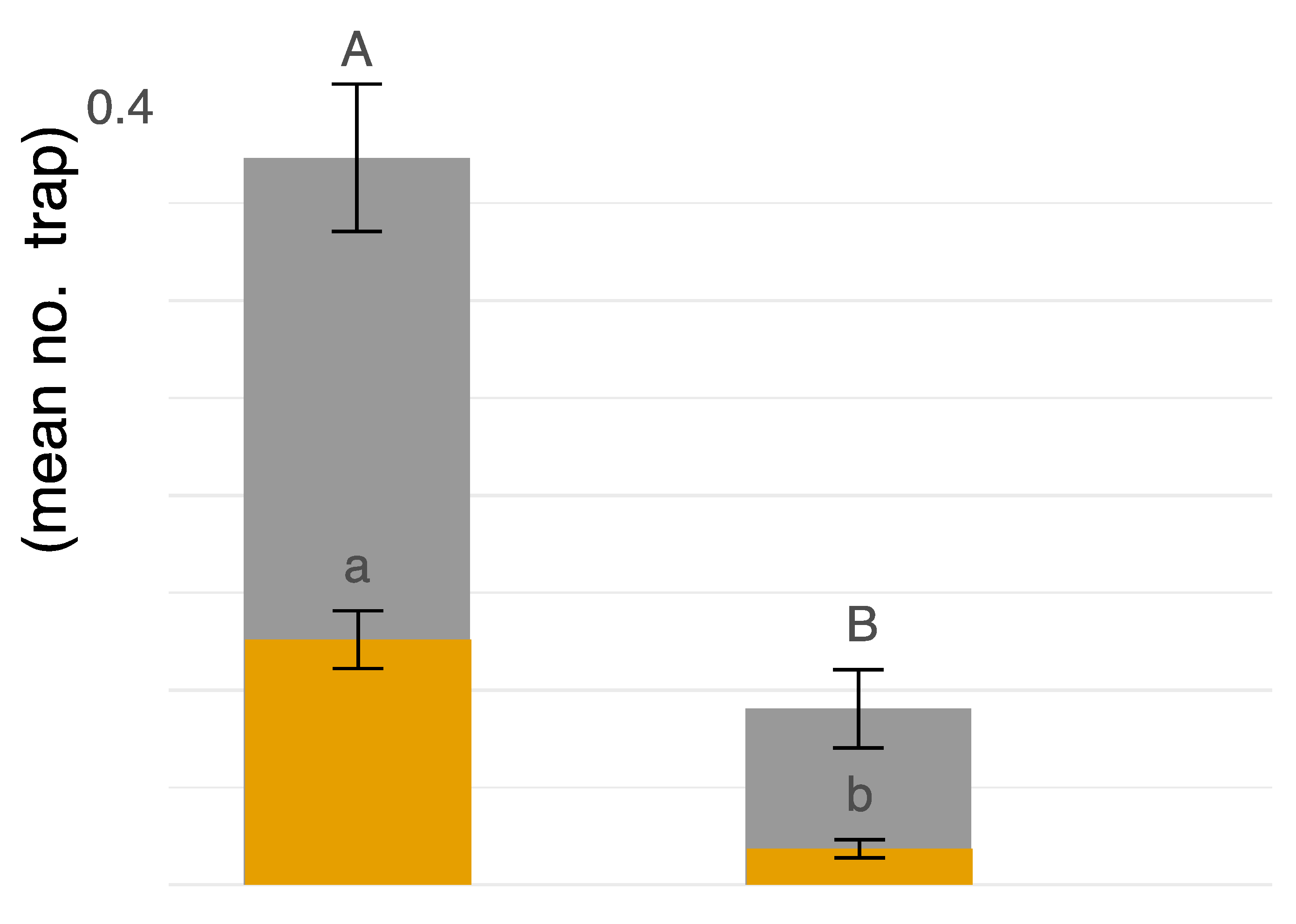Simple Summary
The hunter fly, Coenosia attenuata Stein (1903) (Diptera: Muscidae), is a species of biological control significance typically found in greenhouses. Phylogenetic data suggests Coenosia attenuata originated in the Mediterranean region. Here, we provide the first report of a wild hunter fly population in North America. In 2020 and 2021, Coenosia attenuata was captured in pan traps set in Georgia and South Carolina peach orchards. Specimens collected across multiple sites over two years were identified with morphological keys and confirmed via DNA barcoding, providing strong evidence for an established population in the Southeastern USA.
Abstract
Coenosia attenuata is a member of the tigrina-group of Coenosia (sensu Hennig 1964) and is a capable generalist predator in its larval and adult stages. C. attenuata is common in greenhouses worldwide, however, there are few documented cases of its presence in the wild. Here, we estimated C. attenuata presence in the southeastern USA peach orchards using pan traps. Over two years, a total of 717 specimens were collected from both commercially managed and fungicide-only managed peach orchards. C. attenuata is a known biological control agent in artificial greenhouse settings, but its impact on pest species in the wild is still unknown. For the first time in North America, we document an established wild population of C. attenuata, provide an overview of basic identification, and review potential benefits for biological control.
1. Introduction
The hunter fly, Coenosia attenuata Stein (1903) (Diptera: Muscidae), is a widespread predatory fly often associated with greenhouses [1]. Phylogenetic studies suggest that C. attenuata originated in the Mediterranean region, but is now known to occur in Europe, Asia, the Middle East, Africa, South America, and North America [1]. Across this wide distribution, the species exhibits limited mitochondrial genetic diversity, leading Seabra et al. [1] to propose that much of its current range is due to human-mediated expansion into greenhouses worldwide. Hoebeke et al. [2] hypothesized that the larvae are transported in potted plant soil. The larvae are soil-dwelling, polyphagous predators capable of living for 20–35 days even when prey is scarce [2].
