Simple Summary
RNA interference is an important way to analyze gene function. It is also widely used in honey bees. It was thought that RNA interference with honey bee brain genes could only be achieved by injection. However, this method of injection is very complicated and can easily cause damage to bees. Recent studies have shown that specially treated siRNA can knockdown brain genes in animals by feeding them. Therefore, we used different approaches to deliver modified and unmodified siRNAs to investigate what methods can successfully interfere with honey bee brain genes. The results of this study are helpful to improve the application of RNA interference techniques in honey bees and other insects.
Abstract
RNA interference (RNAi) has been used successfully to reduce target gene expression and induce specific phenotypes in several species. It has proved useful as a tool to investigate gene function and has the potential to manage pest populations and reduce disease pathogens. However, it is not known whether different administration methods are equally effective at interfering with genes in bees. Therefore, we compared the effects of feeding and injection of small interfering RNA (siRNA) on the messenger RNA (mRNA) levels of alpha-aminoadipic semialdehyde dehydrogenase (ALDH7A1), 4-coumarate-CoA ligase (4CL), and heat shock protein 70 (HSP70). Both feeding and injection of siRNA successfully knocked down the gene but feeding required more siRNA than the injection. Our results suggest that both feeding and injection of siRNA effectively interfere with brain genes in bees. The appropriateness of each method would depend on the situation.
1. Introduction
RNA interference (RNAi) was first discovered in transgenic plants [1], followed by the discovery of its use to analyze gene function, and its important applications in several insect species [2,3,4]. In honey bees, RNAi has contributed to the understanding of gene functions in caste differentiation [5,6], sex determination [7,8], lifespan [9,10], social behavior [11], learning, and memory [12,13].
The efficiency and stability of RNAi are of great importance when studying gene functions. RNAi application and efficacy remain variable between insect species, life stages, and genes [14]. Small interfering RNA (siRNA) can induce degradation of the complementary messenger RNA (mRNA) of the target gene, reducing the expression levels of the target gene [15]. Quantifying mRNA levels by quantitative real-time polymerase chain reaction (qRT-PCR) has been widely used to characterize the efficiency of RNAi [12,13,14]. Gene knockdown efficacy depends on the transcript level of the target gene, protein turnover rates, and the efficiency of siRNA uptake by organs or cells [15]. Different interference methods have different locations and times of action on target genes. Two common methods used to administer RNAi in honey bees for gene function studies are via feeding or injection [15]. The responses of cells to these two administration methods are considerably different and lead to significant differences in the effectiveness of RNAi treatments [15,16]. For example, the injection of double-stranded RNA (dsRNA) into the body cavity of a locust caused a higher sensitivity than that induced by the feeding of dsRNA [17]. Although the feeding of dsRNA often requires more dsRNA than injection, this method is both less invasive and has a longer-lasting silencing effect in honey bees [18,19]. Both methods have their merits, but, as far as we know, feeding of RNAi has never been used to suppress gene expression in the brains of honey bees. It is thought that gene expression in the bees’ brains can only be knocked down by local injections [20]. However, chemically modified siRNA has been developed that could successfully interfere with brain genes by intravenous injection [21]. We selected ALDH7A1, 4CL and HSP70 as the target genes, which have been reported to be expressed in honey bees [22]. We also compared the efficiency of feeding and injection of chemically modified siRNA and unmodified siRNA on gene expression in the brains of bees.
ALDH7A1 is a member of the ALDH family and is mainly involved in aldehyde oxidation and aldehyde detoxification [23,24,25]. It affects a large number of neurotransmitters and neurohormones involved in learning, memory, behavior, and energy metabolism [26]. Additionally, ALDH7A1 may be involved in the regulation of honey bee caste differentiation [22]. The 4CL gene is involved in p-coumaric acid synthesis in honey bee larval diets and may be involved in honey bee caste differentiation [27,28,29,30]. HSP70 is a member of the HSP family, which protects cells from both biotic and abiotic stress stimuli [31]. It is involved in the regulation of natural bee metabolism, flight behavior, learning, and memory [32,33,34,35]. Therefore, the ability to interfere with the expression of these genes in the honey bee brain is important for future experiments.
