Establishment of Toxicity and Susceptibility Baseline of Broflanilide for Aphis gossypii Glove
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Chemicals
2.3. Toxicity Bioassays
2.4. Data Analysis
3. Results
3.1. The Toxicity of Broflanilide to Field Populations of Aphis gossypii
3.2. Susceptible Baseline of Aphis gossypii to Broflanilide
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IRAC. Mode of Action Classification Scheme (Version 10.3, June 2022). Available online: http://www.irac-online.org (accessed on 7 September 2022).
- Nakao, T.; Banba, S.; Nomura, M.; Hirase, K. Meta-diamide insecticides acting on distinct sites of RDL GABA receptor from those for conventional noncompetitive antagonists. Insect Biochem. Mol. Biol. 2013, 43, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Ozoe, Y.; Kita, T.; Ozoe, F.; Nakao, T.; Sato, K.; Hirase, K. Insecticidal 3-benzamido-N-phenylbenzamides specifically bind with high affinity to a novel allosteric site in house fly GABA receptors. Pestic. Biochem. Physiol. 2013, 107, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.N.; Li, Y.S.; Yang, M.; Wang, Y.Y. New pesticides registered in China in 2020. Agrochemicals 2021, 60, 79–82. [Google Scholar]
- Li, Y. Pesticide registration and new pesticides registered in China in 2020. World Pestic. 2021, 43, 10–15. [Google Scholar]
- Ngufor, C.; Govoetchan, R.; Fongnikin, A.; Vigninou, E.; Syme, T.; Akogbeto, M.; Rowland, M. Efficacy of broflanilide (vectron T500), a new meta-diamide insecticide, for indoor residual spraying against pyrethroid-resistant malaria vectors. Sci. Rep. 2021, 11, 7976. [Google Scholar] [CrossRef]
- Liu, A.P.; Huang, M.Z.; Wu, M.F.; Long, C.Y.; Wang, H.F.; Ren, Y.G.; Huang, Z.C.; Li, J.M.; Zhang, P.; Liu, X.P. Synthesis and biological activity of broflanilide. Fine Chem. Intermed. 2020, 50, 16–20. [Google Scholar]
- Xu, J.B.; Liu, S.W.; Guo, C.X.; Xu, L.B.; Ban, L.F.; Wu, H.F. Synthesis and insecticidal activity of broflanilide. Agrochemicals 2020, 59, 11–14. [Google Scholar]
- Xu, S.; Wu, Y.J.; Li, B.T.; Shi, X.G.; Xiong, Z.H. Toxicity of broflanilide on major rice pests and its influence on natural enemies in paddy fields. Acta Phytophylacica Sin. 2019, 46, 574–581. [Google Scholar]
- An, J.J.; Gao, Z.L.; Dang, Z.H.; Zhao, Y.J.; Dou, Y.N.; Yan, X.; Hua, J.N.; Pan, W.L.; Guo, J.L.; Li, Y.F. Resistance dynamic of different Aphis gossypii populations in Hebei province to six insecticides. Chin. J. Pestic. Sci. 2021, 23, 1123–1131. [Google Scholar]
- Chen, X.W.; Tie, M.Y.; Chen, A.Q.; Ma, K.S.; Li, F.; Liang, P.Z.; Liu, Y.; Song, D.L.; Gao, X.W. Pyrethroid resistance associated with M918L mutation and detoxifying metabolism in Aphis gossypii from Bt cotton growing regions of China. Pest Manag. Sci. 2017, 73, 2353–2359. [Google Scholar] [CrossRef]