Coenosia attenuata is a generalist predator in both adult and larval stages and, as such, is a biological control agent of multiple larval and adult greenhouse pests [3,4,5,6]. Common prey include: whiteflies (Hemiptera: Aleyrodidae), various Diptera, including fungus gnats (Sciaridae), leaf-miner flies (Agromyzidae), and pomace flies (Drosophilidae) [7,8]. The hunter fly thrives in artificial greenhouse environments and has been documented in New World greenhouses in Ecuador [9], Peru [9], Colombia [10], Costa Rica [11], Chile [12], Venezuela [13], Mexico [7], Honduras [14], Brazil [15], Uruguay [16], and the USA [17]. However, reports of C. attenuata outside of the greenhouse environment are uncommon, especially in the New World. In the first report of the species for North America, most records were greenhouse-based records, except for a single report of specimens captured in malaise traps in suburban Los Angeles, California [17]. The only other records of C. attenuata found in an open field setting in the New World are from South America, where adults were reported on baby’s breath flowers (Caryophyllaceae: Gypsophila paniculata L.) in Northeastern Brazil in 2016 and 2017 [15], and on blueberries (Ericaceae: Vaccinium sp.) in Chile [12].
During insect sampling in peach orchards in South Carolina (SC) and Georgia (GA), USA, in 2020 and 2021, 717 specimens of unknown fly species were collected in colored pan traps. The specimens were identified as Coenosia attenuata using morphological and molecular approaches (Figure 1). Here we document this new occurrence in peach orchard systems, provide a preliminary phenology for the species in peach orchards of the Southeastern USA, provide diagnostic information to assist in the recognition of this species based on the anatomy, and contribute COI barcodes to Genbank.
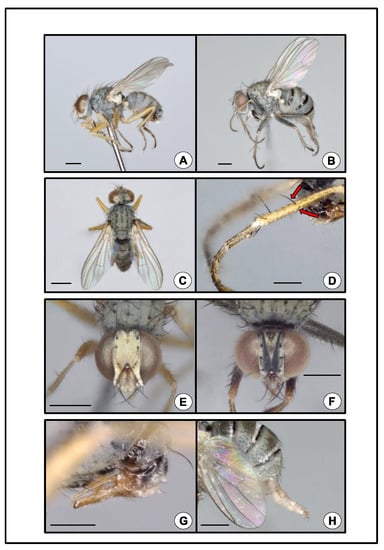
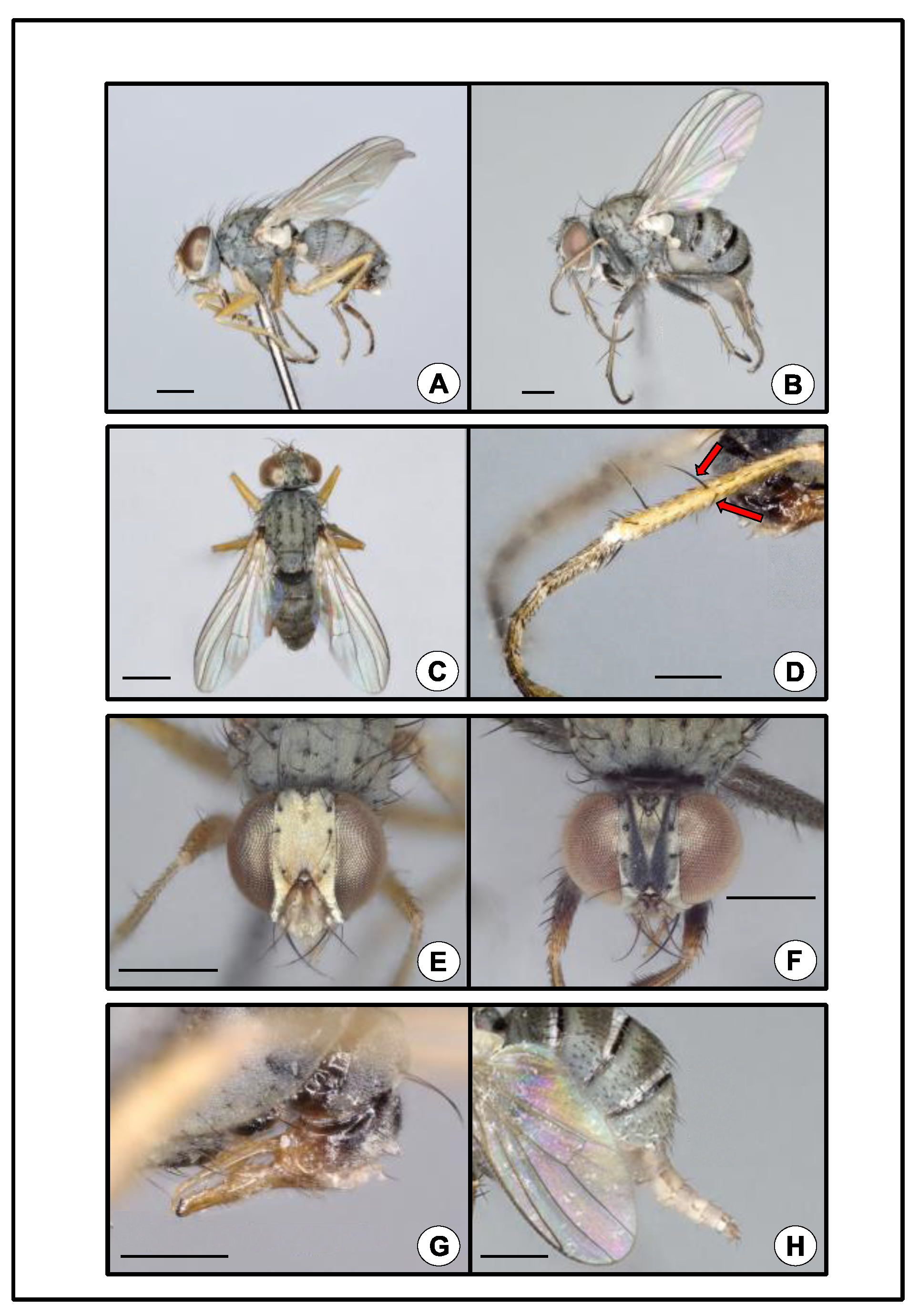
Figure 1.
