Geometric Morphometric Wing Analysis of Avian Malaria Vector, Culiseta longiareolata, from Two Locations in Algeria
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
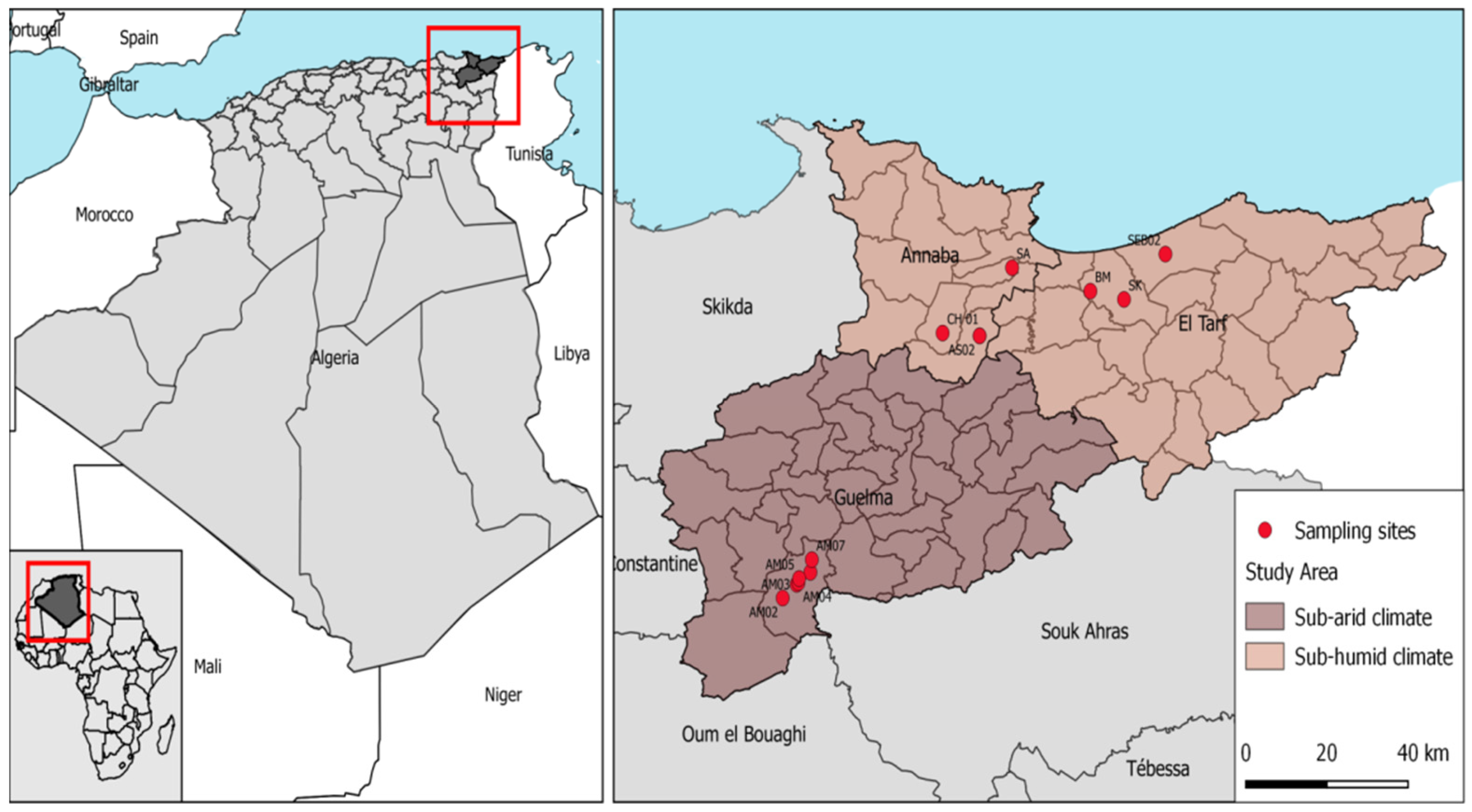
2.1. Study Area
2.2. Mosquitoes Sampling and Identification
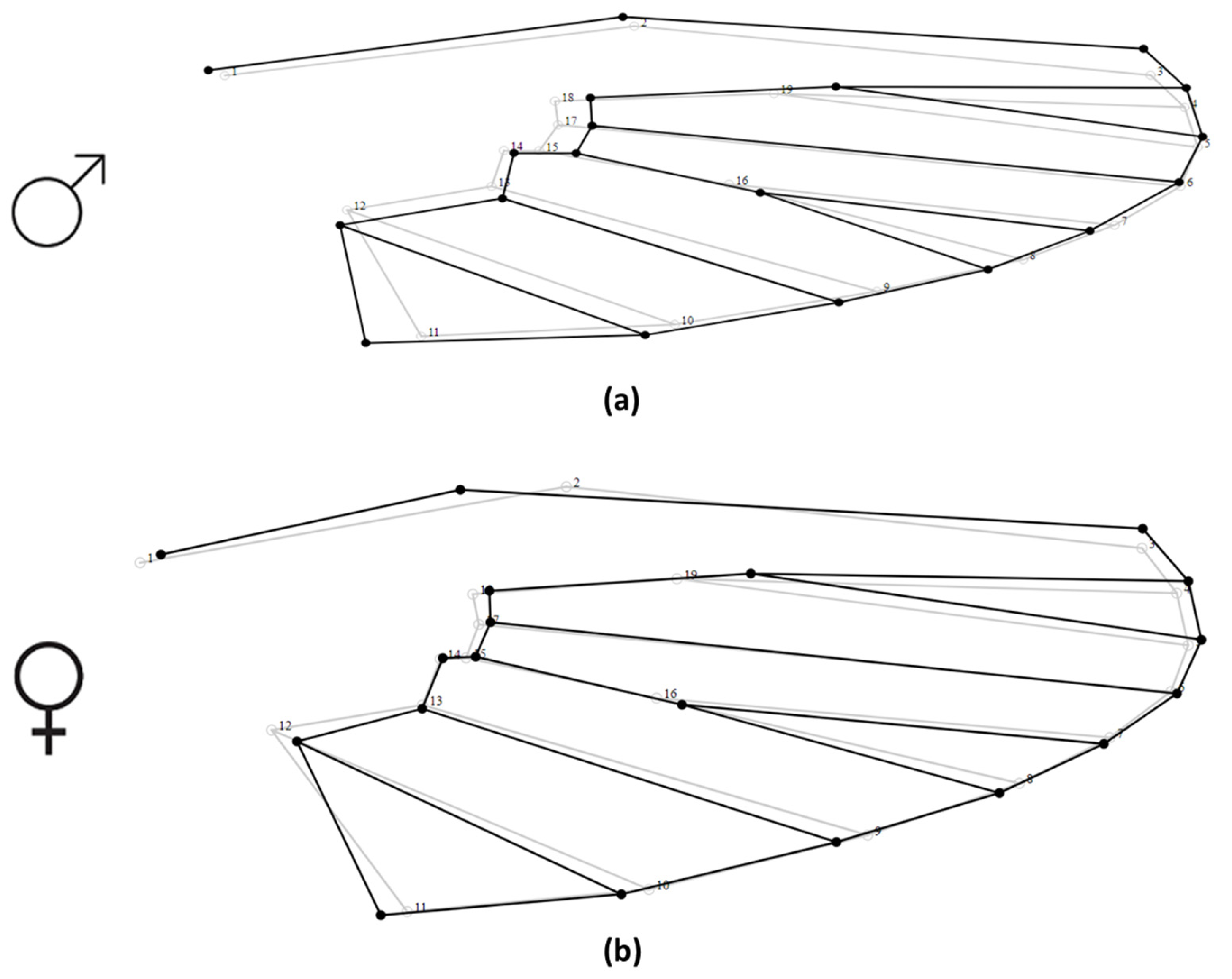
2.3. Wing Preparation
2.4. Data Analysis
3. Results
3.1. Repeatability of Landmarks and Test for Allometry
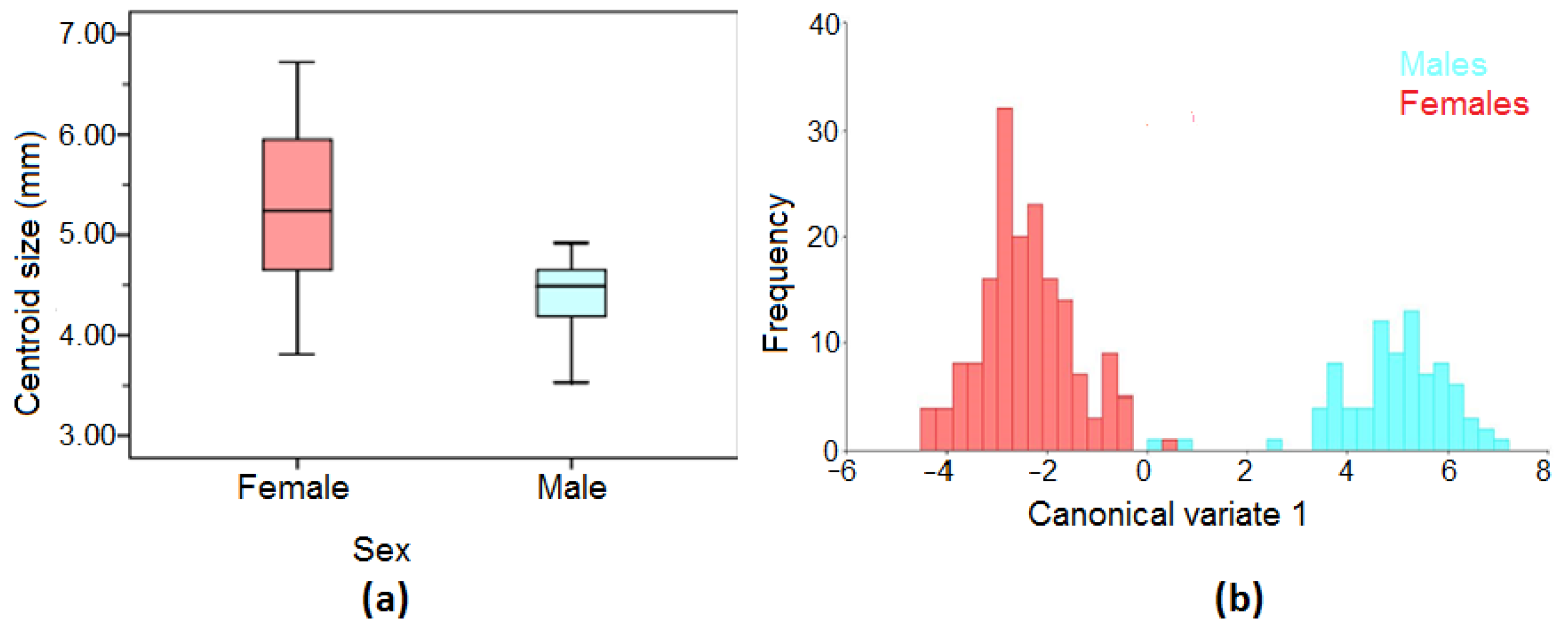
3.2. Sexual Dimorphism
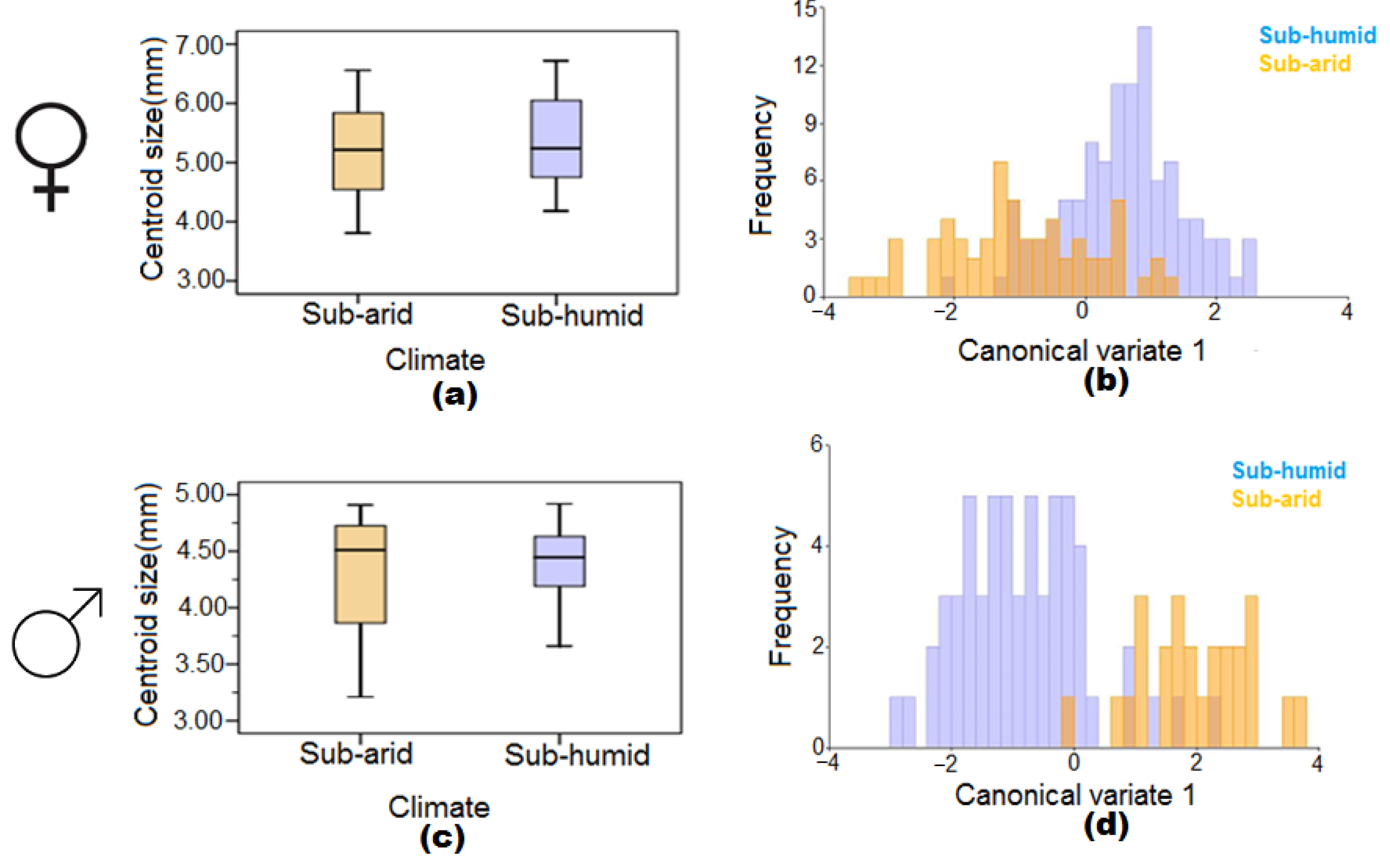
3.3. The Effect of Sub-Arid and Sub-Humid Climatic Conditions
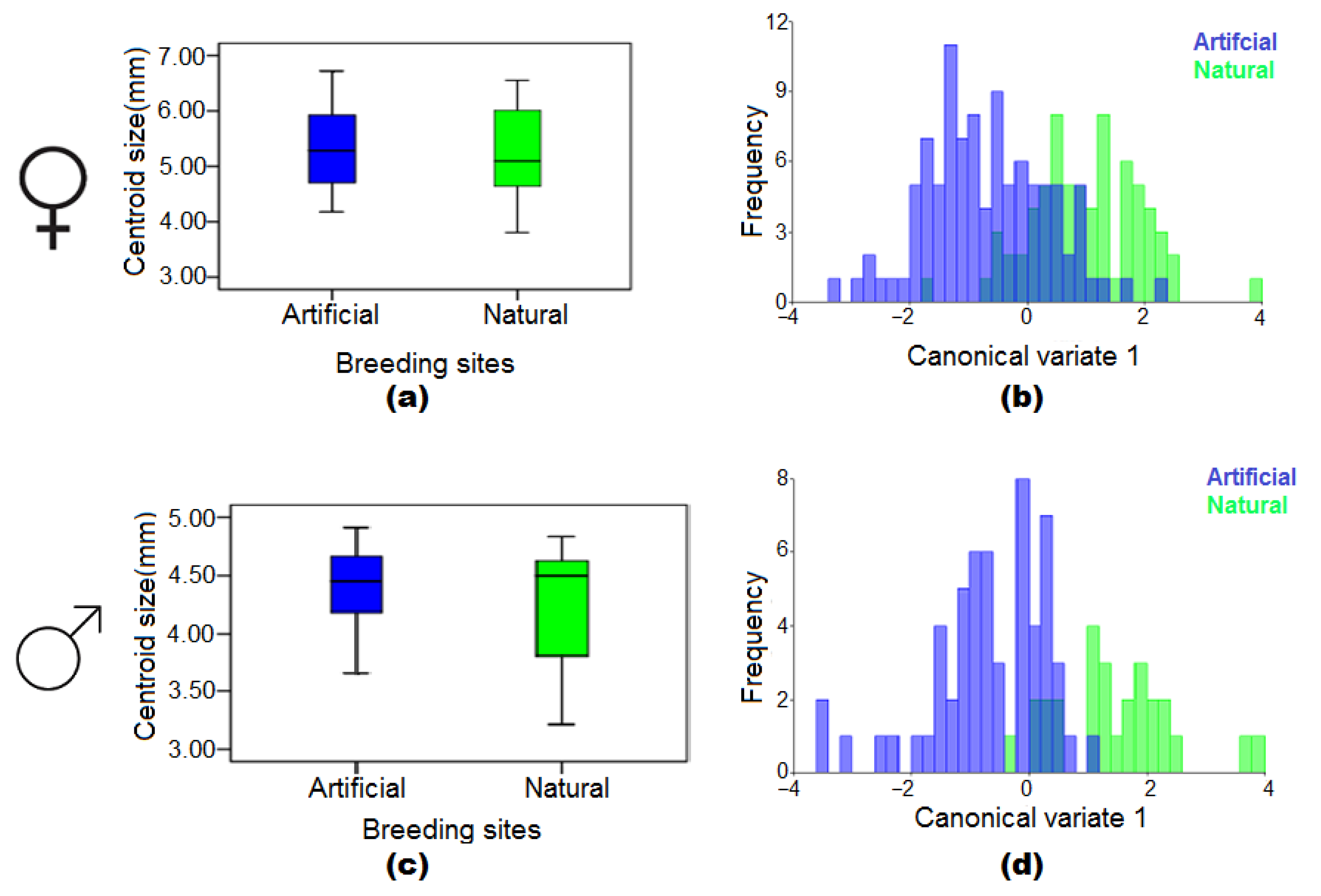
3.4. The Effect of Natural and Artificial Larval Habitats
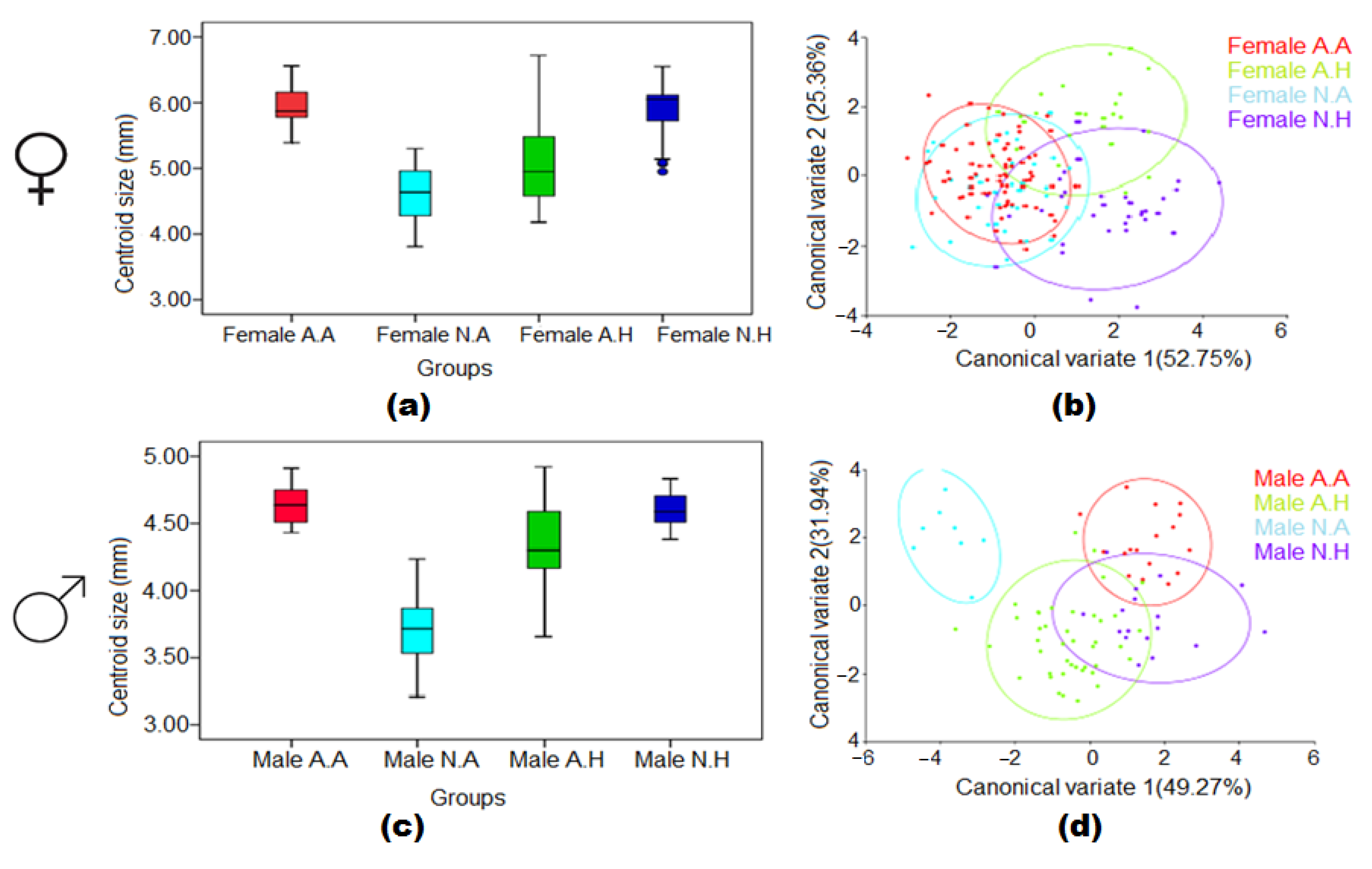
3.5. Combined Effect of Larval Habitat and Climatic Conditions in the Wing Size and Shape
4. Discussion
4.1. Sexual Dimorphism
4.2. Effects of the Climatic Conditions and Larval Habitats
4.3. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sergent, E.; Sergent, É. Observations sur les moustiques des environs d’Alger. Ann. Inst. Pasteur. 1903, 17, 60–67. [Google Scholar]
- Merabti, B.; Lebouz, I.; Adamou, A.; Kouidri, M.; Ouakid, M. Effects of certain natural breeding site characteristics on the distribution of Culicidae (Diptera) mosquito species in southeast Algeria. Afr. Entomol. 2017, 25, 506–514. [Google Scholar] [CrossRef]
- Van Pletzen, R.; Van Der Linde, T.D.K. Studies on the biology of Culiseta longiareolata (Macquart)(Diptera: Culicidae). Bull. Entomol. Res. 1981, 71, 71–79. [Google Scholar] [CrossRef]
- Azari-Hami, S.; Abai, M.; Arzamani, K.; Bakhshi, H.; Karami, H.; Ladonni, H.; Harbach, R. Mosquitoes (Diptera: Culicidae) of North Khorasan Province, northeastern Iran and the zoogeographic affinities of the Iranian and middle Asian mosquito fauna. J. Entomol. 2011, 8, 204–217. [Google Scholar]
- Schaffner, F.; Gatt, P.; Mall, S.; Maroli, M.; Spiteri, G.; Melillo, T.; Zeller, H. Mosquitoes in Malta: Preliminary entomological investigation and risk assessment for vector-borne diseases (Diptera: Culicidae). Bull. Entomol. Soc. Malta 2010, 3, 41–54. [Google Scholar]
- Khaligh, F.G.; Naghian, A.; Soltanbeiglou, S.; Gholizadeh, S. Autogeny in Culiseta longiareolata (Culicidae: Diptera) mosquitoes in laboratory conditions in Iran. BMC Res. Notes 2020, 13, 81. [Google Scholar]
- Becker, N.; Petric, D.; Zgomba, M.; Boase, C.; Madon, M.; Dahl, C.; Kaiser, A. Mosquitoes and Their Contro, 2nd ed.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- González, M.A.; Prosser, S.W.; Hernández-Triana, L.M.; Alarcón-Elbal, P.M.; Goiri, F.; López, S.; Ruiz-Arrondo, I.; Hebert, P.D.N.; García-Pérez, A.L. Avian feeding preferences of Culex pipiens and Culiseta spp. along an urban-to-wild gradient in northern Spain. Front. Ecol. Evol. 2020, 8, 568835. [Google Scholar] [CrossRef]
