Google Scholar] [CrossRef]
- Armitage, P.; Berry, G. Statistical Methods in Medical Research, 3rd ed.; Blackwell Scientific Publications: Oxford, UK, 1994. [Google Scholar]
- Ratnasingham, S.; Hebert, P.D. A DNA-based registry for all animal species: The Barcode Index Number (BIN) system. PLoS ONE 2013, 8, e66213. [Google Scholar] [CrossRef]
- Aguilera-Uribe, M.; Meza-Lázaro, R.N.; Kieran, T.J.; Ibarra-Cerdeña, C.N.; Zaldívar-Riverón, A. Phylogeny of the North-Central American clade of blood-sucking reduviid bugs of the tribe Triatomini (Hemiptera: Triatominae) based on the mitochondrial genome. Infect. Genet. Evol. 2020, 84, 104373. [Google Scholar] [CrossRef]
- Pfeiler, E.; Bitler, B.G.; Ramsey, J.M.; Palacios-Cardiel, C.; Markow, T.A. Genetic variation, population structure, and phylogenetic relationships of Triatoma rubida and T. recurva (Hemiptera: Reduviidae: Triatominae) from the Sonoran Desert, insect vectors of the Chagas’ disease parasite Trypanosoma cruzi. Mol. Phylogenetics Evol. 2006, 41, 209–221. [Google Scholar] [CrossRef]
- Jin, T.; Husseneder, C.; Foil, L. Assigning Culicoides larvae to species using DNA barcoding of adult females and phylogenetic associations. Parasit. Vectors 2022, 15, 349. [Google Scholar] [CrossRef]
- Monroy, M.C.; Penados, D.; Pineda, J.; Ruiz, E.L.; Agreda, E.O.; Alcantara, B.; Rodas, A.; Lange, K.; Weinberg, D.; Bazzani, R.; et al. A multidisciplinary, collaborative, inter-agency and comprehensive approach for the control of Chagas Disease as a public health problem in Guatemala. Acta Trop. 2022, 225, 106157. [Google Scholar] [CrossRef]
- De Fuentes-Vicente, J.A.; Gómez-Gómez, A.; Santos-Hernández, N.G.; Ruiz-Castillejos, C.; Gómez-Sánchez, E.; Vidal-López, D.; Moreno-Rodríguez, A. First report of an infected triatomine bug in an urban area of Tuxtla Gutierrez, Chiapas, México. BIOCYT Biol. Cienc. Tecnol. 2021, 14, 1009. [Google Scholar] [CrossRef]
- Delgado, S.; Ernst, K.C.; Pumahuanca, M.L.H.; Yool, S.R.; Comrie, A.C.; Sterling, C.R.; Levy, M.Z. A country bug in the city: Urban infestation by the Chagas disease vector Triatoma infestans in Arequipa, Peru. Int. J. Health Geogr. 2013, 12, 48. [Google Scholar] [CrossRef]
- Becerril-Flores, M. Descripción de la enfermedad de Chagas en el Valle de Iguala, Guerrero, México. Gac. Médica Mex. 2003, 139, 539–544. [Google Scholar]
- Magallón Gastélum, E.; Magdaleno Peñaloza, N.C.; Katthain Duchateau, G.; Trujillo Contreras, F.; Lozano Kasten, F.J.; Hernández Gutiérrez, R.J. Distribución de los vectores de la enfermedad de Chagas (Hemiptera: Reduviidae: Triatominae), en el estado de Jalisco, México. Rev. Biomed. México. 1998, 9, 151–157. [Google Scholar]
- Dumonteil, E.; Gourbiére, S.; Barrera-Pérez, M.; Rodriguez-Félix, E.; Ruiz-Piña, H.; Baños-Lopez, O.; Rabinovich, J.E. Geographic distribution of Triatoma dimidiata and transmission dynamics of Trypanosoma cruzi in the Yucatan peninsula of Mexico. Am. J. Trop. Med. Hyg. 2002, 67, 176–183. [Google Scholar] [CrossRef]
- Rojas, J.C.; Malo, E.A.; Espinoza-Medinilla, E.; Ondarza, R.N. Sylvatic focus of Chagas’ disease in Oaxaca, Mexico. Ann. Trop. Med. Parasitol. 1989, 83, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Kjos, S.A.; Gillespie, J.J.; Olson, J.K.; Snowden, K.F. Detection of Blastocrithidia spp. (Kinetoplastida: Trypanosomatidae) in Chagas disease vectors from Texas, USA. Vector Borne Zoonotic Dis. 2009, 9, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Adeniran, A.A.; Fernández-Santos, N.A.; Rodríguez-Rojas, J.J.; Treviño-Garza, N.; Huerta-Jiménez, H.; Mis-Ávila, P.C.; Rodríguez-Pérez, M.A. Identification of phlebotomine sand flies (Diptera: Psychodidae) from leishmaniasis endemic areas in southeastern Mexico using DNA barcoding. Ecol. Evol. 2019, 9, 13543–13554. [Google Scholar] [CrossRef] [PubMed]
- Adeniran, A.A.; Hernández-Triana, L.M.; Ortega-Morales, A.I.; Garza-Hernández, J.A.; de la Cruz-Ramos, J.; Chan-Chable, R.J.; Rodríguez-Pérez, M.A. Identification of mosquitoes (Diptera: Culicidae) from Mexico State, Mexico using morphology and COI DNA barcoding. Acta Trop. 2021, 213, 105730. [Google Scholar] [CrossRef]
- La Agencia de Cooperación Internacional del Japón (JICA). Elaboración de la Guía Práctica de Mejoramiento de Vida, “Alli Kawsanamanta”. 2006. Available online: https://www.jica.go.jp/project/spanish/ecuador/001/materials/c8h0vm00008bcae4-att/vida.pdf (accessed on 29 November 2022).