Coenosia attenuata. (A) Male, lateral. Scale bar = 0.5 mm. (B) Female, lateral. Note abdomen distended, exposing three black transverse stripes. Scale bar = 0.5 mm. (C) Male, habitus, dorsal. Scale bar = 0.5 mm. (D) Male, metathoracic tibia and tarsus, lateral. Arrows mark two major diverging setae at midlength. Scale bar = 0.25 mm. (E) Male, head, anterior. Scale bar = 0.5 mm (F) Female, head, anterior. Scale bar = 0.5 mm. (G) Male, abdominal terminalia, genitalia, lateral. Scale bar = 0.25 mm. (H) Female, abdominal terminalia, ovipositor, lateral. Scale bar = 0.5 mm.
2. Materials and Methods
2.1. Field Sites
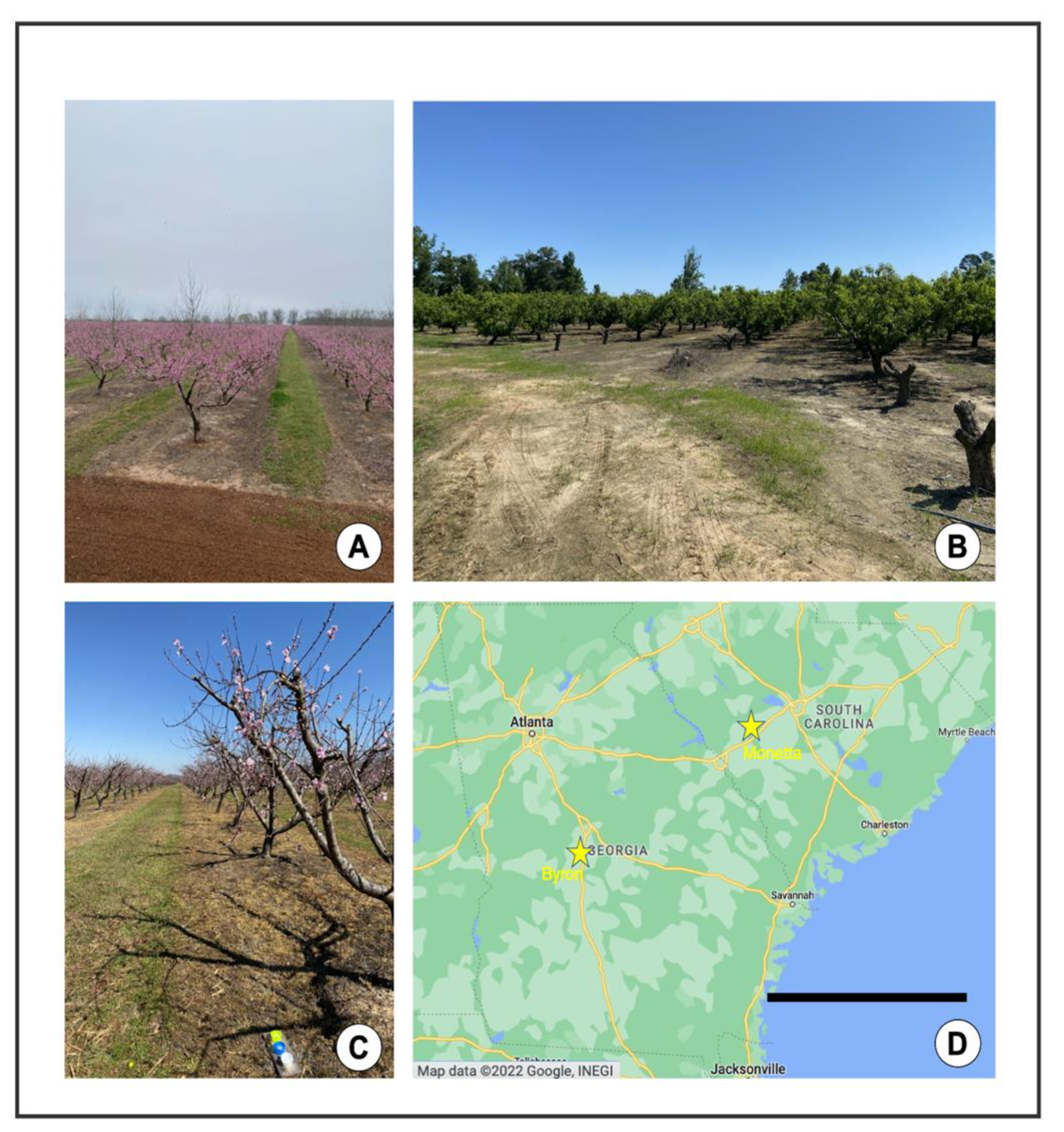
The study was conducted in peach orchards in the Southeastern USA. The climate in the Southeastern USA is highly variable, experiencing extremely high temperatures in the summer and low temperatures in the winter months. There is also considerable humidity, and an average rainfall of between 44 and 52 inches per year [18]. The study sites were located in Byron, GA, and Monetta, SC, approximately 240 km apart (Figure 2). In GA, the six study sites were separated by 1–5 km, and in SC, the study sites were separated by 1–3 km. In an effort to maintain consistency, we selected orchards that were approximately the same size (1–3 ha). Peach orchards have largely sandy soil within the rows where peach trees are planted. The ground within and between rows is naturally well-lit, as trees are typically spaced 5 m apart, separated by grasses that are frequently mowed (Figure 2A–C). The orchards we sampled were managed with recommended practices following the 2022 Southeastern Peach Guide [19], with intensive commercial chemical applications or “high input” management, or “low input” (i.e., fungicide only in 2020, and organic fungicide and insecticide in 2021) management (Figure 2A–C). The high input orchards were treated primarily with a pyrethroid-based spray regimen, as well as supplementary fungicide sprays [19].
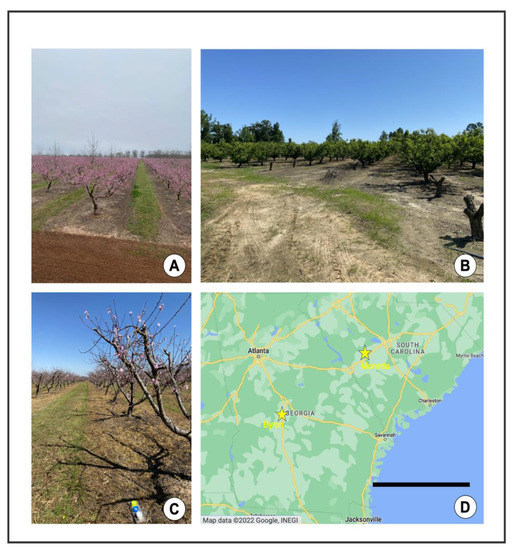
Figure 2.