- La Puente, J.M.-D.; Soriguer, R.; Senar, J.C.; Figuerola, J.; Bueno-Mari, R.; Montalvo, T. Mosquitoes in an Urban Zoo: Identification of Blood Meals, Flight Distances of Engorged Females, and Avian Malaria Infections. Front. Vet. Sci. 2020, 7, 460. [Google Scholar] [CrossRef]
- Toma, L.; Severini, F.; Romi, R.; Goffredo, M.; Torina, A.; Di Luca, M. Checklist of the mosquito species from four Sicilian Islands: Lampedusa, Linosa, Ustica and Pantelleria. J. Entomol. Acarol. Res. 2020, 52, 8968. [Google Scholar]
- Maslov, A.V.; Ward, R.A. Blood-Sucking Mosquitoes of the Subtribe Culisetina (Diptera, Culicidae) in World Fauna. Smithsonian Institution Libraries. 1989. Available online: https://archive.org/details/bloodsuckingmosq00masl/mode/2up (accessed on 20 August 2010).
- Maslov, A.V. Krovososushchie Komary Podtriby Culisetina (Diptera, Culicidae) Mirovoi Fauny; Akademiya Nauk SSSR, Opredeliteli po Faune SSSR, Izdavaemye Zoologicheskim, Instittutom Akademii Nauk SSSR, No. 93; Nauka Publishers: Leningrad, Russia, 1967; Volume 182, p. 1. [Google Scholar]
- Kearney, M.; Porter, W.P.; Williams, C.; Ritchie, S.; Hoffmann, A.A. Integrating biophysical models and evolutionary theory to predict climatic impacts on species’ ranges: The dengue mosquito Aedes aegypti in Australia. Funct. Ecol. 2009, 23, 528–538. [Google Scholar] [CrossRef]
- Murdock, C.C.; Evans, M.V.; McClanahan, T.D.; Miazgowicz, K.L.; Tesla, B. Fine-scale variation in microclimate across an urban landscape shapes variation in mosquito population dynamics and the potential of Aedes albopictus to transmit arboviral disease. PLoS Negl. Trop. Dis. 2017, 11, 0005640. [Google Scholar] [CrossRef]
- Marini, G.; Poletti, P.; Giacobini, M.; Pugliese, A.; Merler, S.; Rosà, R. The Role of Climatic and Density Dependent Factors in Shaping Mosquito Population Dynamics: The Case of Culex pipiens in Northwestern Italy. PLoS ONE 2016, 11, 0154018. [Google Scholar]
- Zelditch, M.L.; Swiderski, D.L.; Sheets, H.D. Geometric Morphometrics of Biologists: A Primer, 2nd ed.; Elsevier Academic Press: Waltham, MA, USA, 2004. [Google Scholar]
- Debat, V.; Béagin, M.; Legout, H.; David, J.R. Allometric and nonallometric components of Drosophila wing shape respond differently to developmental temperature. Evolution 2003, 57, 2773–2784. [Google Scholar]
- Hoffmann, A.A.; Woods, R.E.; Collins, E.; Wallin, K.; White, A.; McKenzie, J.A. Wing shape versus asymmetry as an indicator of changing environmental conditions in insects. Aust. J. Entomol. 2005, 44, 233–243. [Google Scholar] [CrossRef]