Here, we used three genes to study the effect of different siRNA delivery methods on honey bee mortality and gene expression. These data can contribute to better understanding of the importance of siRNA in honey bee RNAi, and inform us about the RNAi methods to be used in different experimental conditions.
2. Materials and Methods
2.1. Insects
We obtained the honey bees (Apis mellifera) for this study from Jiangxi Agricultural University (28.46 μN, 115.49 μE), Nanchang, China, in 2021. A frame of capped brood was removed from a colony and placed in a cage within a 34 °C humidified incubator overnight. Honey bees were collected within eight hours of emergence to ensure that they were the same age. Newly emerged honey bees were kept in a humidified incubator for six days before the experimental treatment began. The honey bees were starved for 3 h prior to injection and feeding of siRNA.
2.2. siRNA Preparation and Injection
ALDH7A1-specific siRNA (forward: GCAUGGAUUCAAUGGG-CAUTT, reverse: AUGCCCAUUGAAUCCAUGCTT), HSP70-specific siRNA (forward: GCUCGAUGCAACCAAUUATT, reverse: UAAUUGGUUAGCAUCGAGCTT) and 4CL-specific siRNA (forward: GGUGAAAGAUAUGCUAAUATT, reverse: UAUUAGCAUAUCUUUCACCTT) sequences were designed by siDirect (http://sidirect2.rnai.jp/; accessed on 13 September 2022) and DSIR (http://biodev.extra.cea.fr/DSIR/DSIR.html; accessed on 13 September 2022). GenePharma (Shanghai, China; Shanghai Jima Pharmaceutical Technology) helped us synthesize 2′-O-methyl (2′-Ome) modified and unmodified siRNAs. Negative control siRNA is widely used as a control and has no effect on gene expression in bees [36]. Therefore, in this experiment, siRNA-NC (forward: UUCUCCGAACGUGUCACGUTT, reverse: ACGUGACACGUUCGGAGAATT) was used in the control group.
During siRNA injecting, honey bees were tied inside a copper tube and then placed under a stereomicroscope with 1.5 mm sponge double-sided tape under the bee’s brain (to ensure that the bee’s brain did not move around; the sides of the proboscis could also be fastened with a pin). The fluff was scraped from the bee’s brain with a 5 mL syringe needle. The tip of a 5 mL syringe was used to make a crack of about 1 mm in front of the median ocellus. (The tip should not be too deep in the bee’s brain to ensure survival). The siRNA was injected into the bee’s brain through the fissure in the bee’s brain using a microinjector (FemtoJet 4i, Eppendorf; Figure S1a). Honey bees in the experimental group were injected with 1 μL of siRNA solution (siRNA-ALDH7A1, siRNA-4CL, siRNA-HSP70) by microinjector. siRNA solutions were diluted in ddH2O to six different concentrations (0.5 μg/μL, 1 μg/μL, 2 μg/μL, 5 μg/μL, 10 μg/μL, and 15 μg/μL). The honey bees in the control group were treated the same as the honey bees in the experimental group, except that the honey bees in the control group were injected siRNA-NC. After the injection, Vaseline was applied to the fissure in the bees’ brains to avoid infection. One hundred honey bees were injected for each group. Fifty of the honey bees were used for survival analysis and the remaining honey bees were used for qRT-PCR.
During siRNA feeding, honey bees in the experimental group were fed 5 μL of siRNA solution (siRNA-ALDH7A1, siRNA-4CL, siRNA-HSP70) by a pipettor (Figure S1b). siRNA solutions were diluted in ddH2O to six different concentrations (0.1 μg/μL, 0.2 μg/μL, 0.4 μg/μL, 1 μg/μL, 2 μg/μL, and 3 μg/μL). The honey bees in the control group were treated the same as the honey bees in the experimental group, except that the honey bees in the control group were fed siRNA-NC. If a honey bee could not completely eat all 5 μL of siRNA solution, this honey bee was abandoned. One hundred honey bees were fed for each group. Fifty of the honey bees were used for survival analysis and the remaining honey bees were used for qRT-PCR.
At 8 h, 16 h, 24 h, 48 h, and 72 h after injection and feeding, the brains of the honey bees were dissected, and knockdowns were verified using qRT-PCR.