- Wang, Z.J.; Liang, C.R.; Shang, Z.Y.; Yu, Q.T.; Xue, C.B. Insecticide resistance and resistance mechanisms in the melon aphid, Aphis gossypii, in Shandong, China. Pestic. Biochem. Physiol. 2021, 172, 104768. [Google Scholar] [CrossRef] [PubMed]
- Subbaratnam, G.V.; Radhika, P. Global view of insecticide resistance management in cotton aphid review. Pest Manag. Econ. Zool. 2005, 13, 163–183. [Google Scholar]
- Somar, R.O.; Zamani, A.A.; Alizadeh, M. Joint action toxicity of imidacloprid and pymetrozine on the melon aphid, Aphis gossypii. Crop Prot. 2019, 124, 104850. [Google Scholar] [CrossRef]
- Ebert, T.A.; Cartwright, B. Biology and ecology of Aphis gossypii Glover (Homoptera: Aphididae). Southwest. Entomol. 1997, 22, 116–153. [Google Scholar]
- Fukuto, T.R. Mechanism of action of organophosphorus and carbamate insecticides. Environ. Health Perspect. 1990, 87, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.Y.; Guo, Q.L.; Xia, X.M.; Wang, H.Y.; Liu, T.X. Resistance of Aphis gossypii (Homoptera: Aphididae) to selected insecticides on cotton from five cotton production regions in Shandong, China. J. Pestic. Sci. 2007, 32, 372–378. [Google Scholar] [CrossRef]
- Koo, H.N.; An, J.J.; Park, S.E.; Kim, J.I.; Kim, G.H. Regional susceptibilities to 12 insecticides of melon and cotton aphid, Aphis gossypii (Hemiptera: Aphididae) and a point mutation associated with imidacloprid resistance. Crop Prot. 2014, 55, 91–97. [Google Scholar] [CrossRef]
- Bass, C.; Denholm, I.; Williamson, M.S.; Nauen, R. The global status of insect resistance to neonicotinoid insecticides. Pestic. Biochem. Physiol. 2015, 121, 78–87. [Google Scholar] [CrossRef]
- Sun, Y.Q.; Feng, G.L.; Yuan, J.G.; Gong, K.Y. Insecticide resistance of cotton aphid in North China. Entomol. Sin. 1994, 1, 242–250. [Google Scholar] [CrossRef]
- Patima, W.; Guo, P.P.; Ma, S.J.; Gao, X.W.; Zhang, L.J.; Zhang, S.; Ma, D.Y. Resistance of different field populations of Aphis gossypii to ten insecticides in Xinjiang. Plant Prot. 2019, 45, 273–278. [Google Scholar]
- Chen, X.; Tang, C.; Ma, K.; Xia, J.; Song, D.; Gao, X.W. Overexpression of UDP-glycosyltransferase potentially involved in insecticide resistance in Aphis gossypii Glover collected from Bt cotton fields in China. Pest Manag. Sci. 2020, 76, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Moores, G.D.; Gao, X.W.; Denholm, I.; Devonshire, A.L. Characterization of insensitive acetylcholinesterase in insecticide-resistant cotton aphids, Aphis gossypii glover (Homoptera: Aphididae). Pestic. Biochem. Physiol. 1996, 56, 102–110. [Google Scholar] [CrossRef]