- Garzón, M.J.; Schweigmann, N. Wing morphometrics of Aedes (Ochlerotatus) albifasciatus (Macquart, 1838) (Diptera: Culicidae) from different climatic regions of Argentina. Parasit. Vectors 2018, 11, 303. [Google Scholar] [CrossRef]
- Motoki, M.T.; Suesdek, L.; Bergo, E.S.; Sallum, M.A.M. Wing geometry of Anopheles darlingi Root (Diptera: Culicidae) in five major Brazilian ecoregions. Infect. Genet. Evol. 2012, 12, 1246–1252. [Google Scholar] [CrossRef]
- Carvajal, T.M.; Ho, H.; Hernandez, L.F.; Viacrusis, K.; Amalin, D.; Watanabe, K. An Ecological Context Toward Understanding Dengue Disease Dynamics in Urban Cities: A Case Study in Metropolitan Manila, Philippines, in Health in Ecological Perspectives in the Anthropocene; Springer: Berlin/Heidelberg, Germany, 2018; pp. 117–131. [Google Scholar]
- Daget, P. Le bioclimatméditerranéen: Analyse des formesclimatiques par le systèmed’Emberger. J. Veg. 1977, 34, 87–103. [Google Scholar] [CrossRef]
- Le Houerou, H.N.; Hoste, C.H. Rangeland production and annual rainfall relations in the Mediterranean Basin and in the African SaheloSudanian zone. J. Range Manag. 1977, 30, 181–189. [Google Scholar]
- Semahi, S.; Zemmouri, N.; Singh, M.K.; Attia, S. Comparative bioclimatic approach for comfort and passive heating and cooling strategies in Algeria. Built. Environ. 2019, 161, 106271. [Google Scholar] [CrossRef]
- Layadi, M.; Hireche, H.; Djorfi, S.E. L’apport des Conditions Hydroclimatologiques dans L’étude du Contextehydrogéologique des Sources d’eau de la Régiond’ainmakhlouf, (Wilaya de Guelma); Université de Jijel: Jijel, Algeria, 2020. [Google Scholar]
- Chemam, A.; Hadjzobir, S.; Daif, M.; Altenberger, U.; Günter, C. Provenance analyses of the heavy-mineral beach sands of the Annaba coast, northeast Algeria, and their consequences for the evaluation of fossil placer deposit. J. Earth Syst. Sci. 2018, 127, 118. [Google Scholar] [CrossRef]
- Ouchene-Khelifi, N.; Ouchene, N.; Dahmani, H.; Dahmani, A.; Sadi, M.; Douifi, M. Fasciolosis due to Fasciola hepatica in ruminants in abattoirs and its economic impact in two regions in Algeria. Trop. Biomed. 2018, 35, 181–187. [Google Scholar] [PubMed]
- Papierok, B.; Croset, H.; Rioux, J.A. Estimation de l’effectif des populations larvaires d’Aedes (O.) cataphylla Dyar, 1916 (Diptera, Culicidae): 2. Méthode utilisant le “coup de louche” ou “dipping”. Sér. Ent. Med. Parasitol. 1975, 13, 47–51. [Google Scholar]
- Brunhes, J.; Rhaim, A.; Geoffroy, B.; Angel, G.; Hervy, J.J.M. Frensh, The mosquitoes of Mediterranean Africa: CD-ROM of Identification and Teaching Software; Research Institute for Development: Paris, France, 1999. [Google Scholar]
