The Role of Tire Leachate in Condition-Specific Competition and the Persistence of a Resident Mosquito from a Competitively Superior Invader
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tire Condition Experiment
2.2. Competition Trial
2.3. Oviposition Choice Trial
2.4. Statistical Analyses
3. Results
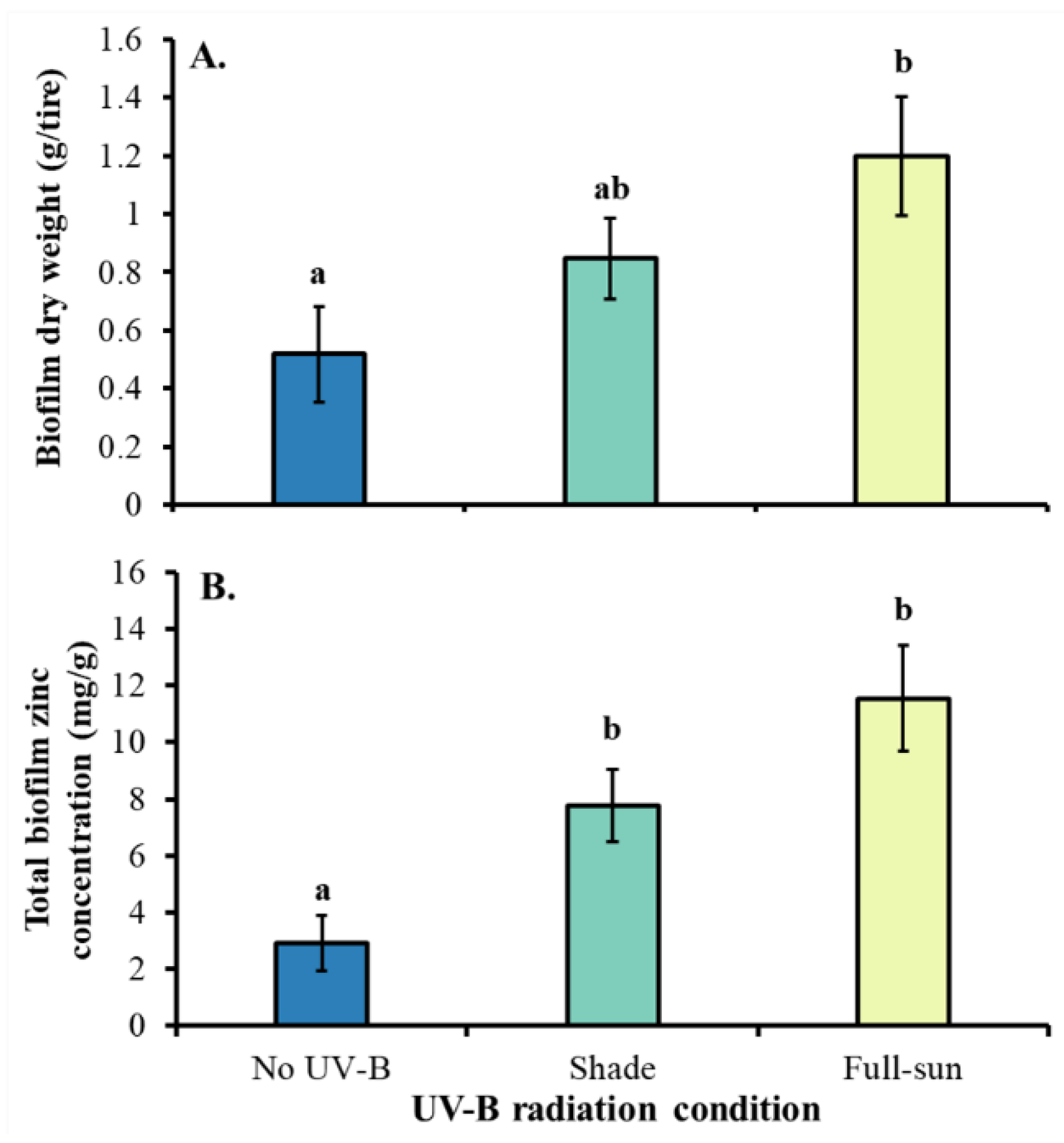
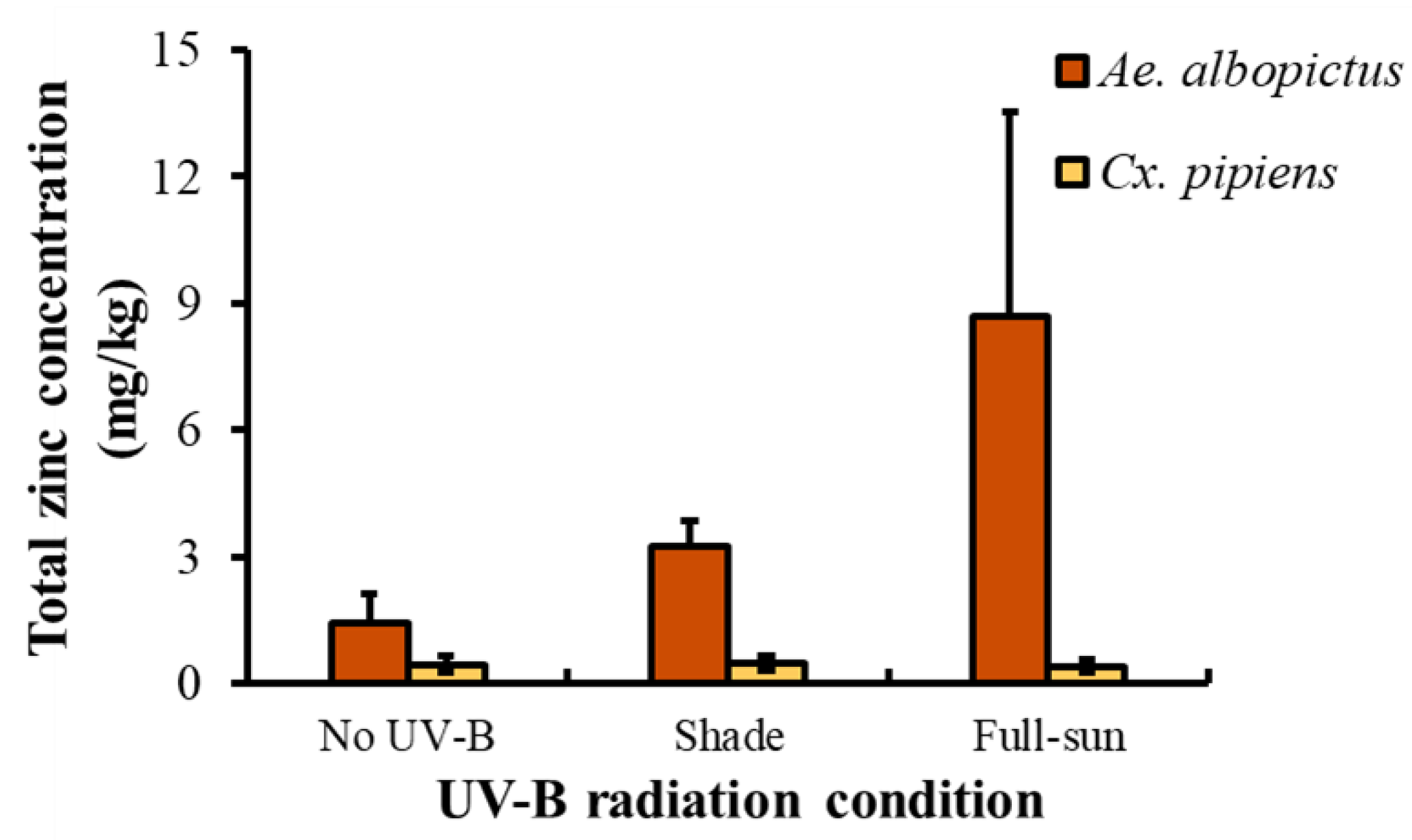
3.1. Tire Condition Experiment
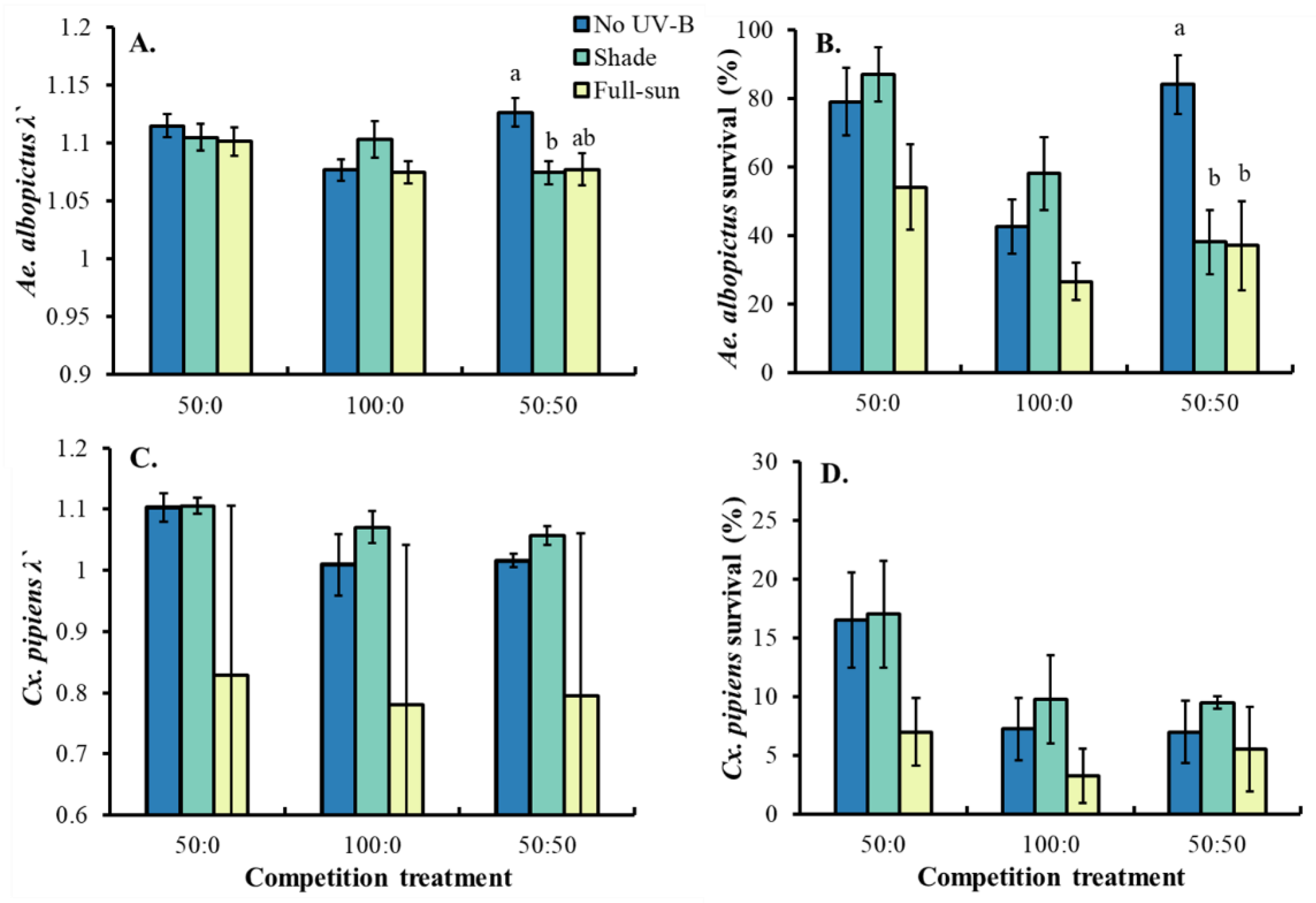
3.2. Competition Trial
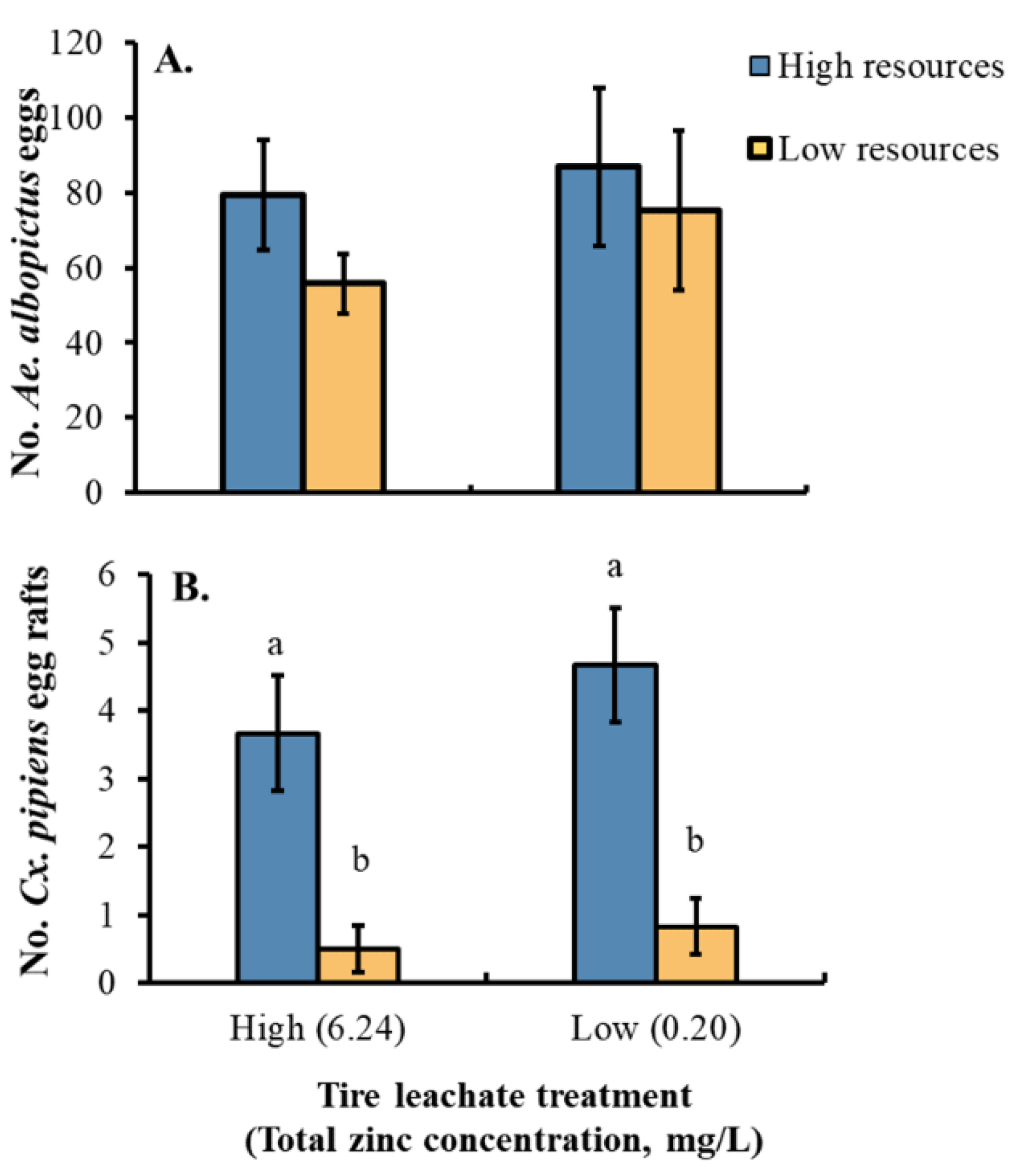
3.3. Oviposition Choice Trial
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kesavaraju, B.; Juliano, S.A. No evolutionary response to four generations of laboratory selection on antipredator behavior of Aedes albopictus: Potential implications for biotic resistance to invasion. J. Med. Entomol. 2009, 46, 772–781. [Google Scholar] [CrossRef]
- Davis, M.A. Invasion Biology; Oxford University Press: New York, NY, USA, 2009. [Google Scholar]
- Lockwood, J.L.; Cassey, P.; Blackburn, T.M. The more you introduce the more you get: The role of colonization pressure and propagule pressure in invasion ecology. Divers. Distrib. 2009, 15, 904–910. [Google Scholar] [CrossRef]
- Tilman, D. Resource Competition and Community Structure; Princeton University Press: Princeton, NJ, USA, 1982. [Google Scholar]
- Chase, J.M.; Leibold, M.A. Ecological Niches: Linking Classical and Contempory Approaches; University of Chicago Press: Chicago, IL, USA, 2003. [Google Scholar]
- Case, T. Invasion resistance, species build-up and community collapse in metapopulation models with interspecies competition. Biol. J. Linn. Soc. 1991, 42, 239–266. [Google Scholar] [CrossRef]
- Lounibos, L.P.; Bargielowski, I.; Carrasquilla, M.C.; Nishimura, N. Coexistance of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in peninsular Florida two decades after competitive displacements. J. Med. Entomol. 2016, 53, 1385–1390. [Google Scholar] [CrossRef]
- Blaustein, L.; Chase, J.M. Interactions between mosquito larvae and species that share the same trophic level. Annu. Rev. Entomol. 2007, 52, 489–507. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, K.S.; Kesavaraju, B.; Juliano, S.A. Condition specific competition in container mosquitoes: The role of non-competing life-history stages. Ecology 2005, 86, 3289–3295. [Google Scholar] [CrossRef]
- Leisnham, P.T.; LaDeau, S.L.; Juliano, S.A. Spatial and temporal habitat segregation of mosquitoes in urban Florida. PLoS ONE 2014, 9, e91655. [Google Scholar] [CrossRef] [PubMed]