Field sites. (A) Commercial peach farm (high input), Byron, GA, 3/9/2021. (B) Organic peach farm (low input), Monetta, SC, 5/13/2021. (C) Commercial peach farm, Byron, GA, 3/16/2021. (D) Locations of field sites in Southeastern U.S. Map data © 2022 Google, INEGI (Google Maps) [20] Scale bar = 240 km.
2.2. Sample Collection
We used pan traps colored with blue, white, or yellow fluorescent paint to estimate the activity of flower-visiting insects, such as hymenoptera, diptera, and lepidoptera [21,22]. Traps were constructed of plastic bowls (7.1 cm diameter) filled with dish soap (to break surface tension) and water. Twenty-seven traps were deployed per field site and were collected after 24 h. Traps were deployed in sets of three (blue, white, and yellow grouped together) and placed under nine trees in a transect pattern. Three trees along the edge of the orchard, three at 25 m towards the interior, and three at 50 m toward the interior. A total of 2244 traps were deployed over 17 sample dates. Traps were deployed monthly between July and October in 2020, and from March–September in 2021. During this study, many other insects were collected, however here we only report on Coenosia attenuata.
2.3. Sample Processing
While sorting the pan traps, we discovered an unusual dipteran, and initially we sent representative specimens to E. Richard Hoebeke (University of Georgia Collection of Arthropods) and Adrian C. Pont (Oxford University) for identification. Hoebeke and Pont confirmed the identification of the fly as Coenosia attenuata. Remaining traps were then sorted to document all specimens of this fly, and subsequent specimens were morphologically identified by the authors using Hoebeke et al.’s guide [2]. Coenosia attenuata is a member of the tigrina-group of Coenosia (sensu Hennig 1964) [23], which includes Old World species that can be diagnosed by the occurrence of two major diverging bristles near the midlength of the anterior and anterodorsal surfaces of the hind tibia (Figure 1D). Two other species of this group have been introduced into North America, Coenosia tigrina F. (1775) and Coenosia humilis Meigen (1826) [2]. Coenosia attenuata can be distinguished from these two species based on its smaller size, having a body length of 2.5–3.0 mm for males and 3.0–4.0 mm for females, (C. tigrina: 4.75–5.75 mm, male and 5.75–7.0 mm, female; C. humilis: 3.0–3.5 mm, male and 3.0–5.5 mm, female), and by the yellow color of the femora in males (C. tigrina femora are black with a reddish-yellow apex; C. humilis femora are black) [2]. In addition, the form of the male genitalia of C. attenuata is diagnostic (Figure 1G). A detailed taxonomic description and natural history summary for C. attenuata are given by Hoebeke et al. [2].
Three male specimens were identified using DNA barcoding of the cytochrome oxidase 1 (CO1) gene. Genomic DNA was extracted from individual flies (whole body extraction) using the Qiagen DNeasy Blood and Tissue kit, following the manufacturer’s protocol. Extracted DNA was stored at −20 °C. A negative extraction control that contained all Qiagen buffers for extraction and Proteinase K solutions was included. The DNA barcode region of the CO1 gene was amplified using standard DNA barcoding primers [24], following the protocol described by Cutler et al. [25]. PCR products were sent for purification and sequencing at Eurofins (©Eurofins Scientific 2021). Purified PCR products were sequenced bidirectionally. Using Codon Code Aligner version 9.0.1, forward and reverse sequences were assembled, aligned, and edited to trim the remaining primer sequences. Prepared sequences were then “blasted” against all sequence records in public databases of the Barcode of Life Data System (BOLD) and the National Center for Biotechnology Information (NCBI).
A representative male and female were imaged by transferring preserved specimens with 75% ethanol to amyl acetate for 48 h before being mounted on a minute pin and air dried. Specimens were photographed using the automated image rendering of a Keyence VHX-7000 digital imaging microscope (Keyence, Itasca, IL, USA). Voucher specimens were deposited in the University of Georgia Collection of Arthropods (UGCA) in Athens, GA, USA.
2.4. Statistical Analyses
We fitted several statistical models to test if hunter-fly counts were influenced by sampling date, trap color, or management intensity (i.e., high or low chemical input), and to assess male:female sex ratios. In each of the models, we fitted general mixed effect models (GLMMs) using the GLIMMIX procedure. AR1 covariance structure of errors was used in testing for seasonal variation, which considered repeated measures. To test if hunter fly counts were influenced by pan trap color, we fitted a GLMM of hunter fly pooled counts by field. Trap color was the fixed effect, and transect and date were set as random effects. To ask whether hunter flies in the Southeast are multivoltine, we assessed significant seasonal variation in hunter fly counts for each year using GLMMs with date as the fixed effect and transects nested in fields used as random effects. Lastly, management intensity (i.e., high or low chemical input) in orchards may affect the abundance of hunter flies. Therefore, in similar structured models, we analyzed the effect of management and field position (i.e., transect) on hunter fly counts using GLMMs with management intensity, transect, and the interaction between management intensity and transect as fixed effect terms with date nested within field as random effects. The differences in the model structure of fixed effects and random effects were needed to test different hypotheses. In all cases, natural log transformed hunter fly counts were used as the response variable, which improved normality and homogeneity of variance. The statistical analyses were conducted in SAS version [9.4] (2013) by SAS Institute Inc., Cary, NC, USA.