2.3. RNA Preparation and qRT-PCR Assay
Total RNA was extracted from the pooled brains with Trizol (Transgen; Beijing, China), and reverse transcribed to obtain cDNA using the PrimeScript™RT reagent kit (Takara; Tokyo Japan). The obtained cDNA was used for qRT-PCR analysis. The qRT-PCR analysis was performed using the ABI 7500 real-time quantitative PCR system (ABI; Waltham, MA, USA) to detect the expression levels of genes, with GAPDH as an internal control. Two bee brains were pooled as a sample. Four biological replicates were performed for each sample, and each biological replicate included three technical replicates. Primers were designed by Premier 5.0 software based on the sequences. The primers for the qRT-PCR assay are provided in Table 1. The cycle threshold value for each sample was obtained by calculating the mean of technical replicates. The data were analyzed by 2−ΔΔCT. When the p value is less than 0.05 on an ANOVA test, it is considered as a significant difference.

Table 1.
Primer sequences for quantitative qRT-PCR.
2.4. Effects of Different Modes of siRNA Delivery on the Survival of Honey Bees
To confirm whether the different ways of delivering siRNA influence the survival of honey bees, honey bees were collected and fed using the previous experimental method. At six days old, the experimental group of honey bees was administered RNAi by the method mentioned in Section 2.2. They were then re-placed in an incubator for rearing (n = 50 per cage). The control group received no treatment. The number of dead honey bees was recorded at 12 noon each day and the dead bees were removed. When all the honey bees were dead, the data were counted and analyzed.
2.5. Data Analysis and Statistics
An ANOVA test was conducted to analyze the difference between mRNA levels. The results were expressed as mean ± SE. The Kaplan–Meier method was used to analyze the differences between the control and treatment groups. A value of p < 0.05 was considered statistically significant. All statistical data were analyzed with SPSS 25.0 (IBM, New York, NY, USA).
3. Results
3.1. The Effects of RNA Interference Methods on the Survival of Honey Bees
Recent studies have shown that both injection-induced damage and high doses of the reagent can cause a rapid increase in honey bee mortality. The high mortality rate of bees may have an impact on subsequent experiments. Therefore, we compared the effects of feeding and injecting high concentrations of siRNA on honey bee mortality.
The different delivery methods, and whether the siRNA was modified or not, had no effect on the survival rate of honey bees (log-rank, chi-square = 2.99, df = 4, p = 0.56, Figure 1a; log-rank, chi-square = 0.77, df = 4, p = 0.94, Figure 1b; log-rank, chi-square = 0.85, df = 4, p = 0.93, Figure 1c). The pairwise comparison between samples is shown in supplementary Table S1.
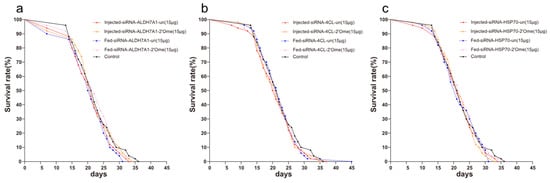
Figure 1.
Survival curves of honey bees after the injection and feeding of siRNA. (a) Effects of different delivery methods and different modified siRNAs on the survival rate of honey bees. Red solid line represents the injection of unmodified siRNA-ALDH7A1; Orange solid line represents the injection of 2′Ome modified siRNA-ALDH7A1; Blue dashed line represents the feeding of unmodified siRNA-ALDH7A1; Pink dashed line represents the feeding of 2′Ome modified siRNA-ALDH7A1; Black solid line represents honey bees without any treatment. (b) siRNA-4CL and (c) siRNA-HSP70 were used, otherwise consistent with (a). Each group has 50 honey bees.