- Chesson, P. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 2000, 31, 343–366. [Google Scholar] [CrossRef]
- Chesson, P.; Huntly, N. The roles of harsh and fluctuating conditions in the dynamics of ecological communities. Am. Nat. 1997, 150, 519–553. [Google Scholar] [CrossRef]
- Lawton, J.H.; Hassell, M.P. Asymmetrical competition in insects. Nature 1981, 289, 793–795. [Google Scholar] [CrossRef]
- Juliano, S.A. Species interactions among larval mosquitoes: Context dependence across habitat gradients. Annu. Rev. Entomol. 2009, 54, 37–56. [Google Scholar] [CrossRef]
- Costanzo, K.S.; Muturi, E.J.; Lampman, H.L.; Alto, B.W. The effects of resource type and ratio on competition with Aedes albopictus and Culex pipiens (Diptera: Culicidae). J. Med. Entomol. 2011, 48, 29–38. [Google Scholar] [CrossRef] [PubMed]
- USTMA. U.S. Scrap Tire Management Summary; United States Tire Manufacturers Association: Washington, DC, USA, 2019; Available online: https://www.ustires.org/sites/default/files/2019%20USTMA%20Scrap%20Tire%20Management%20Summary%20Report.pdf (accessed on 2 September 2022).
- Councell, T.B.; Dukenfield, K.U.; Landa, E.R.; Callender, E. Tire-wear particles as a source of zinc to the environment. Environ. Sci. Technol. 2004, 38, 4206–4214. [Google Scholar] [CrossRef] [PubMed]
- Day, K.E.; Holtze, K.E.; Metcalfe-Smith, J.L.; Bishop, C.T.; Dutka, B.J. Toxicity of leachate from automobile tires to aquatic biota. Chemosphere 1993, 27, 665–675. [Google Scholar] [CrossRef]
- Selbes, M.; Yilmaz, O.; Khan, A.A.; Karanfil, T. Leaching of DOC, DN, and inorganic constituents from scrap tires. Chemosphere 2015, 139, 617–623. [Google Scholar] [CrossRef]
- Capolupo, M.; Sørensen, L.; Jayasena, K.D.R.; Booth, A.M.; Fabbri, E. Chemical composition and ecotoxicity of plastic and car tire rubber leachates to aquatic organisms. Water Res. 2020, 169, 115270. [Google Scholar] [CrossRef]
- Andrady, A.L.; Hamid, H.S.; Torikai, A. Effects of climate change and UV-B on materials. Photochem. Photobiol. Sci. 2003, 2, 68–72. [Google Scholar] [CrossRef]
- Andrady, A.L.; Hamid, S.H.; Hu, X.; Torikai, A. Effects of increased solar ultraviolet radiation on materials. J. Photochem. Photobiol. Sci. 1998, 46, 96–103. [Google Scholar] [CrossRef]
- Hartwell, S.I.; Jordahl, D.M.; Dawson, C.E.O. The effect of salinity on tire leachate toxicity. Water Air Soil Pollut. 2000, 121, 119–131. [Google Scholar] [CrossRef]
- Gualtieri, M.; Andrioletti, M.; Mantecca, P.; Vismara, C.; Camatini, M. Impact of tire debris on in vitro and in vivo systems. Part. Fibre Toxicol. 2005, 2, 1. [Google Scholar] [CrossRef]