- LeOra. Polo-Plus, POLO for Windows LeOra Software. 2002. Available online: www.LeOra-Software.com (accessed on 17 January 2018).
- Nakao, T.; Banba, S. Broflanilide: A meta-diamide insecticide with a novel mode of action. Bioorg. Med. Chem. 2016, 24, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Hu, F.; Wang, P.; Fu, W.; Liu, X.Y. Broflanilide effectively controls Helicoverpa armigera and Spodoptera exigua exhibiting diverse susceptibilities to chlorantraniliprole and emamectin benzoate. Pest Manag. Sci. 2021, 77, 1262–1272. [Google Scholar] [CrossRef]
- Shen, N.; Liu, H.Y.; Mou, T.Y.; Ma, Y.B.; Li, Y.; Song, Z.J.; Tang, T.; Han, Z.J.; Zhao, C.Q. Novel meta-diamide insecticide, broflanilide, suppresses the population of common cutworm Spodoptera litura through its lethal and sublethal effects. Pest Manag. Sci. 2022, 78, 1081–1089. [Google Scholar] [CrossRef]
- Snetselaar, J.; Rowland, M.W.; Manunda, B.J.; Kisengwa, E.M.; Small, G.J.; Malone, D.J.; Mosha, F.W.; Kirby, M.J. Efficacy of indoor residual spraying with broflanilide (tenebenal), a novel meta-diamide insecticide, against pyrethroid-resistant anopheline vectors in northern Tanzania: An experimental hut trial. PLoS ONE 2021, 16, e0248026. [Google Scholar] [CrossRef]
- Jia, Z.Q.; Zhan, E.L.; Zhang, S.G.; Jones, A.K.; Zhu, L.; Wang, Y.N.; Huang, Q.T.; Han, Z.J.; Zhao, C.Q. Sublethal doses of broflanilide prevents molting in the fall armyworm, Spodoptera frugiperda via altering molting hormone biosynthesis. Pestic. Biochem. Physiol. 2022, 181, 105017. [Google Scholar] [CrossRef]
- Jia, Z.Q.; Zhan, E.L.; Lian, S.G.; Wang, Y.; Song, P.P.; Jones, A.K.; Han, Z.J.; Zhao, C.Q. Broflanilide prolongs the development of fall armyworm Spodoptera frugiperda by regulating biosynthesis of juvenile hormone. Entomol. Gen. 2022, 42, 761–769. [Google Scholar] [CrossRef]
- Shen, N.; Li, Y.; Leviticus, K.; Chang, X.L.; Tang, T.; Cui, L.; Han, Z.J.; Zhao, C.Q. Effect of broflanilide on the phytophagous mite Tetranychus urticae and the predatory mite Typhlodromips swirskii. Pest Manag. Sci. 2021, 77, 2964–2970. [Google Scholar] [CrossRef]
- Xie, G.; Zhou, W.W.; Jin, M.X.; Yu, A.L.; Rao, L.; Jia, H.R.; Luo, J.; He, Y.C.; Li, B.T. Residues analysis and dissipation dynamics of broflanilide in rice and its related environmental samples. Int. J. Anal. Chem. 2020, 14, 8845387. [Google Scholar] [CrossRef]
- Ma, K.S.; Tang, Q.L.; Zhang, B.Z.; Liang, P.; Wang, B.M.; Gao, X.W. Overexpression of multiple cytochrome P450 genes associated with sulfoxaflor resistance in Aphis gossypii Glover. Pestic. Biochem. Physiol. 2019, 157, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.D.; Liang, P.Z.; Zhang, L.; Lv, H.X.; Gao, X.W.; You, H.; Li, J.H.; Ma, K.S. Susceptibility baseline of Aphis gossypii Glover (Hemiptera: Aphididae) to the novel insecticide afidopyropen in China. Crop Prot. 2022, 151, 105834. [Google Scholar] [CrossRef]