3. Results
Coenosia attenuata is a small, light gray fly with a muscoid body form (Figure 1A–C). The tarsi are long with black setulae and at rest are held slightly curved, giving a raptorial appearance (Figure 1A,B,D). The sexes are dimorphic. Males are smaller than females (see above). The male head has a bright silvery-white pruinose vertex, frons, parafacials, and lunule (Figure 1E). The female head is pruinose and gray with two dark converging longitudinal stripes on the frons (Figure 1F). The legs are yellowish in males with slightly darker tarsi (Figure 1A,D). The legs of females are grayish, especially on the femora (Figure 1B). The abdomen is uniformly light gray in males (Figure 1A,C,G) or has some indistinct darker blotches, but in females, the abdomen bears three distinct black transverse stripes (Figure 1B,H). The morphological characteristics described above, and the COI sequence analysis confirmed the collected specimens to be C. attenuata. All three individuals that were barcoded yielded a 658-bp fragment (Genbank accession numbers: ON257860–ON257862), and were consistent (i.e., 100% overlap and >99% identity match) with public sequences for C. attenuata on both BOLD and Genbank.
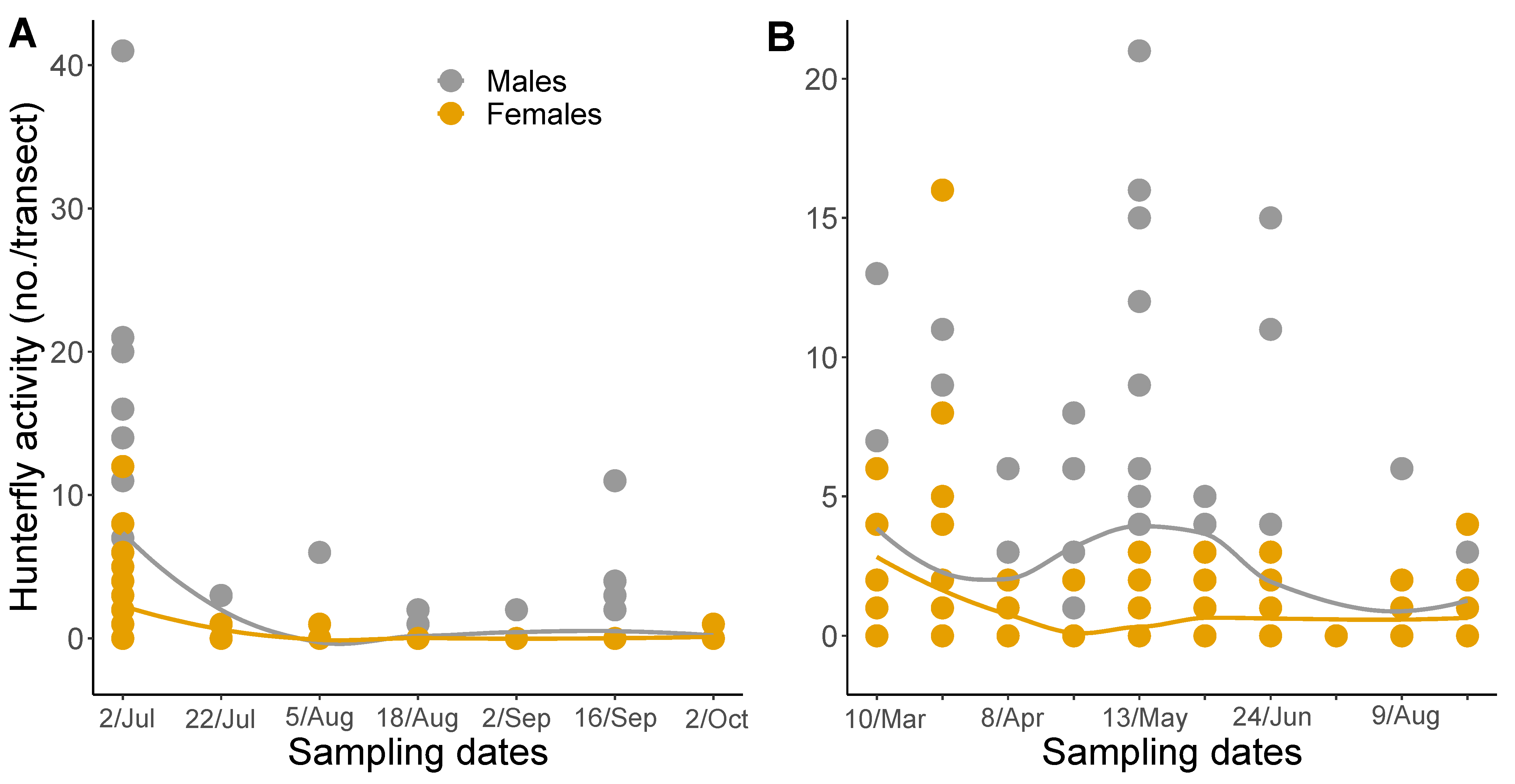
Overall, we captured 609 individuals in GA and 108 in SC for a total of 717 C. attenuata over the two-year study. In 2020, we captured a total of 231 and in 2021, 486 C. attenuata. For both male and female hunter flies, in 2020 there was significant variation in counts throughout the season (F6,30 = 5.57, p = 0.0006; F6,30 = 8.67, p ≤ 0.0001, respectively), with a peak in July (Figure 3A). In 2020, there were significantly more flies caught on July 2nd than on all other sample dates, expect for another peak in capture on September 16th (Tukey-Kramer). There was marginally significant variation in seasonal data for male and female hunter flies in 2021 (F9,34 = 1.95, p = 0.0783; F9,34 = 1.98, p = 0.0734, respectively; Figure 3B).
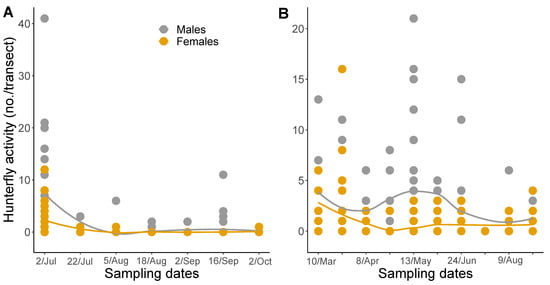
Figure 3.
Seasonal summary of mean counts of Coenosia attenuata observed in pan traps combined by transect for (A) 2020 and (B) 2021. Line color indicates: Male = gray, Female = gamboge. Data points represent the mean sum total of hunter flies captured in blue, white, and yellow pan traps for a total of nine traps per field. The solid lines are loess smoothing curves with corresponding shaded 95% CI to display the estimated fluctuation in captures over the season.