- Gualtieri, M.; Andrioletti, M.; Vismara, C.; Milani, M.; Camatini, M. Toxicity of tire debris leachates. Environ. Int. 2005, 31, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Crampton, M.; Ryan, A.; Eckert, C.; Baker, K.H.; Herson, D.S. Effects of leachate from crumb rubber and zinc in green roofs on the survival, growth, and resistance characteristics of Salmonella enterica subsp. enterica serovar Typhimurium. Appl. Environ. Microbiol. 2014, 80, 2804–2810. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wik, A.; Dave, G. Environmental labeling of car tires–toxicity to Daphnia magna can be used as a screening method. Chemosphere 2005, 58, 645–651. [Google Scholar] [CrossRef]
- Wik, A.; Dave, G. Acute toxicity of leachates of tire wear material to Daphnia magna–variability and toxic components. Chemosphere 2006, 64, 1777–1784. [Google Scholar] [CrossRef] [PubMed]
- Wik, A.; Dave, G. Occurrence and effects of tire wear particles in the environment–a critical review and an initial risk assessment. Environ. Pollut. 2009, 157, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Marwood, C.; McAtee, B.; Kreider, M.; Ogle, R.S.; Finley, B.; Sweet, L.; Panko, J. Acute aquatic toxicity of tire and road wear particles to alga, daphnid, and fish. Ecotoxicology 2011, 20, 2079–2089. [Google Scholar] [CrossRef]
- Villena, O.C.; Terry, I.; Iwata, K.; Landa, E.R.; LaDeau, S.L.; Leisnham, P.T. Effects of tire leachate on the invasive mosquito Aedes albopictus and the native congener Aedes triseriatus. PeerJ 2017, 5, e3756. [Google Scholar] [CrossRef]
- Villena, O.C.; Momen, B.; Sullivan, J.; Leisnham, P.T. Effects of ultraviolet radiation on metabolic rate and fitness of Aedes albopictus and Culex pipiens mosquitoes. PeerJ 2018, 6, e6133. [Google Scholar] [CrossRef]
- Sanchez-Bayo, F.; Van den Brink, P.; Mann, R. Ecological Impacts of Toxic Chemicals; Bentham Science Publisher Ltd.: Bussum, Netherlands, 2011. [Google Scholar]
- Lefcort, H.; Hancock, K.A.; Maur, K.M.; Rostal, D.C. The effects of used motor oil, silt, and the water mold Saprolegnia parasitica on the growth and survival of mole salamanders (genus Ambystoma). Arch. Environ. Contam. Toxicol. 1997, 32, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Lefcort, H.; Wehner, E.A.; Cocco, P.L. Pre-exposure to heavy metal pollution and the odor of predation decrease the ability of snails to avoid stressors. Arch. Environ. Contam. Toxicol. 2013, 64, 273–280. [Google Scholar] [CrossRef]