- Rinkevich, F.D.; Du, Y.Z.; Dong, K. Diversity and convergence of sodium channel mutations involved in resistance to pyrethroids. Pestic. Biochem. Physiol. 2013, 106, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.G.; Zhu, Y.K.; Xia, X.M.; Qiao, K.; Wang, H.Y.; Wang, K.Y. The mutation in nicotinic acetylcholine receptor beta 1 subunit may confer resistance to imidacloprid in Aphis gossypii (Glover). J. Food Agric. Environ. 2012, 10, 1227–1230. [Google Scholar]
- Sun, X.; Wei, R.; Li, L.H.; Zhu, B.; Liang, P.; Gao, X.W. Resistance and fitness costs in diamondback moths after selection using broflanilide, a novel meta-diamide insecticide. Insect Sci. 2022, 29, 188–198. [Google Scholar] [CrossRef]
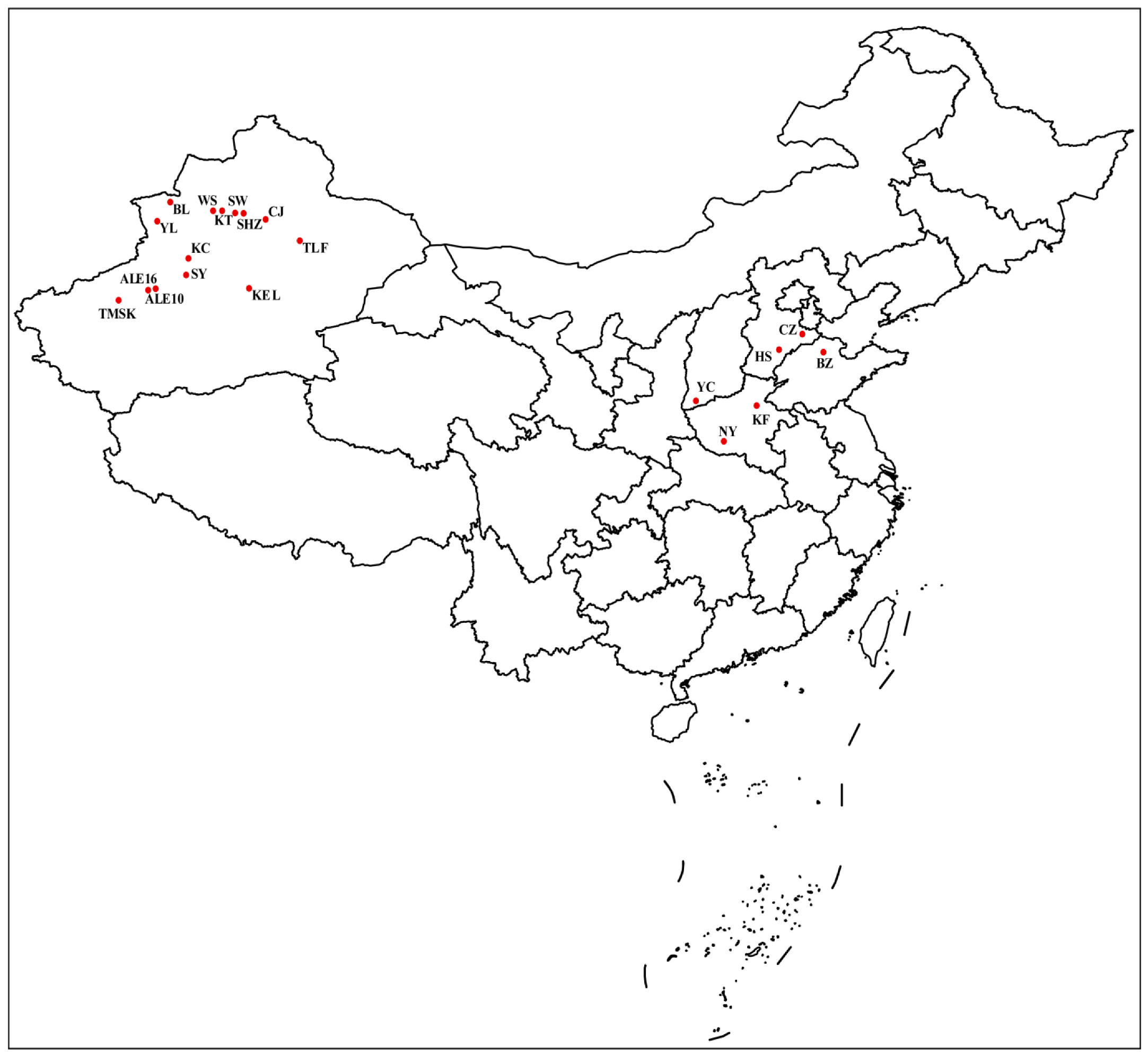

| Populations | Location (City, Province) | Longitude and Latitude | Collecting Date |
|---|---|---|---|
| CZ | Cangzhou, Hebei | 116.87° E, 38.31° N | 3 September 2021 |
| HS | Hengshui, Hebei | 115.58° E, 37.55° N | 21 July 2021 |
| KF | Kaifeng, Henan | 114.35° E, 34.79° N | 10 September 2021 |
| NY | Nanyang, Henan | 112.54° E, 33.00° N | 2 September 2021 |
| BZ | Binzhou, Shandong | 118.02° E, 37.43° N | 6 August 2021 |
| YC | Yuncheng, Shanxi | 111.00° E, 35.02° N | 5 August 2021 |
| KEL | Kuerle, Xinjiang | 86.39° E, 40.59° N | 30 July 2021 |
| ALE10 | Alaer10, Xinjiang | 81.24° E, 40.56° N | 30 July 2021 |
| ALE16 | Alaer16, Xinjiang | 80.84° E, 40.50° N | 30 July 2021 |
| BL | Bole, Xinjiang | 82.05° E, 44.85° N | 13 July 2021 |
| CA | Changji, Xinjiang | 87.31° E, 44.01° N | 13 July 2021 |
| KC | Kuche, Xinjiang | 83.05° E, 42.08° N | 30 July 2021 |
| KT | Kuitun, Xinjiang | 84.90° E, 44.43° N | 13 July 2021 |
| SW | Shawan, Xinjiang | 85.62° E, 44.33° N | 13 July 2021 |
| SY | Shaya, Xinjiang | 82.92° E, 41.25° N | 30 July 2021 |
| SHZ | Shihezi, Xinjiang | 86.08° E, 44.31° N | 13 July 2021 |
| TMSK | Tumushuke, Xinjiang | 79.21° E, 40.00° N | 30 July 2021 |
| TLF | Tulufan, Xinjiang | 89.19° E, 42.94° N | 17 September 2021 |
| WS | Wusu, Xinjiang | 84.68° E, 44.44° N | 13 July 2021 |
| YL | Yili, Xinjiang | 81.32° E, 43.92° N | 17 September 2021 |
| Populations | Slope ± SE a | LC50 (95%CL) b μg mL−1 | RR c | LC90 (95%CL) b μg mL−1 | χ2(df) d | p Value |
|---|---|---|---|---|---|---|
| ALE10 | 1.58 ± 0.20 | 0.20 (0.09–0.32) | 0.49 | 1.28 (0.87–2.13) | 16.03 (12) | 0.19 |
| ALE16 | 1.24 ± 0.12 | 0.44 (0.28–0.66) | 1.07 | 4.77 (2.95–9.21) | 14.04 (12) | 0.30 |
| BL | 1.91 ± 0.20 | 0.45 (0.30–0.62) | 1.10 | 2.13 (1.57–3.16) | 17.74 (14) | 0.22 |