3.2. The Effects of ALDH7A1 RNAi Knockdown on mRNA Levels
We quantified the mRNA transcripts of ALDH7A1 in the honey bee brains 8 h, 16 h, 24 h, 48 h, and 72 h after administering RNAi using qRT-PCR. At 16 h after the injection, the qRT-PCR results showed that different doses of siRNA reduced the expression of ALDH7A1. The effect lasted up to 72 h (Figure 2a–f; Table S2). The injection of 2′Ome modified siRNA (siRNA-ALDH7A1-2′Ome) and unmodified siRNA (siRNA-ALDH7A1-un) had the same effect on the expression of the ALDH71 gene. Feeding low doses of siRNA-ALDH7A1-un or siRNA-ALDH7A1-2′Ome had no effect on ALDH7A1 expression (Figure 1g–i). The expression of ALDH7A1 was affected by feeding high doses of siRNA-ALDH7A1-2′Ome but was not affected by feeding high doses of siRNA-ALDH7A1-un (Figure 2j–l). Both the feeding and injection of 2′Ome modified siRNA successfully reduced ALDHA71 mRNA (Figure 2; Table S2). However, the knockdown required different siRNA dosages. When injecting siRNA-ALDH7A1-2′Ome, only 1 μg RNA was required to produce the best knockout effect, while 10 μg siRNA-ALDH7A1-2′Ome was required when feeding.
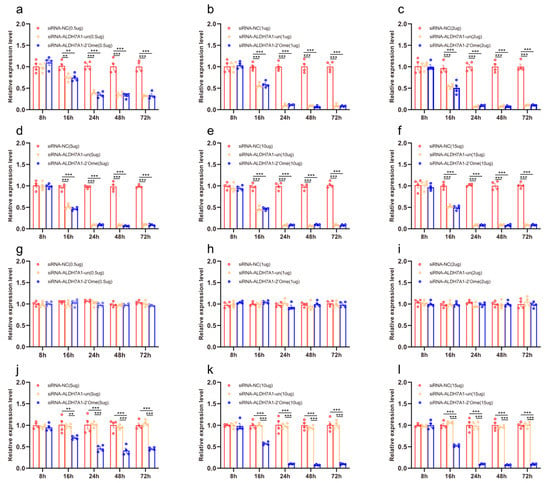
Figure 2.
The effects of siRNA on ALDH7A1 mRNA transcripts in honey bee brains. The mRNA levels of ALDH7A1 were analyzed at 8 h, 16 h, 24 h, 48 h, and 72 h after injection of 0.5 μg (a), 1 μg (b), 2 μg (c), 5 μg (d), 10 μg (e), and 15 μg (f) siRNA, or after feeding of 0.5 μg (g), 1 μg (h), 2 μg (i), 5 μg (j), 10 μg (k), and 15 μg (l) siRNA. The red column shows the relative expression of siRNA-NC injected. The orange column shows the relative expression of siRNA-ALDH7A1-un injected. The blue column shows the relative expression of siRNA-ALDH7A1-2′Ome injected. Each circle represents a biological repeat. ** indicates p < 0.01; *** indicates p < 0.001, tested by ANOVA test. Detailed data are shown in Table S2.
3.3. The Effects of 4CL RNAi Knockdown on mRNA Levels
We quantified the mRNA transcripts of 4CL in the honey bee brains 8 h, 16 h, 24 h, 48 h, and 72 h after administering RNAi using qRT-PCR. At 16 h after the injection, the qRT-PCR results showed that all doses of siRNA except 0.5 μg reduced the expression of 4CL. The effect lasted up to 72 h (Figure 3b–f; Table S3). The injection of 2′Ome modified siRNA (siRNA-4CL-2′Ome) and unmodified siRNA (siRNA-4CL-un) had the same effect on the expression of the 4CL gene. Feeding low doses of siRNA-4CL or siRNA-4CL-2′Ome had no effect on the gene expression of 4CL (Figure 1g–i). The expression of 4CL was affected by feeding high doses of siRNA-4CL-2′Ome but was not affected by feeding high doses of siRNA-4CL-un (Figure 3j–l). Both the feeding and injection of siRNA successfully reduced 4CL mRNA (Figure 3; Table S3). However, the knockdown required different siRNA dosages. When injecting siRNA-4CL-2′Ome, only 2 μg RNA was required to produce the best knockout effect, while 10 μg siRNA-4CL-2′Ome was required when feeding.