- Pollino, C.A.; Georgiades, E.; Holdway, D.A. Physiological changes in reproductively active rainbowfish (Melanotaenia fluviatilis) following exposure to naphthalene. Ecotox. Environ. Safe. 2009, 72, 1265–1270. [Google Scholar] [CrossRef]
- Aguirre, A.A.; Lutz, P. Marine turtles as sentinels of ecosystem health: Is fibropapillomatosis an indicator? EcoHealth 2004, 1, 275–283. [Google Scholar] [CrossRef]
- Zychowski, G.V.; Godard-Codding, C.A.J. Reptilian exposure to polycyclic aromatic hydrocarbons and associated effects. Environ. Toxicol. Chem. 2017, 36, 25–35. [Google Scholar] [CrossRef]
- Neff, J.M.; Stout, S.A.; Gunster, D.G. Ecological risk assessment of polycyclic aromatic hydrocarbons in sediments: Identifying sources and ecological hazard. Integr. Environ. Assess. Manag. 2005, 1, 22–33. [Google Scholar] [CrossRef]
- Honda, M.; Suzuki, N. Toxicities of polycyclic aromatic hydrocarbons for aquatic animals. Int. J. Environ. Res. Public Health 2020, 17, 1363. [Google Scholar] [CrossRef] [PubMed]
- Girardin, V.; Grung, M.; Meland, S. Polycyclic aromatic hydrocarbons: Bioaccumulation in dragonfly nymphs (Anisoptera), and determination of alkylated forms in sediment for an improved environmental assessment. Sci. Rep. 2020, 10, 10958. [Google Scholar] [CrossRef] [PubMed]
- Rohr, J.; Crumrine, P. Effects of a herbicide and an insecticide on pond community structure and processes. Ecol. Appl. 2005, 15, 1135–1147. [Google Scholar] [CrossRef]
- De Hoop, L.; De Troch, M.; Hendriks, A.J.; De Laender, F. Modeling toxic stress by atrazine in a marine consumer-resource system. Environ. Toxicol. Chem. 2013, 32, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Yee, D.A.; Kneitel, J.M.; Juliano, S.A. Environmental correlates of abundances of mosquito species and stages in discarded vehicle tires. J. Med. Entomol. 2010, 47, 53–62. [Google Scholar] [CrossRef]
- Lounibos, L.P. Invasions by insect vectors of human disease. Annu. Rev. Entomol. 2002, 47, 233–266. [Google Scholar] [CrossRef]
- Benedict, M.Q.; Levine, R.S.; Hawley, W.A.; Lounibos, L.P. Spread of the tiger: Global risk of invasion by the mosquito Aedes albopictus. Vector-Borne Zoonotic Dis. 2007, 7, 76–85. [Google Scholar] [CrossRef]
- Sprenger, D.; Wuithiranyagool, T. The discovery and distribution of Aedes albopictus in Harris County, Texas. J. Am. Mosq. Control Assoc. 1986, 2, 217–219. [Google Scholar] [PubMed]
- Vinogradova, E.B. Culex Pipiens Pipiens Mosquitoes: Taxonomy, Distribution, Ecology, Physiology, Genetics, Applied Importance and Control; Pensoft: Sofia, Bulgaria, 2000. [Google Scholar]