- Agencia de Cooperación Internacional del Japón (JICA) and de Nicaragua. Manual de Mejoramiento de Viviendas. 2013. Available online: https://www.jica.go.jp/project/nicaragua/001/materials/ku57pq0000126ws5-att/manual_de_mejoramientos_de_viviendas.pdf (accessed on 28 November 2022).
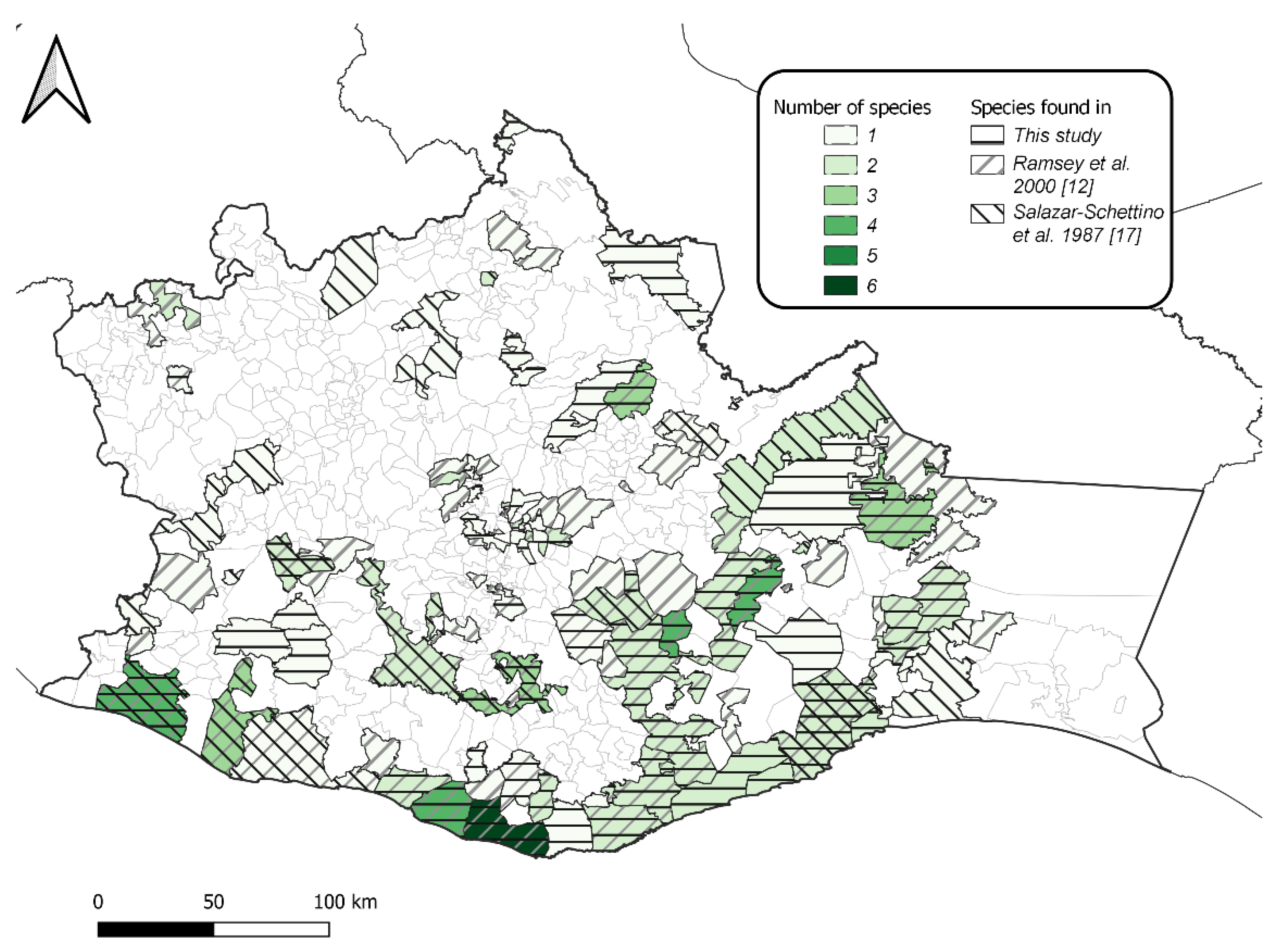
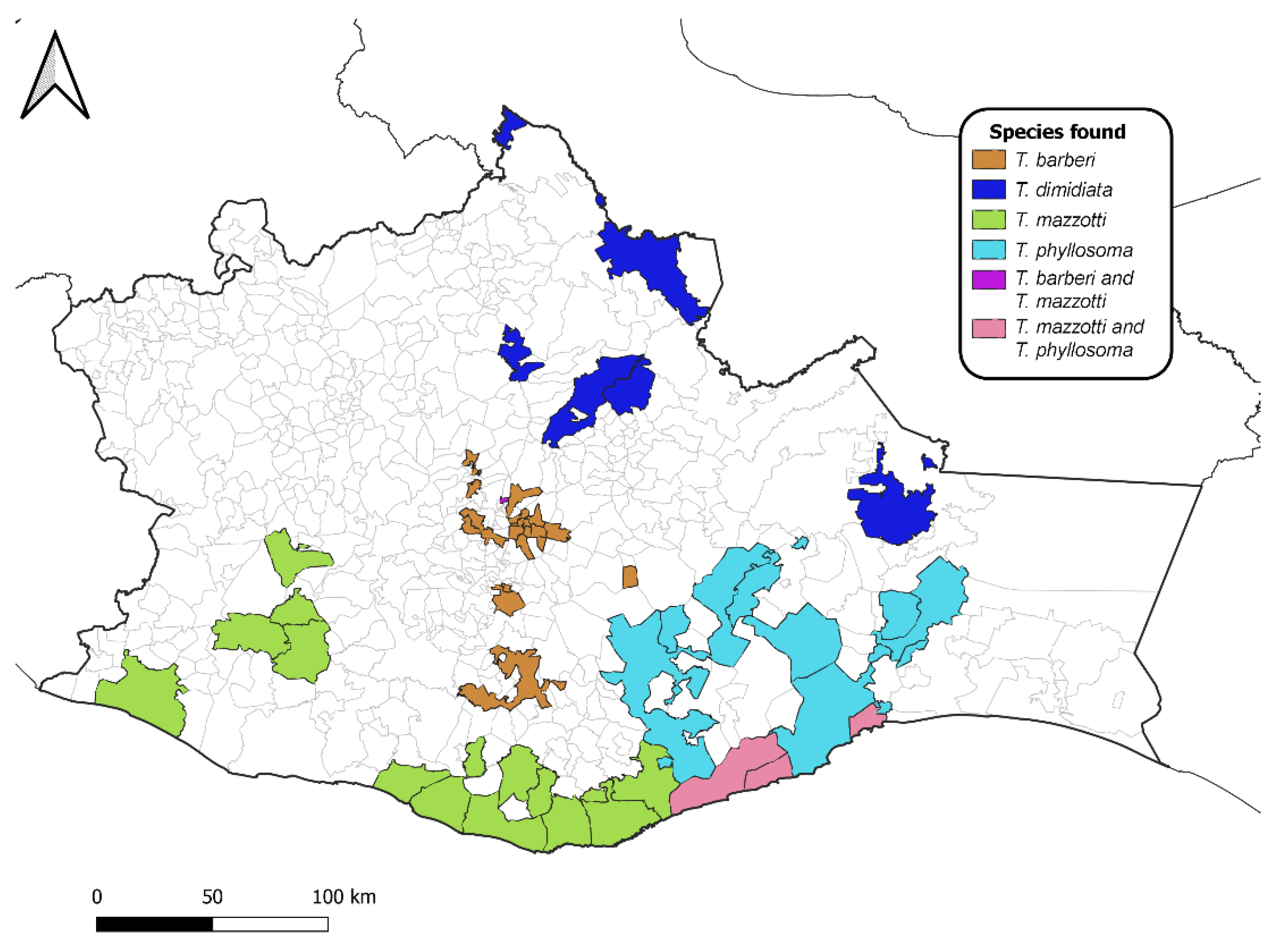
| Species | No. of Municipalities | No. of Positive Households Sampled | Colonization Index & | Crowding Index $ | No. of Infected Triatomines/No. Examined | Infectivity Rate CI * |
|---|---|---|---|---|---|---|
| T. phyllosoma | 12 | 57 | 0.14 | 2.0 | 41/83 | 49% (38–60%) |
| T. barberi | 20 | 77 | 0.72 | 4.3 | 49/171 | 28% (22–35%) |
| T. mazzotti | 21 | 167 | 0.03 | 1.1 | 31/177 | 17% (12–23%) |
| T. dimidiata | 7 | 8 | 0.50 | 1.4 | 0/10 | 0% (17% #) |
| Unidentified Triatoma spp. | 25 | 72 | 1.0 | 1.8 | 98/333 | 29% (24–34%) |
| Overall | 60 | 381 | 0.38 | 2.0 | 219/774 | 28% (25–31%) |
| Adult Life Stage | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Households Sampled | Collection Site | T. mazzotti | T. barberi | T. phyllosoma | T. dimidiata | Unidentified Triatoma spp. | Total | ||||