- Himmi, O.; Dakki, M.; Trari, B.; Agbani, M.A. Les Culicidae du Maroc:Clésd’identification, avec donnéesbiologiques et écologiques. Série Zool. Rabat 1995, 44, 1–51. [Google Scholar]
- Lorenz, C.; Suesdek, L. Evaluation of Chemical Preparation on Insect Wing Shape for Geometric Morphometrics. Am. J. Trop. Med. Hyg. 2013, 89, 928–931. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, C.; Almeida, F.; Almeida-Lopes, F.; Louise, C.; Pereira, S.N.; Petersen, V.; Vidal, P.O.; Virginio, F.; Suesdek, L. Geometric morphometrics in mosquitoes: What has been measured? Infect. Genet. Evol. 2017, 54, 205–215. [Google Scholar] [CrossRef]
- Rohlf, F.J. tpsDig, Digitize Landmarks and Outlines, Version 2.31; Stony Brook; Department of Ecology and Evolution, State University of New York: New York, NY, USA, 2017. [Google Scholar]
- Arnqvist, G.; Mårtensson, T. Measurement error in geometric morphometrics: Empirical strategies to assess and reduce its impact on measures of shape. Acta Zool Acad. Sci. Hung. 1998, 44, 73–96. [Google Scholar]
- Zelditch, M.L.; Swiderski, D.L.; Sheets, H.D. A Practical Companion to Geometric Morphometrics for Biologists: Running Analyses in Freely-Available Software. Available online: http://booksite.elsevier.com/9780123869036/content/Workbook.pdf2012 (accessed on 1 March 2022).
- SPSS. IBM SPSS Statistics for Windows, Version 23.0; IBM Corp.: Armonk, NY, USA, 2015. [Google Scholar]
- Adams, D.C.; Rohlf, F.J.; Slice, D.E. Geometric morphometrics: Ten years of progress following the ‘revolution’. Ital. J. Zool. 2004, 71, 5–16. [Google Scholar]
- Bookstein, F.L. Landmark methods for forms without landmarks: Morphometrics of group differences in outline shape. Med. Image Anal. 1997, 1, 225–243. [Google Scholar]
- Klingenberg, C.P. MorphoJ: An integrated software package for geometric morphometrics. Mol. Ecol. Resour. 2011, 11, 353–357. [Google Scholar]
- Gilchrist, G.W.; Huey, R.B. Plastic and Genetic Variation in Wing Loading as a Function of Temperature Within and Among Parallel Clines in Drosophila subobscura. Integr. Comp. Biol. 2004, 44, 461–470. [Google Scholar] [CrossRef]
- Siomava, N.; Wimmer, E.A.; Posnien, N. Extensive sexual wing shape dimorphism in Drosophila melanogaster, Ceratitis capitata, and Musca domestica. BioRxiv 2017, 135749. [Google Scholar] [CrossRef]
- Kelly, C.D. Size and shape assortative mating in Japanese beetles (Popillia japonica). Behav. Ecol. 2020, 31, 1073–1083. [Google Scholar] [CrossRef]
- Devicari, M.; Lopes, A.R.; Suesdek, L. Dimorfismo sexual alar em Aedes scapularis (Diptera: Culicidae). Biota Neotrop. 2011, 11, 165–169. [Google Scholar] [CrossRef]