- Costanzo, K.S.; Mormann, K.; Juliano, S.A. Asymmetrical competition and patterns of abundance of Aedes albopictus and Culex pipiens (Diptera: Culicidae). J. Med. Entomol. 2005, 42, 559–570. [Google Scholar] [CrossRef]
- Leisnham, P.T.; LaDeau, S.L.; Saunders, M.E.M.; Villena, O.C. Condition-specific competitive effects of the invasive mosquito Aedes albopictus on the resident Culex pipiens among different urban container habitats may explain their coexistence in the field. Insects 2021, 12, 993. [Google Scholar] [CrossRef] [PubMed]
- Carrieri, M.; Bacchi, M.; Bellini, R.; Maini, S. On the competition occurring between Aedes albopictus and Culex pipiens (Diptera: Culicidae) in Italy. Environ. Entomol. 2003, 32, 1313–1322. [Google Scholar] [CrossRef]
- Marini, G.; Guzzetta, G.; Baldacchino, F.; Arnoldi, D.; Montarsi, F.; Capelli, G. The effect of interspecific competition on the temporal dynamics of Aedes albopictus and Culex pipiens. Parasites Vectors 2017, 10, 102. [Google Scholar] [CrossRef]
- Müller, R.; Knautz, T.; Vollroth, S.; Berger, R.; Kreb, A.; Reuss, F.; Groneberg, D.A.; Kuch, U. Larval superiority of Culex pipiens to Aedes albopictus in a replacement series experiment: Prospects for coexistence in Germany. Parasites Vectors 2018, 11, 80. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, H.; Sadraei, J.; Moosa-Kazemi, S. The morphological variations of Culex pipiens larvae (Diptera: Culicidae) in Yazd Province, Central Iran. Iran. J. Arthropod-Borne Dis. 2010, 4, 42–49. [Google Scholar] [PubMed]
- Allgood, D.W.; Yee, D.A. Oviposition preference and offspring performance in container breeding mosquitoes: Evaluating the effects of organic compounds and laboratory colonisation. Ecol. Entomol. 2017, 42, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Merritt, R.W.; Dadd, R.H.; Walker, E.D. Feeding behavior, natural food, and nutritional relationships of larval mosquitoes. Annu. Rev. Entomol. 1992, 37, 349–376. [Google Scholar] [CrossRef] [PubMed]
- Alto, B.W.; Lampman, R.L.; Kesavaraju, B.; Muturi, E.J. Pesticide-induced release from competition among competing Aedes aegypti and Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 2013, 50, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, R.R.; Gottfried, K.L.; Apperson, C.S.; Davis, B.S.; Erwin, P.C.; Smith, A.B.; Panella, N.A.; Powell, E.E.; Nasci, R.S. The first isolation of La Crosse virus from naturally occurring infected Aedes albopictus. Emerg. Infect. Dis. 2001, 7, 807–811. [Google Scholar] [CrossRef]