Coenosia attenuata was found at both high-input and low-input managed peach orchards during both years of the study. Our sampling efforts over two years produced a greater number of specimens from the high input sites (n = 595) than the low input sites (n = 122) (Figure 4). Trap color appears to be a significant predictor of hunter-fly counts observed in traps for both males (F2,255 = 34.78, p ≤ 0.0001) and females (F2,255 = 16.47, p ≤ 0.0001). A greater number of hunter flies were observed in white bowls (n = 585) as compared to either blue (n = 45) or yellow (n = 77) (t = −7.65, df = 255, p ≤ 0.0001; t = 6.70, df = 255, p ≤ 0.0001, respectively).
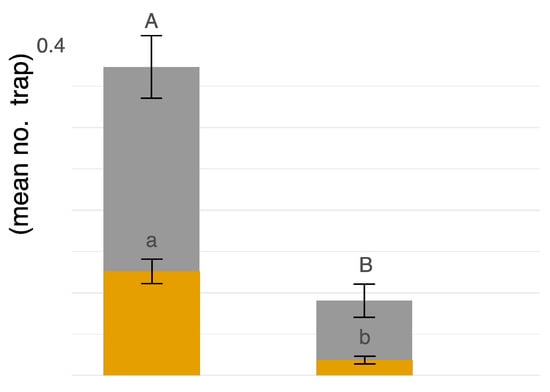
Figure 4.
Male = grey, Female = gamboge. On the x-axis, “high” is high-input commercial chemical management, “low” is low-input fungicide-only chemical management. On the y-axis, blue, white, and yellow traps were pooled together, and displayed as average count of flies per trap location. The uppercase letters represent linear contrasts for males and lowercase letters for females in relation to management strategy (α = 0.05).
The number of male hunter flies captured was influenced by management strategy (F1,179 = 16.64, p ≤ 0.0001; Figure 4), no significant influence of field transects (F2,179 = 2.50, p = 0.0847), and no interaction between management and field transects (F2,179 = 1.49, p = 0.2272) (Figure 4). More males were observed in high input management than in low input (t = 4.08, p ≤ 0.0001) (Figure 4). For females, management strategy had a significant effect on the number of flies captured (F1,179 = 13.61, p ≤ 0.0001), with no transect effect (F2,179 = 0.98, p = 0.3773) or interaction between management and field transect (F2,179 = 1.12, p = 0.3301). The management effect is explained by higher numbers of female hunter flies observed in the high input system (t = 5.29, p ≤ 0.0001) (Figure 4). Lastly, abundance patterns showed male hunter flies were more commonly captured, providing evidence of male biased sex ratios with on average 3.145 males per female (X2 = 191.98, p < 0.0001) (Figure 4).
4. Discussion
Here, we report the first wild population of C. attenuata, the hunter fly, in the peach agroecosystems of Georgia and South Carolina, USA. The number of flies captured varied according to management, sex ratio, as well as trap color. Overall, C. attenuata was present in high-input, commercially managed orchards, USDA organically certified orchards, and in low-input, fungicide-only managed research orchards. In Turkey, C. attenuata was monitored in a greenhouse that underwent chemical treatments to combat whiteflies. All four chemical treatments of insecticides and fungicides appeared to have low-to-no impact on hunter fly populations [4]. This suggests that C. attenuata may be tolerant of some insecticide and fungicide applications, demonstrating their potential value as a biological control agent in commercially managed field settings.
As a predator, adult hunter flies rely primarily on visual cues. Their hunting behavior involves perching in well-lit areas, waiting for flying insects to venture nearby, darting out to capture the prey in the air, and returning to their perch [26,27]. This precise mid-air attack is called “hawking” hunting behavior, and is a strategy shared with other insects, such as Anisoptera (Odonata) and Asilidae (Diptera) [26]. Hunter flies can attack prey from any angle (launching from the ceiling, a wall, or the ground), minimizing their flight time and efficiently expending energy. Adults kill their prey by stabbing the cervix (neck) area with their proboscis, sometimes partially decapitating their prey, before returning to their perch to drink the nutrients [28]. Hunter flies were recorded attacking flying insects near their perch without attempting to eat them, exhibiting almost territorial behavior [29]. Interestingly, Mateus et al. [28] found that an attack was not provoked if prey walked near C. attenuata; only a nearby flight would trigger an attack. This provoked hunting behavior of adults was observed again in laboratory feeding experiments with different prey species. In a controlled laboratory experiment, hunter fly adults preyed most heavily on adult fungus gnats, followed by adult shore flies and adult whiteflies [30,31]. Whiteflies are mostly sedentary when feeding, so it would follow that they are attacked less often by the hunter flies [28]. We did not monitor the feeding habits of the C. attenuata in this study. However, many of the known prey taxa of C. attenuata were present in our pan trap bycatch. It is possible that C. attenuata are drawn to the prey taxa attracted to the pan traps, using these pseudo “flowers” as a hunting site [15,32]. The feeding habits and biological control contributions of C. attenuata in the southeastern USA’s peach orchards should be considered in future studies.
The number of hunter flies captured in peach orchards varied seasonally. Capture data suggest anecdotal peaks in activity during early April, late May, and early July (Figure 3). Under laboratory conditions, the lifecycle of C. attenuata takes 26 days to complete [33]. The only record of a wild population of C. attenuata phenology suggests the fly is multivoltine in Turkish cotton fields [34]. Wild populations of a closely related species, Coenosia tigrina, in Michigan onion fields were also found to be multivoltine [35]. Similarly, species of another hunter-fly genus, Limnophora Robineau-Desvoidy (1830) (Muscidae: Coenosiinae), were found to be multivoltine in tufa barriers in Croatia [36]. However, our current data do not suggest multiple statistically significant peaks in counts throughout 2020 and 2021, so we cannot conclude if C. attenuata are multivoltine in the southeastern part of the USA.