- Vidal, P.O.; Peruzin, M.C.; Suesdek, L. Wing diagnostic characters for Culex quinquefasciatus and Culex nigripalpus (Diptera, Culicidae). Rev. Bras. Entomol. 2011, 55, 134–137. [Google Scholar] [CrossRef]
- Wormington, J.D.; Juliano, S.A. Sexually dimorphic body size and development time plasticity in Aedes mosquitoes (Diptera: Culicidae). Evol. Ecol. Res. 2014, 16, 223–234. [Google Scholar]
- Virginio, F.; Vidal, P.O.; Suesdek, L. Wing sexual dimorphism of pathogen-vector culicids. Parasit. Vectors 2015, 8, 159. [Google Scholar]
- Carvajal, T.M.; Amalin, D.M.; Watanabe, K. Watanabe, Wing geometry and genetic analyses reveal contrasting spatial structures between male and female Aedes aegypti (L.) (Diptera: Culicidae) populations in metropolitan Manila, Philippines. Infect. Genet. Evol. 2021, 87, 104676. [Google Scholar] [CrossRef]
- López-Mercadal, J.; Wilke, A.B.B.; Barceló, C.; Miranda, M.A. Evidence of Wing Shape Sexual Dimorphism in Aedes (Stegomyia) albopictus in Mallorca, Spain. Front. Ecol. Evol. 2021, 9, 569034. [Google Scholar]
- Vidal, P.O.; Carvalho, E.; Suesdek, L. Temporal variation of wing geometry in Aedes albopictus. Mem. Inst. Oswaldo Cruz. 2012, 107, 1030–1034. [Google Scholar]
- Carvajal, T.M.; Hernandez, L.F.T.; Ho, H.T.; Cuenca, M.G.; Orantia, B.M.C.; Estrada, C.R.; Viacrusis, K.M.; Amalin, D.M.; Watanabe, K. Spatial analysis of wing geometry in dengue vector mosquito, Aedes aegypti (L.) (Diptera: Culicidae), populations in Metropolitan Manila, Philippines. J. Vector Borne Dis. 2016, 53, 127–135. [Google Scholar]
- Christe, R.O.; Wilke, A.B.B.; Vidal, P.O.; Marrelli, M.T. Wing sexual dimorphism in Aedes fluviatilis (Diptera: Culicidae). Infect. Genet. Evol. 2016, 45, 434–436. [Google Scholar] [CrossRef] [PubMed]
- Chaiphongpachara, T.; Laojun, S. Variation over time in wing size and shape of the coastal malaria vector Anopheles (Cellia) epiroticus Linton and Harbach (Diptera: Culicidae) in Samut Songkhram, Thailand. J. Adv. Vet. Anim. Res. 2019, 6, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Manimegalai, K.; Arunachalam, M. Udayakumari, Morphometric geometric study of wing shape in Culex quinquefasciatus Say (Diptera: Culicidae) from Tamil Nadu, India. J. Threat. Taxa 2009, 1, 263–268. [Google Scholar] [CrossRef]
- Dhivya, R.; Manimegalai, K. Wing shape analysis of the Japanese encephalitis vector Culex gelidus (Diptera: Culicidae) at the Foot Hill of southern western Ghats, India. World J. Zool. 2013, 8, 119–125. [Google Scholar]
- Wiklund, C. Sexual selection and the evolution of butterfly mating systems. In Butterflies; Ecology and Evolution Taking Flight; The University of Chicago Press: Chicago, IL, USA, 2003; pp. 67–90. [Google Scholar]