- Kim, C.H.; Lampman, R.; Muturi, E.J. Bacterial communities and midgut microbiota associated with mosquito populations from waste tires in east-central Illinois. J. Med. Entomol. 2005, 52, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Farajollahi, A.; Fonseca, D.M.; Kramer, L.D.; Kilpatrick, M.A. “Bird biting” mosquitoes and human disease: A review of the role of Culex pipiens complex mosquitoes in epidemiology. Infect. Genet. Evol. 2011, 11, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Chancey, C.; Grinev, A.; Volkova, E.; Rios, M. The global ecology and epidemiology of West Nile virus. BioMed Res. Int. 2015, 2015, 376230. [Google Scholar] [CrossRef]
- Kilpatrick, A.M.; Meola, M.A.; Moudy, R.M.; Kramer, L.D. Temperature, viral genetics, and the transmission of West Nile virus by Culex pipiens mosquitoes. PLOS Pathog. 2008, 4, e1000092. [Google Scholar] [CrossRef] [PubMed]
- Tiawsirisup, S.; Platt, K.B.; Evans, R.B.; Rowley, W.A. A comparision of West Nile virus transmission by Ochlerotatus trivittatus (COQ.), Culex pipiens (L.), and Aedes albopictus (Skuse). Vector-Borne Zoonotic Dis. 2005, 5, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, A.; Bolzoni, L.; Chadwick, E.A.; Capelli, G.; Montarsi, F.; Grisenti, M.; de la Puente, J.M.; Muñoz, J.; Figuerola, J.; Soriguer, R.; et al. Understanding West Nile virus ecology in Europe: Culex pipiens host feeding preference in a hotspot of virus emergence. Parasites Vectors 2015, 8, 213. [Google Scholar] [CrossRef] [PubMed]
- Grant, R.H.; Apostol, K.G.; Schmitz, H.F. Physiological Impacts of Short-Term UV Irradiance Exposures on Cultivars of Glycine Max. In UV Radiation in Global Climate Change: Measurements, Modeling and Effects on Ecosystems; Gao, W., Slusser, J.R., Schmoldt, D.L., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 458–487. [Google Scholar]
- Sullivan, J.H.; Pope, L.C.; Sutherland, B.M.; Bennett, P.V.; Blum, J.E.; Stapleton, A.E.; Gitz, D.C. Assessment of DNA Damage as a Tool to Measure UV-B Tolerance in Soybean Lines Differing in Foliar Flavonoid Composition. In UV Radiation in Global Climate Change: Measurements, Modeling and Effects on Ecosystems; Gao, W., Slusser, J.R., Schmoldt, D.L., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 437–457. [Google Scholar]
- Maciá, A. Differences in performance of Aedes aegypti larvae raised at different densities in tires and ovitraps under field conditions in Argentina. J. Vector Ecol. 2006, 31, 371–377. [Google Scholar] [CrossRef]
- USEPA. SW-846 Test Method 3015A: Microwave Assisted Acid Digestion of Aqueous Samples and Extracts; Environmental Protection Agency: Washington, DC, USA, 2007.