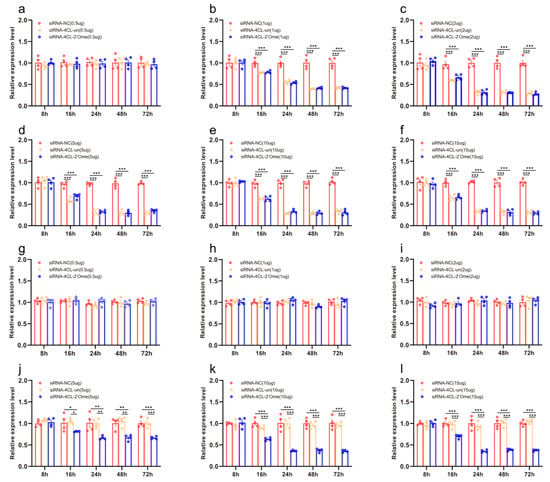
Figure 3.
The effects of siRNA on 4CL mRNA transcripts in honey bee brains. The mRNA levels of 4CL were analyzed at 8 h, 16 h, 24 h, 48 h, and 72 h after injection of 0.5 μg (a), 1 μg (b), 2 μg (c), 5 μg (d), 10 μg (e), and 15 μg (f) siRNA, or after feeding of 0.5 μg (g), 1 μg (h), 2 μg (i), 5 μg (j), 10 μg (k), and 15 μg (l) siRNA. The red column shows the relative expression of siRNA-NC injected. The orange column shows the relative expression of siRNA-4CL-un injected. The blue column shows the relative expression of siRNA-4CL-2′Ome injected. Each circle represents a biological repeat. * Indicates p < 0.05, ** indicates p < 0.01, *** indicates p < 0.001, tested by ANOVA test. Detailed data are shown in Table S3.
3.4. The Effects of HSP70 RNAi Knockdown on mRNA Levels
We quantified the mRNA transcripts of HSP70 in the honey bee brains 8 h, 16 h, 24 h, 48 h, and 72 h after administering RNAi using qRT-PCR. At 8 h after the injection, the qRT-PCR results showed that different doses of siRNA reduced the expression of HSP70. The effect lasted up to 72 h (Figure 4a–f; Table S4). The injection of 2′Ome modified siRNA (siRNA-HSP70-2′Ome) and unmodified siRNA (siRNA-HSP70-un) had the same effect on the expression of the HSP70 gene. Feeding low doses of siRNA had no effect on the gene expression of HSP70 (Figure 4g–i). The expression of HSP70 was affected by feeding high doses of siRNA-HSP70-2′Ome but was not affected by feeding high doses of siRNA-HSP70-un (Figure 4j–l). Both the feeding and injection of siRNA successfully reduced HSP70 mRNA (Figure 4; Table S4). However, the knockdown required different siRNA dosages. When injecting siRNA-HSP70-2′Ome, only 1 μg RNA was required to produce the best knockout effect, while 10 μg siRNA-HSP70-2′Ome was required when feeding.
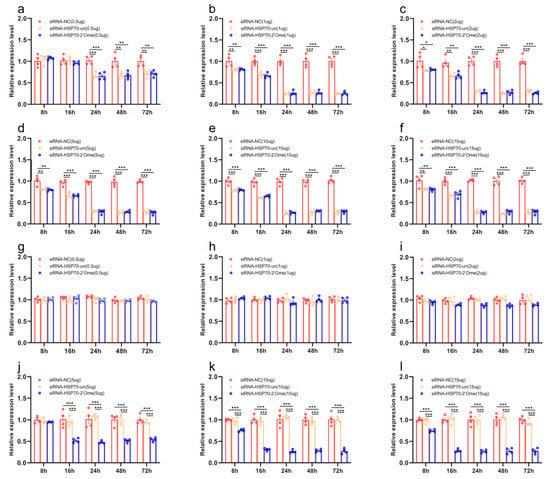
Figure 4.
The effects of siRNA on HSP70 mRNA transcripts in honey bee brains. The mRNA levels of HSP70 were analyzed at 8 h, 16 h, 24 h, 48 h, and 72 h after injection of 0.5 μg (a), 1 μg (b), 2 μg (c), 5 μg (d), 10 μg (e), and 15 μg (f) siRNA, or after feeding of 0.5 μg (g), 1 μg (h), 2 μg (i), 5 μg (j), 10 μg (k), and 15 μg (l) siRNA. The red column shows the relative expression of siRNA-NC injected. The orange column shows the relative expression of siRNA-HSP70-un injected. The blue column shows the relative expression of siRNA-HSP70-2′Ome injected. Each circle represents a biological repeat. * Indicates p < 0.05, ** indicates p < 0.01, *** indicates p < 0.001, tested by ANOVA test. Detailed data are shown in Table S4.