- Outomuro, D.; Söderquist, L.; Nilsson-Örtman, V.; Cortázar-Chinarro, M.; Lundgren, C.; Johansson, F. Antagonistic natural and sexual selection on wing shape in a scrambling damselfly. Evolution 2016, 70, 1582–1595. [Google Scholar] [CrossRef]
- Sanford, M.R.; Demirci, B.; Marsden, C.D.; Lee, Y.; Cornel, A.J.; Lanzaro, G.C. Morphological Differentiation May Mediate Mate-Choice between Incipient Species of Anopheles gambiae ss. PLoS ONE 2011, 6, 27920. [Google Scholar]
- Klingenberg, C.P.; Debat, V.; Roff, D.A. Quantitative genetics of shape in cricket wings: Developmental integration in a functional structure. Evol. Int. J. Org. Evol. 2010, 64, 2935–2951. [Google Scholar]
- Yeap, H.L.; Endersby, N.M.; Johnson, P.H.; Ritchie, S.A.; Hoffmann, A.A. Body size and wing shape measurements as quality indicators of Aedes aegypti mosquitoes destined for field release. Am. J. Trop. Med. Hyg. 2013, 89, 78–92. [Google Scholar] [CrossRef]
- Fairbairn, D.J. Allometry for sexual size dimorphism: Pattern and process in the coevolution of body size in males and females. Annu. Rev. Ecol. Syst. 1997, 28, 659–687. [Google Scholar] [CrossRef]
- Nasci, R.S. The size of emerging and host-seeking Aedes aegypti and the relation of size to blood-feeding success in the field. J. Am. Mosq. Control Assoc. 1986, 2, 61–62. [Google Scholar]
- Vidal, P.O.; Suesdek, L. Comparison of wing geometry data and genetic data for assessing the population structure of Aedes aegypti. Infect. Genet. Evol. 2012, 12, 591–596. [Google Scholar]
- Parker, A.T.; Gardner, A.M.; Perez, M.; Allan, B.F.; Muturi, E.J. Container Size Alters the Outcome of Interspecific Competition Between Aedes aegypti (Diptera: Culicidae) and Aedes albopictus. J. Med. Entomol. 2019, 56, 708–715. [Google Scholar] [CrossRef]
- Phanitchat, T.; Apiwathnasorn, C.; Sungvornyothin, S.; Samung, Y.; Dujardin, S.; Dujardin, J.-P.; Sumruayphol, S. Geometric morphometric analysis of the effect of temperature on wing size and shape in Aedes albopictus. Med. Vet. Entomol. 2019, 33, 476–484. [Google Scholar] [CrossRef]
- Hassall, C. Strong geographical variation in wing aspect ratio of a damselfly, Calopteryx maculata (Odonata: Zygoptera). Peer J. 2015, 3, e1219. [Google Scholar]
- Azevedo, R.B.R.; James, A.C.; McCabe, J.; Partridge, L. Latitudinal variation of wing: Thorax size ratio and wing-aspect ratio in Drosophila melanogaster. Evolution 1998, 52, 1353–1362. [Google Scholar] [CrossRef]
- Machida, W.; Tidon, R.; Klaczko, J. Wing plastic response to temperature variation in two distantly related Neotropical Drosophila species (Diptera, Drosophilidae). Can. J. Zool. 2021, 100, 82–89. [Google Scholar]