- USEPA. Method 200.7, Revision 4.4: Determination of metals and trace elements in water and wastes by inductively coupled plasma-atomic emission spectrometry. In Methods for the Determination of Metals in Environmental Samples, Supplement I; Environmental Protection Agency: Cincinnati, OH, USA, 1994. [Google Scholar]
- Freed, T.Z.; Leisnham, P.T. Roles of spatial partitioning, competition, and predation in the North American invasion of an exotic mosquito. Oecologia 2014, 175, 601–611. [Google Scholar] [CrossRef]
- Freed, T.Z.; Kesavaraju, B.; Leisnham, P.T. Effects of competition and predation by native mosquitoes on the North American invasion of Aedes japonicus japonicus (Diptera: Culicidae). J. Med. Entomol. 2014, 51, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Griswold, M.W.; Lounibos, L.P. Competitive outcomes of aquatic container Diptera depend on predation and resource levels. Ann. Entomol. Soc. Am. 2005, 98, 673–681. [Google Scholar] [CrossRef][Green Version]
- Juliano, S.A. Species introduction and replacement among mosquitoes: Interspecific resource competition or apparent competition? Ecology 1998, 79, 255–268. [Google Scholar] [CrossRef]
- Vinogradova, E.; Karpova, S. Effect of photoperiod and temperature on the autogeny rate, fecundity and wing length in the urban mosquito, Culex pipiens pipiens f. molestus (Diptera, Culicidae). Int. J. Dipterol. Res. 2006, 17, 3–12. [Google Scholar]
- Lounibos, L.; Suárez, S.; Menéndez, Z.; Nishimura, N.; Escher, R.; Connell, S.O.; Rey, J. Does temperature affect the outcome of larval competition between Aedes aegypti and Aedes albopictus? J. Vector Ecol. 2002, 27, 86–95. [Google Scholar] [PubMed]
- Leisnham, P.T.; Scott, B.; Baldwin, A.H.; LaDeau, S.L. Effects of detritus on the mosquito Culex pipiens: Phragmites and Schedonorus (Festuca) invasion affect population performance. Int. J. Environ. Res. Public Health 2019, 16, 4118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, J.; Li, C.; Momen, B.; Kohanski, R.; Pick, L. Deletion of Drosophila insulin-like peptides causes growth defects and metabolic abnormalities. Proc. Natl. Acad. Sci. USA 2009, 106, 19617–19622. [Google Scholar] [CrossRef] [PubMed]
- Braissant, O.; Wirz, D.; Gopfert, B.; Daniels, A.U. Use of isothermal microcalorimeter to monitor microbial activities. FEMS Microbiol. Lett. 2010, 303, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Yee, D.A.; Glasgow, W.C.; Ezeakacha, N.F. Quantifying species traits related to oviposition behavior and offspring survival in two important disease vectors. PLoS ONE 2020, 16, e0250288. [Google Scholar] [CrossRef]
- Allgood, D.W. Influence of Detritus Levels and Organic Pollution on Interspecific Resource Competition, Oviposition Behavior, and Larval Survival of Two Tire-Inhabiting Mosquito Species (Diptera: Culicidae); University of Southern Mississippi: Hattiesburg, MS, USA, 2011. [Google Scholar]
- SAS Institute. SAS User’s Guide: Statistics, Version 9.1.; SAS Institute: Cary, NC, USA, 2003. [Google Scholar]
- Scheiner, S.M. MANOVA: Multiple Response Variables and Multispecies Interactions. In Design and Analysis of Ecological Experiments, 2nd ed.; Scheiner, S.M., Gurevitch, J., Eds.; Oxford University Press: Oxford, UK, 2001; pp. 99–133. [Google Scholar]
- Dayton, P.; Dayton, P.K. Competition, disturbance, and community organization: The provision and subsequent utilization of space in a rocky intertidal community. Ecol. Monogr. 1971, 41, 351–389. [Google Scholar] [CrossRef]
- Juliano, S.A. Invasion ecology of Asian tiger mosquito: Egg mortality alters the outcome of competition among larvae. Ecol. Soc. Am. Annu. Meet. Abstr. 2002, 87, 371. [Google Scholar]
- Malenke, J.R.; Newbold, N.; Clayton, D.H. Condition-specific competition governs the geographic distribution and diversity of ectoparasites. Am. Nat. 2011, 177, 522–534. [Google Scholar] [CrossRef]
- Aboelkheir, M.G.; Lima Junior, J.G.; Toledo Filho, R.D.; Souza Junior, F.G.; dos Santos Siqueira, C.Y. Thermo-oxidative degradation of vulcanized SBR: A comparison between ultraviolet (UV) and microwave as recovery techniques. J. Polym. Res. 2021, 28, 141. [Google Scholar] [CrossRef]
- Grech, M.G.; Juliano, S.A. Complex effects of superior competitors and resources on Culex restuans (Diptera: Culicidae) oviposition. J. Med. Entomol. 2018, 55, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Williams-Newkirk, A.; Kitron, U.; Chaves, L. Seasonal weather, nutrients, and conspecific presence impacts on the southern house mosquito oviposition dynamics in combined sewage overflows. J. Med. Entomol. 2014, 46, 1328–1338. [Google Scholar] [CrossRef]
- Silberbush, A.; Tsurim, I.; Rosen, R.; Margalith, Y.; Ovadia, O. Species-specific non-physical interference competition among mosquito larvae. PLoS ONE 2014, 9, e88650. [Google Scholar] [CrossRef]
- Broadie, K.S.; Bradshaw, W.E. Mechanisms of interference competition in the western treehole mosquito, Aedes sierrensis. Ecol. Entomol. 1991, 16, 145–154. [Google Scholar] [CrossRef]
- Sunahara, T.; Mogi, M. Priority effects of bamboo-stump mosquito larvae: Influences of water exchange and leaf litter input. Ecol. Entomol. 2002, 27, 347–354. [Google Scholar] [CrossRef]
- Talaga, S.; Dejean, A.; Mouza, C.; Dumont, Y.; Leroy, C. Larval interference competition between the native Neotropical mosquito Limatus durhamii and the invasive Aedes aegypti improves the fitness of both species. Insect Sci. 2018, 25, 1102–1107. [Google Scholar] [CrossRef]
- Juliano, S.A.; Ribeiro, G.S.; Maciel-De-Freitas, R.; Castro, M.G.; Codeco, C.; Lourenco-de-Oliveira, R.; Lounibos, L.P. She’s a femme fatale: Low-density larval development produces good disease vectors. Mem. Do Inst. Oswaldo Cruz 2014, 109, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Kellough, R.M. The Effects of Scrap Automobile Tires in Water; Waste Management Branch: Toronto, ON, Canada, 1991; p. 10. [Google Scholar]
- Abernethy, S.G.; Montemayor, B.P.; Penders, J.W. The Aquatic Toxicity of Scrap Automobile Tires; Ontario Ministry of Environment and Energy: Guelph, ON, Canada, 1996.