4. Discussion
In this study, we investigated the efficiency of gene knockdown using different dosages and different administration methods (via injection and feeding) in honey bee brains at mRNA levels. We determined the optimal time-window and dosage for studying the functions of the honey bee brain using RNAi.
In the experiment using the siRNA injection, we compared the efficacy of different RNAi molecules. Although the initial time of effect is different, they can effectively reduce the expression of target genes, and the effect can last for at least 48 h. In general, the knockdown effect is also different in different target genes of the same organism, depending on the specific structure of the siRNA and the molecular dose of RNAi [37]. Several researches have indicated that there is also great variability in the knockdown effect of siRNA due to the different regions of the targeted gene [20,38]. This would explain why HSP70 expression began to decline as early as 8 h after siRNA injection. However, the expression of ALDH7A1 and 4CL did not begin to decrease until 16 h later. Moreover, the expression of HSP70 was decreased by injection of 0.5 μg siRNA, while ALDH7A1 and 4CL needed an injection of 1 μg siRNA.
Although modified siRNA has been shown to interfere with brain genes by injection in mammals [39,40], the feeding of siRNA has not been previously reported to inhibit gene expression in honey bee brains [41]. This may be due to the low accumulation and poor stability of siRNA in the brain. Researchers are working on ways to deliver siRNA systematically, efficiently, and safely to the brain. Presently, there are two main methods. Firstly, siRNA can be encapsulated in nanoparticles to avoid degradation. It interacts with cell-surface receptors expressed in the brain to provide cell uptake of siRNA. Alternatively, siRNA can be chemically modified so that it can enter specific tissues [21,42,43,44]. Our results clearly show that feeding 2′Ome-modified siRNA can reduce gene expression in the honey bee brain, whereas unmodified siRNA cannot.
We found no difference in honey bee mortality between the siRNA-treated bees and the control group. This indicated that neither of the siRNA administration methods affected the survival rate of the honey bees. When siRNA was injected, only 1 μg was required to achieve the highest level of interference, while 10 μg was required when feeding siRNA. This suggests that the effect of gene knockdown depends on the delivery mode and dose of siRNA, which is consistent with Mittal’s view [37].
Injection and feeding each have their advantages and disadvantages. Injection has an important advantage in that it allows researchers to deliver the siRNA immediately to the tissue or into the hemolymph and hence avoiding possible barriers such as the blood-brain barrier or the gut epithelium which could be a problem in feeding. Another advantage is that the exact amount of dsRNA brought into an organism is known, in contrast to delivery by soaking or in some cases by feeding. However, this method has some disadvantages. The work itself is more delicate than other methods. Factors such as the choice of needle, the angle of the injection, and the volume and position of the injection are all very important and vary greatly between organisms. For example, in Acyrthosiphon pisum, the volume of the injection has been reported to be critical to the survival of the aphid after injection [45]. Damage to the cuticle caused by the injection may stimulate immune function, which could further complicate the interpretation of the results [46,47].
Feeding also has many advantages. It is easy to manipulate, convenient, and causes less damage to the insect [48,49]. It also has advantages in small insects, which are harder to manipulate using microinjections [49,50]. This method is also very suitable for the screening of pest control genes [47]. However, feeding is not suitable for all species. For example, the dsRNA designed for Spodoptera litura did not succeed in disrupting the target [51]. Sometimes feeding is less effective than injection, such as in Caenorhabditis elegans [52] and Rhodnius prolixus [50]. In addition, the RNAi efficiency of siRNA ingestion in different species may vary depending on the intestinal environment. Another limitation of siRNA feeding is the difficulty of determining the amount of siRNA that enters the insect through ingestion, which may affect many investigations. In addition, from the results of this article, to interfere with gene expression in the honey bees’ brain by feeding, modified siRNA must be used. Therefore, the appropriateness of the injection compared to the feeding of siRNA needs to be decided based on the requirements of each experiment.