The sex ratio of reported male and female hunter flies differs between a greenhouse, laboratory, and field settings as well as according to sample methodology. In this study, the sex ratio of hunter flies was male-biased; 3.145 Male:Female (Figure 4), while greenhouse systems appeared to be female-biased; 0.25 M:F ratio [28]. Furthermore, under optimized laboratory-rearing conditions, emerging flies had a sex ratio of 0.67 and 0.69 M:F [32,37]. With the number of confounding variables between studies, it is difficult to speculate on what caused the different sex ratios. Environmental conditions may have affected the sex ratio (e.g., the moist soil in greenhouses, or coconut fiber/black peat mixture for rearing, versus the dry sandy substrate of peach farms). Alternatively, sampling methodology may affect sex-specific capture rates (e.g., suction sampling, sticky cards, and pan traps). In addition, we found that both male and female hunter flies were most abundant in the white-colored pan traps. However, factors that contribute to different sex ratios of C. attenuata should be explored in future studies.
5. Conclusions
At this time, we cannot predict the impact of the Coenosia attenuata establishment in the southeastern part of the USA’s agroecosystems. However, based on hunter-fly biology and life history traits, this species should be considered a potential biological control agent. As such, future studies in the region should be mindful of this potentially significant addition to the local insect fauna.
Author Contributions
Conceptualization, A.D.K. and J.V.M.; methodology, A.D.K., B.R.B., J.M.S. and T.D.G.; data management and analysis, A.D.K., J.M.S. and X.L.; molecular analysis, J.M.S. and T.D.G.; writing original draft, A.D.K.; writing—review and editing, J.V.M., B.R.B., J.M.S. and T.D.G.; photos and figures, A.D.K. and J.V.M.; funding acquisition, B.R.B., J.M.S. and J.V.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a Georgia Specialty Crop Block Grant 17BLAA3101 to B. Blaauw and J. M. Schmidt and the USDA National Institute of Food and Agriculture Hatch project GEO00886 to J. V. McHugh.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank E. Richard Hoebeke (Georgia Museum of Natural History, UGCA) and Adrian C. Pont (Oxford University Museum of Natural History) for providing preliminary identifications and reviewing the original manuscript. We would also like to thank cooperating peach producers, and Melissa Thompson for their help in the Schmidt molecular laboratory. Many undergraduates helped with sampling and sample processing. We thank Reilly Farrell, Emmalee Milner, Rachel Perez, Olivia Centanni, Matthew Greer, and Jack Layman.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Seabra, S.G.; Brás, P.G.; Martins, J.; Martins, R.; Wyatt, N.; Shirazi, J.; Rebelo, M.T.; Franco, J.C.; Mateus, C.; Figueiredo, E. Phylogeographical patterns in Coenosia attenuata (Diptera: Muscidae): A widespread predator of insect species associated with greenhouse crops. Biol. J. Linn. Soc. 2015, 114, 308–326. [Google Scholar] [CrossRef]
- Ugine, T.A.; Sensenbach, E.J.; Sanderson, J.P.; Wraight, S.P. Biology and feeding requirements of larval hunter flies Coenosia attenuata (Diptera: Muscidae) reared on larvae of the fungus gnat Bradysia impatiens (Diptera: Sciaridae). J. Econ. Entomol. 2010, 103, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Kühne, S. Open rearing of generalist predators: A strategy for improvement of biological pest control in greenhouses. Phytoparasitica 1998, 26, 277–281. [Google Scholar] [CrossRef]
- Pohl, D.; Kühne, S.; Karaca, İ.; Moll, E. Review of Coenosia attenuata Stein and its first record as a predator of important greenhouse pests in Turkey. Phytoparasitica 2012, 40, 63–68. [Google Scholar] [CrossRef]
- Zou, D.; Coudron, T.A.; Zhang, L.; Xu, W.; Xu, J.; Wang, M.; Xiao, X.; Wu, H. Effect of prey species and prey densities on the performance of adult Coenosia attenuata. Insects 2021, 12, 669. [Google Scholar] [CrossRef]
- Zou, D.; Coudron, T.A.; Xu, W.; Xu, J.; Wu, H. Performance of the tiger-fly Coenosia attenuata Stein reared on the alternative prey, Chironomus plumosus (L.) larvae in coir substrate. Phytoparasitica 2021, 49, 83–92. [Google Scholar] [CrossRef]
- Bautista-Martínez, N.; Illescas-Riquelme, C.P.; de Jesus García-Ávila, C. First report of “hunter-fly” Coenosia attenuata (Diptera: Muscidae) in Mexico. Fla. Entomol. 2017, 100, 174–175. [Google Scholar] [CrossRef]
- Tellez, M.D.M.; Tapia, G.; Gamez, M.; Cabello, T.; van Emden, H.F. Predation of Bradysia sp.(Diptera: Sciaridae), Liriomyza trifolii (Diptera: Agromyzidae) and Bemisia tabaci (Hemiptera: Aleyrodidae) by Coenosia attenuata (Diptera: Muscidae) in greenhouse crops. Eur. J. Entomol. 2009, 106, 199–204. [Google Scholar] [CrossRef]
- Martinez-Sanchez, A.; Marcos-Garcia, M.A.; Pont, A.C. Coenosia attenuata Stein, 1903 (Diptera, Muscidae) nueva especie para la fauna neotropical. Boll. Di Zool. Agrar. E Di Bachic. 2002, 34, 269–272. [Google Scholar]
- Pérez, M.M. Estudio de la morfología externa de los adultos de la mosca cazadora Coenosia attenuata Stein, 1903 (Diptera: Muscidae), y primer reporte para Colombia. Rev. De La Fac. De Cienc. Básicas 2006, 2, 68–87. [Google Scholar]