- Demari-Silva, B.; Suesdek, L.; Sallum, M.A.M.; Marrelli, M.T. Wing geometry of Culex coronator (Diptera: Culicidae) from south and southeast Brazil. Parasit. Vectors 2014, 7, 174. [Google Scholar]
- Gómez, G.F.; Márquez, E.J.; Gutiérrez, L.A.; Conn, J.E.; Correa, M.M. Geometric morphometric analysis of Colombian Anopheles albimanus (Diptera: Culicidae) reveals significant effect of environmental factors on wing traits and presence of a metapopulation. Acta Trop. 2014, 135, 75–85. [Google Scholar] [CrossRef]
- Ayala, D.; Caro-Riaño, H.; Dujardin, J.-P.; Rahola, N.; Simard, F.; Fontenille, D. Chromosomal and environmental determinants of morphometric variation in natural populations of the malaria vector Anopheles funestus in Cameroon. Infect. Genet. Evol. 2011, 11, 940–947. [Google Scholar] [CrossRef]
- Aytekin, S.; Aytekin, A.M.; Alten, B. Effect of different larval rearing temperatures on the productivity (R o) and morphology of the malaria vector Anopheles superpictus Grassi (Diptera: Culicidae) using geometric morphometrics. J. Vector Ecol. 2009, 34, 32–42. [Google Scholar]
- Kennington, W.J.; Killeen, J.R.; Goldstein, D.B.; Partridge, L. Rapid laboratory evolution of adult wing area in Drosophila melanogaster in response to humidity. Evolution 2003, 57, 932–936. [Google Scholar] [CrossRef] [PubMed]
- Vargas, R.E.M.; Ya-Umphan, P.; Phumala-Morales, N.; Komalamisra, N.; Dujardin, J.-P. Climate associated size and shape changes in Aedes aegypti (Diptera: Culicidae) populations from Thailand. Infect. Genet. Evol. 2010, 10, 580–585. [Google Scholar]
- Hidalgo, K.; Dujardin, J.-P.; Mouline, K.; Dabiré, R.K.; Renault, D.; Simard, F. Seasonal variation in wing size and shape between geographic populations of the malaria vector, Anopheles coluzzii in Burkina Faso (West Africa). Acta Trop. 2015, 143, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Gleiser, R.M.; Urrutia, J.; Gorla, D.E. Body size variation of the floodwater mosquito Aedes albifasciatus in Central Argentina. Med. Vet. Entomol. 2000, 14, 38–43. [Google Scholar] [CrossRef]
- Alto, B.W.; Juliano, S.A. Precipitation and temperature effects on populations of Aedes albopictus (Diptera: Culicidae): Implications for range expansion. J. Med. Entomol. 2001, 38, 646–656. [Google Scholar] [CrossRef]
- Multini, L.C.; Wilke, A.B.B.; Marrelli, M.T. Urbanization as a driver for temporal wing-shape variation in Anopheles cruzii (Diptera: Culicidae). Acta Trop. 2019, 190, 30–36. [Google Scholar]
- Sendaydiego, J.P.; Torres, M.A.J.; Demayo, C.G. Describing wing geometry of Aedes aegypti using landmark-based geometric morphometrics. Int. J. Biosci. Biochem. Bioinform. 2013, 3, 379. [Google Scholar]