| BZ | 3.02 ± 0.33 | 0.76 (0.60–0.92) | 1.85 | 2.02 (1.64–2.69) | 18.41 (16) | 0.30 |
| CJ | 2.90 ± 0.37 | 0.51 (0.38–0.63) | 1.24 | 1.41 (1.15–1.84) | 12.01 (16) | 0.74 |
| CZ | 2.42 ± 0.25 | 0.37 (0.27–0.46) | 0.90 | 1.25 (0.97–1.75) | 14.25 (12) | 0.29 |
| HS | 4.46 ± 0.69 | 0.36 (0.26–0.45) | 0.88 | 0.70 (0.56–0.90) | 15.72 (16) | 0.47 |
| KC | 2.47 ± 0.42 | 1.48 (0.97–1.96) | 3.61 | 4.90 (3.60–8.30) | 14.35 (14) | 0.42 |
| KEL | 4.37 ± 0.75 | 0.55 (0.45–0.64) | 1.34 | 1.09 (0.91–1.46) | 11.56 (12) | 0.48 |
| KF | 1.71 ± 0.23 | 0.25 (0.12–0.40) | 0.61 | 1.40 (0.88–3.00) | 15.31 (10) | 0.12 |
| KT | 3.92 ± 0.47 | 1.10 (0.89–1.30) | 2.68 | 2.34 (1.94–3.04) | 15.21 (14) | 0.36 |
| NY | 2.74 ± 0.46 | 0.46 (0.27–0.63) | 1.12 | 1.35 (1.00–2.10) | 16.03 (14) | 0.31 |
| SHZ | 2.19 ± 0.49 | 0.68 (0.14–1.12) | 1.66 | 2.61 (1.78–5.05) | 18.47 (13) | 0.14 |
| SW | 6.56 ± 1.10 | 0.89 (0.74–1.01) | 2.17 | 1.39 (1.20–1.80) | 14.93 (13) | 0.31 |
| SY | 1.97 ± 0.38 | 0.42 (0.14–0.65) | 1.02 | 1.90 (1.31–4.12) | 18.96 (13) | 0.12 |
| TLF | 2.02 ± 0.26 | 0.34 (0.23–0.44) | 0.83 | 1.45 (1.11–2.08) | 10.64 (13) | 0.64 |
| TMSK | 3.21 ± 0.70 | 0.67 (0.31–0.89) | 1.63 | 1.67 (1.30–2.81) | 15.62 (12) | 0.21 |
| WS | 2.08 ± 0.34 | 0.60 (0.32–0.86) | 1.46 | 2.47 (1.88–3.42) | 10.15 (13) | 0.68 |
| YC | 2.37 ± 0.33 | 0.41 (0.24–0.57) | 1.00 | 1.42 (1.05–2.14) | 17.94 (15) | 0.27 |
| YL | 3.69 ± 0.57 | 0.43 (0.31–0.53) | 1.05 | 0.95 (0.78–1.22) | 7.96 (13) | 0.85 |
| SBL e | 2.12 ± 0.08 | 0.41 (0.37–0.44) | 1.00 | 1.63 (1.52–1.78) | 168.12 (174) | 0.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Cheng, S.; Chen, Z.; Guo, T.; Liang, P.; Zhen, C.; Wang, J.; Zhang, L.; Liang, P.; Gao, X. Establishment of Toxicity and Susceptibility Baseline of Broflanilide for Aphis gossypii Glove. Insects 2022, 13, 1033. https://doi.org/10.3390/insects13111033
Li R, Cheng S, Chen Z, Guo T, Liang P, Zhen C, Wang J, Zhang L, Liang P, Gao X. Establishment of Toxicity and Susceptibility Baseline of Broflanilide for Aphis gossypii Glove. Insects. 2022; 13(11):1033. https://doi.org/10.3390/insects13111033
Chicago/Turabian StyleLi, Ren, Shenhang Cheng, Zhibin Chen, Tianfeng Guo, Pingzhuo Liang, Congai Zhen, Jinghui Wang, Lei Zhang, Pei Liang, and Xiwu Gao. 2022. "Establishment of Toxicity and Susceptibility Baseline of Broflanilide for Aphis gossypii Glove" Insects 13, no. 11: 1033. https://doi.org/10.3390/insects13111033
APA StyleLi, R., Cheng, S., Chen, Z., Guo, T., Liang, P., Zhen, C., Wang, J., Zhang, L., Liang, P., & Gao, X. (2022). Establishment of Toxicity and Susceptibility Baseline of Broflanilide for Aphis gossypii Glove. Insects, 13(11), 1033. https://doi.org/10.3390/insects13111033