- Hernández-Ramírez, J.; Monge, M.P.; Casasola, L.E.; Solano, N.B.; Umaña, F.M. Presencia de la ‘mosca tigre’en Costa Rica. Actual. Fitosanit. 2008, 33, 1–4. [Google Scholar]
- Couri, M.S.; Salas, C. First record of Coenosia attenuata Stein (Diptera, Muscidae) from Chile, with biological notes. Rev. Bras. Entomol. 2010, 54, 144–145. [Google Scholar] [CrossRef]
- Solano-Rojas, Y.; Pont, A.; De Freitas, J.; Moros, G.; Goyo, Y. First record of Coenosia attenuata Stein, 1903 (Diptera: Muscidae) in Venezuela. In Anales de Biología; Servicio de Publicaciones de la Universidad de Murcia: Murcia, Spain, 2017; pp. 223–226. [Google Scholar]
- Orozco, J. DNA barcoding reveals the first record of Coenosia attenuata Stein (Diptera: Muscidae) in Honduras. CEIBA 2018, 55, 70–72. [Google Scholar] [CrossRef]
- Couri, M.S.; Sousa, V.R.; Lima, R.M.; Dias-Pini, N.S. The predator Coenosia attenuata Stein (Diptera, Muscidae) on cultivated plants from Brazil. An. Da Acad. Bras. De Ciências 2018, 90, 179–183. [Google Scholar] [CrossRef]
- Giambiasi, M.; Rodríguez, A.; Arruabarrena, A.; Buenahora, J. First report of Coenosia attenuata (Stein, 1903) (Diptera, Muscidae) in Uruguay, confirmed by DNA barcode sequences. Check List 2020, 16, 749–752. [Google Scholar] [CrossRef]
- Hoebeke, E.R.; Sensenbach, E.J.; Sanderson, J.P.; Wraight, S.P. First report of Coenosia attenuata Stein (Diptera: Muscidae), an Old Worldhunter fly’in North America. Proc.-Entomol. Soc. Wash. 2003, 105, 769. [Google Scholar]
- Konrad, C.E.; Fuhrmann, C.M. Climate of the southeast USA: Past, present, and future. In Climate of the Southeast United States; Springer: Berlin/Heidelberg, Germany, 2013; pp. 8–42. [Google Scholar]
- Blaauw, B.; Brannen, P.; Lockwood, D.; Schnabel, G.; Ritchie, D. 2022 Southeastern peach, nectarie, and plum pest management and culture guide. UGA Coop. Ext. Bull. 2022, 1171, 1–78. [Google Scholar]
- Google. Google Maps. Map Data © 2022 Google, INEGI. Available online: https://www.google.com/maps/@32.8502955,-81.4519247,6.98z (accessed on 6 July 2022).
- Campbell, J.W.; Hanula, J. Efficiency of Malaise traps and colored pan traps for collecting flower visiting insects from three forested ecosystems. J. Insect Conserv. 2007, 11, 399–408. [Google Scholar] [CrossRef]
- Schlueter, M.A.; Stewart, N.G. Native bee (Hymenoptera: Apoidea) abundance and diversity in north Georgia apple orchards throughout the 2010 growing season (March to October). Southeast. Nat. 2015, 14, 721–739. [Google Scholar] [CrossRef]
- Hennig, W.; Lindner, E.E. Muscidae Die Flieg. Der Palaearktischen Reg. 1964, 63, 481–528. [Google Scholar]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- Cutler, G.C.; Gariepy, T.D.; De Silva, E.C.A.; Hillier, N.K. High rates of parasitism of blueberry spanworm (Lepidoptera: Geometridae) by Ichneumonidae and Tachinidae in commercial lowbush blueberry fields. J. Pest Sci. 2015, 88, 219–223. [Google Scholar] [CrossRef]
- Cicero, J.M.; Adair, M.M.; Adair, R.C.; Hunter, W.B.; Avery, P.B.; Mizell, R.F. Predatory behavior of long-legged flies (Diptera: Dolichopodidae) and their potential negative effects on the parasitoid biological control agent of the Asian citrus psyllid (Hemiptera: Liviidae). Fla. Entomol. 2017, 100, 485–487. [Google Scholar] [CrossRef]
- Pons, L. Greenhouse Pests Beware. Agric. Res. 2005, 53, 7. [Google Scholar]
- Mateus, C. Bioecology and behaviour of Coenosia attenuata in greenhouse vegetable crops in the Oeste region, Portugal. Bull. Insectol 2012, 65, 257–263. [Google Scholar]
- Martinez, M.; Cocquempot, C. La mouche Coenosia attenuata, un nouvel auxiliaire prometteur en culture protégée. PHM Rev. Hortic. 2000, 414, 50–52. [Google Scholar]
- Sensenbach, E.J. Coenosia Attenuata Stein (Diptera: Muscidae): A Predatory Fly in North American Greenhouses; Cornell University, August: Ithaca, NY, USA, 2004. [Google Scholar]
- Sensenbach, E.; Wraight, S.; Sanderson, J. Biology and predatory feeding behavior of larvae of the hunter fly Coenosia attenuata. IOBC/Wprs Bull. 2005, 28, 229–232. [Google Scholar]
- Zou, D.; Coudron, T.A.; Xu, W.; Gu, X.; Wu, H. Development of immature tiger-fly Coenosia attenuata (Stein) reared on larvae of the fungus gnat Bradysia impatiens (Johannsen) in coir substrate. Phytoparasitica 2017, 45, 75–84. [Google Scholar] [CrossRef]
- Kühne, S. Räuberische Fliegen der Gattung Coenosia Meigen, 1826 (Diptera: Muscidae) und die Möglichkeit ihres Einsatzes bei der Biologischen Schädlingsbekämpfung; Ampyx-Verlag: Halle/Saale, Germany, 2000. [Google Scholar]
- Pohl, D.; Uygur, F.; Sauerborn, J. Note: Fluctuations in population of the first recorded predatory fly Coenosia attenuata in cotton fields in Turkey. Phytoparasitica 2003, 31, 446–449. [Google Scholar] [CrossRef]
- Drummond, F.A.; Groden, E.; Haynes, D.; Edens, T.C. Some Aspects of the Biology of a Predaceous Anthomyiid Fly, Coenosia tigrina. Great Lakes Entomol. 1989, 22, 2. [Google Scholar]
- Ivković, M.; Pont, A.C. Long-time emergence patterns of Limnophora species (Diptera, Muscidae) in specific karst habitats: Tufa barriers. Limnologica 2016, 61, 29–35. [Google Scholar] [CrossRef]
- Martins, J.; Mateus, C.; Ramos, A.C.; Figueiredo, E. An optimized method for mass rearing the tiger-fly, Coenosia attenuata (Diptera: Muscidae). Eur. J. Entomol. 2015, 112, 470–476. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).