- Yee, D.A. Tires as habitats for mosquitoes: A review of studies within the eastern United States. J. Med. Entomol. 2008, 45, 581–593. [Google Scholar] [PubMed]
- LaDeau, S.L.; Leisnham, P.T.; Biehler, D.; Bodner, D. Higher mosquito production in low-income neighborhoods of Baltimore and Washington, DC: Understanding ecological drivers and mosquito-borne disease risk in temperate Cities. Int. J. Environ. Res. Public Health 2013, 10, 1505–1526. [Google Scholar] [CrossRef] [PubMed]
- Bennett, K.L.; Gómez Martínez, C.; Almanza, A.; Rovira, J.R.; Mcmillan, W.O.; Enriquez, V.; Barraza, E.; Diaz, M.; Sanchez-Galan, J.E.; Whiteman, A.; et al. High infestation of invasive Aedes mosquitoes in used tires along the local transport network of Panama. Parasites Vectors 2019, 12, 1–10. [Google Scholar] [CrossRef]
- Kwon, E.; Castaldi, M.J. Fundamental understanding of the thermal degradation mechanisms of waste tires and their air pollutant generation in a N2 atmosphere. Environ. Sci. Technol. 2009, 43, 5996–6002. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.E.; Castaldi, M.J. Mechanistic understanding of polycyclic aromatic hydrocarbons (PAHs) from the thermal degradation of tires under various oxygen concentration atmospheres. Environ. Sci. Technol. 2012, 46, 12921–12926. [Google Scholar] [CrossRef]
- Zheng, S.; Liao, M.; Chen, Y.; Brook, M.A. Dissolving used rubber tires. Green Chem. 2020, 22, 94–102. [Google Scholar] [CrossRef]

| Variable | λ’ | Survival | Female Developmental Time | Female Wing Length | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| df | F | p-Value | df | F | p-Value | df | F | p-Value | df | F | p-Value | |
| Ae. albopictus | ||||||||||||
| UV-B condition | 2,24 | 2.68 | 0.089 | 2,24 | 7.29 | 0.003 | 2,24 | 2.83 | 0.079 | 2,24 | 0.24 | 0.790 |
| Competition | 2,24 | 3.04 | 0.066 | 2,24 | 8.29 | 0.001 | 2,24 | 1.02 | 0.375 | 2,24 | 1.45 | 0.255 |
| UV-B condition x competition | 4,24 | 3.10 | 0.034 | 4,24 | 3.30 | 0.027 | 4,24 | 1.74 | 0.175 | 4,24 | 0.41 | 0.800 |
| Cx. pipiens | ||||||||||||
| UV-B condition | 2,24 | 2.97 | 0.066 | 2,24 | 5.91 | 0.008 | 2,21 | 0.37 | 0.692 | 2,21 | 13.7 | 0.002 |
| Competition | 2,24 | 0.15 | 0.752 | 2,24 | 3.79 | 0.037 | 2,21 | 5.38 | 0.013 | 2,21 | 6.11 | 0.008 |
| UV-B condition x competition | 4,24 | 0.01 | 0.990 | 4,24 | 0.28 | 0.888 | 4,21 | 1.13 | 0.370 | 4,21 | 2.61 | 0.064 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villena, O.C.; Sullivan, J.H.; Landa, E.R.; Yarwood, S.A.; Torrents, A.; Zhang, A.; Leisnham, P.T. The Role of Tire Leachate in Condition-Specific Competition and the Persistence of a Resident Mosquito from a Competitively Superior Invader. Insects 2022, 13, 969. https://doi.org/10.3390/insects13110969
Villena OC, Sullivan JH, Landa ER, Yarwood SA, Torrents A, Zhang A, Leisnham PT. The Role of Tire Leachate in Condition-Specific Competition and the Persistence of a Resident Mosquito from a Competitively Superior Invader. Insects. 2022; 13(11):969. https://doi.org/10.3390/insects13110969
Chicago/Turabian StyleVillena, Oswaldo C., Joseph H. Sullivan, Edward R. Landa, Stephanie A. Yarwood, Alba Torrents, Aijun Zhang, and Paul T. Leisnham. 2022. "The Role of Tire Leachate in Condition-Specific Competition and the Persistence of a Resident Mosquito from a Competitively Superior Invader" Insects 13, no. 11: 969. https://doi.org/10.3390/insects13110969
APA StyleVillena, O. C., Sullivan, J. H., Landa, E. R., Yarwood, S. A., Torrents, A., Zhang, A., & Leisnham, P. T. (2022). The Role of Tire Leachate in Condition-Specific Competition and the Persistence of a Resident Mosquito from a Competitively Superior Invader. Insects, 13(11), 969. https://doi.org/10.3390/insects13110969