| Nymph Stage * | |||||||||||
| V | IV | III | II | ||||||||
| 196 | Intradomicile | + | 18 | 2 | 16 | 0 | 6 | 3 | 2 | 1 | 48+ |
| − | 45 | 1 | 14 | 1 | 11 | 12 | 11 | 0 | 95− | ||
| NF | 69 | 3 | 9 | 5 | 5 | 8 | 8 | 4 | 111 NF | ||
| 176 | Peridomicile | + | 13 | 47 | 25 | 0 | 14 | 28 | 26 | 6 | 159+ |
| − | 7 | 65 | 10 | 1 | 29 | 31 | 48 | 15 | 206− | ||
| NF | 20 | 53 | 7 | 2 | 14 | 10 | 17 | 4 | 127 NF | ||
| 9 | ND | + | 0 | 0 | 0 | 0 | 2 | 4 | 2 | 4 | 12+ |
| − | 0 | 0 | 2 | 0 | 1 | 1 | 0 | 3 | 7− | ||
| NF | 5 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 9 NF | ||
| 381 | Total | 177 | 171 | 83 | 10 | 82 | 98 | 115 | 38 | 774 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Santos, N.A.; Trujillo-García, J.C.; Hamer, S.A.; Wei, L.; Martínez-Montoya, H.; Tamez-Guerra, P.; Hamer, G.L.; Rodríguez-Pérez, M.A. Domestic Triatoma spp. Infections with Trypanosoma cruzi, Household Infestations, and Molecular Identification in Oaxaca, México. Insects 2022, 13, 1134. https://doi.org/10.3390/insects13121134