- Damiens, D.; Lebon, C.; Wilkinson, D.A.; Dijoux-Millet, D.; Le Goff, G.; Bheecarry, A.; Gouagna, L.C. Cross-Mating Compatibility and Competitiveness among Aedes albopictus Strains from Distinct Geographic Origins-Implications for Future Application of SIT Programs in the South West Indian Ocean Islands. PLoS ONE 2016, 11, 0163788. [Google Scholar] [CrossRef]
- Strickman, D.; Kittayapong, P. Dengue and its vectors in Thailand: Calculated transmission risk from total pupal counts of Aedes aegypti and association of wing-length measurements with aspects of the larval habitat. Am. J. Trop. Med. Hyg. 2003, 68, 209–217. [Google Scholar]
- Darriet, F. Development of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) larvae feeding on the plant material contained in the water. Ann. Community Med. Pract. 2016, 2, 1014. [Google Scholar]
- Stephens, C.R.; Juliano, S.A. Wing shape as an indicator of larval rearing conditions for Aedes albopictus and Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 2012, 49, 927–938. [Google Scholar]
- Oliveira-Christe, R.; Wilke, A.B.B.; Marrelli, M.T. Microgeographic Wing-Shape Variation in Aedes albopictus and Aedes scapularis (Diptera: Culicidae) Populations. Insects 2020, 11, 862. [Google Scholar] [CrossRef]
- Loetti, V.; Schweigmann, N.; Burroni, N. Development rates, larval survivorship and wing length of Culex pipiens (Diptera: Culicidae) at constant temperatures. J. Nat. Hist. 2011, 45, 2203–2213. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boumaza, M.; Merabti, B.; Adjami, Y.; Ouakid, M.L.; Carvajal, T.M. Geometric Morphometric Wing Analysis of Avian Malaria Vector, Culiseta longiareolata, from Two Locations in Algeria. Insects 2022, 13, 1031. https://doi.org/10.3390/insects13111031
Boumaza M, Merabti B, Adjami Y, Ouakid ML, Carvajal TM. Geometric Morphometric Wing Analysis of Avian Malaria Vector, Culiseta longiareolata, from Two Locations in Algeria. Insects. 2022; 13(11):1031. https://doi.org/10.3390/insects13111031
Chicago/Turabian StyleBoumaza, Mounir, Brahim Merabti, Yasmine Adjami, Mohamed Laid Ouakid, and Thaddeus M. Carvajal. 2022. "Geometric Morphometric Wing Analysis of Avian Malaria Vector, Culiseta longiareolata, from Two Locations in Algeria" Insects 13, no. 11: 1031. https://doi.org/10.3390/insects13111031
APA StyleBoumaza, M., Merabti, B., Adjami, Y., Ouakid, M. L., & Carvajal, T. M. (2022). Geometric Morphometric Wing Analysis of Avian Malaria Vector, Culiseta longiareolata, from Two Locations in Algeria. Insects, 13(11), 1031. https://doi.org/10.3390/insects13111031