Fernández-Santos NA, Trujillo-García JC, Hamer SA, Wei L, Martínez-Montoya H, Tamez-Guerra P, Hamer GL, Rodríguez-Pérez MA. Domestic Triatoma spp. Infections with Trypanosoma cruzi, Household Infestations, and Molecular Identification in Oaxaca, México. Insects. 2022; 13(12):1134. https://doi.org/10.3390/insects13121134
Chicago/Turabian StyleFernández-Santos, Nadia A., Josefina C. Trujillo-García, Sarah A. Hamer, Lihua Wei, Humberto Martínez-Montoya, Patricia Tamez-Guerra, Gabriel L. Hamer, and Mario A. Rodríguez-Pérez. 2022. "Domestic Triatoma spp. Infections with Trypanosoma cruzi, Household Infestations, and Molecular Identification in Oaxaca, México" Insects 13, no. 12: 1134. https://doi.org/10.3390/insects13121134
APA StyleFernández-Santos, N. A., Trujillo-García, J. C., Hamer, S. A., Wei, L., Martínez-Montoya, H., Tamez-Guerra, P., Hamer, G. L., & Rodríguez-Pérez, M. A. (2022). Domestic Triatoma spp. Infections with Trypanosoma cruzi, Household Infestations, and Molecular Identification in Oaxaca, México. Insects, 13(12), 1134. https://doi.org/10.3390/insects13121134