5. Conclusions
The results showed that the injection of unmodified or 2′Ome-modified siRNA could reduce the expression of honey bee brain genes. However only feeding 2′Ome-modified siRNA could reduce the expression of bee brain genes. Feeding unmodified siRNA did not reduce gene expression in the bee brain. siRNA feeding and siRNA injection had no significant effect on honey bee mortality, but less siRNA was required for siRNA injection.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/insects13100928/s1, Figure S1. (a) Schematic diagram of honey bee injection. The green arrow shows the microinjector used to inject the bees with siRNA. (b) Schematic diagram of honey bee feeding. The green arrow shows the pipettor used to inject the bees with siRNA. Table S1: Pairwise comparison of the effects of different delivery methods and different modified siRNA methods on honey bee mortality. Table S2: Effects of different delivery methods and different modified siRNA methods on the expression of ALDH7A1 in honey bees. Table S3: Effects of different delivery methods and different modified siRNA methods on the expression of 4CL in honey bees. Table S4: Effects of different delivery methods and different modified siRNA methods on the expression of HSP70 in honey bees.
Author Contributions
Conceptualization, Y.Z., Z.L., Z.-L.W., L.-Z.Z. and Z.-J.Z.; methodology, Y.Z.; software, Y.Z.; validation, Z.-L.W. and Z.-J.Z.; formal analysis, Y.Z. and Z.L.; investigation, Z.-J.Z.; resources, Z.-J.Z.; data curation, Y.Z. and Z.L.; writing—original draft preparation, Y.Z.; writing—review and editing, Y.Z. and Z.L.; visualization, Y.Z. and Z.L.; supervision, Z.-J.Z.; project administration, Z.-J.Z.; funding acquisition, Z.-J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (32172790, 31872432), and the Earmarked Fund for China Agriculture Research System (CARS-44-KXJ15).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article.
Acknowledgments
The authors are thankful to the National Natural Science Foundation of China (32172790, 31872432), and the Earmarked Fund for China Agriculture Research System (CARS-44-KXJ15).
Conflicts of Interest
The authors declare that there is no conflict of interests.
References
- Matzke, M.; Primig, M.; Trnovsky, J.; Matzke, A. Reversible methylation and inactivation of marker genes in sequentially transformed tobacco plants. EMBO J. 1989, 8, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Bellés, X. Beyond Drosophila: RNAi in vivo and functional genomics in insects. Annu. Rev. Entomol. 2010, 55, 111–128. [Google Scholar] [CrossRef]
- Huvenne, H.; Smagghe, G. Mechanisms of dsRNA uptake in insects and potential of RNAi for pest control: A review. J. Insect Physiol. 2010, 56, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Pitts, R.J.; Bohbot, J.D.; Jones, P.L.; Wang, G.; Zwiebel, L.J. Distinct olfactory signaling mechanisms in the malaria vector mosquito Anopheles gambiae. PLoS Biol. 2010, 8, e1000467. [Google Scholar] [CrossRef]
- Ament, S.A.; Corona, M.; Pollock, H.S.; Robinson, G.E. Insulin signaling is involved in the regulation of worker division of labor in honey bee colonies. Proc. Natl. Acad. Sci. USA 2008, 105, 4226–4231. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Azevedo, S.V.; Hartfelder, K.; Amdam, G.V. Insulin-like peptides (AmILP1 and AmILP2) differentially affect female caste development in the honey bee (Apis mellifera L.). J. Exp. Biol. 2013, 216, 4347–4357. [Google Scholar] [CrossRef] [PubMed]
- Beye, M.; Hasselmann, M.; Fondrk, M.K.; Page, R.E., Jr.; Omholt, S.W. The gene csd is the primary signal for sexual development in the honeybee and encodes an SR-type protein. Cell 2003, 114, 419–429. [Google Scholar] [CrossRef]
- Kohno, H.; Kubo, T. mKast is dispensable for normal development and sexual maturation of the male European honeybee. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Paoli, P.P.; Wakeling, L.A.; Wright, G.A.; Ford, D. The dietary proportion of essential amino acids and Sir2 influence lifespan in the honeybee. Age 2014, 36, 1239–1247. [Google Scholar] [CrossRef]
- Seehuus, S.-C.; Norberg, K.; Gimsa, U.; Krekling, T.; Amdam, G.V. Reproductive protein protects functionally sterile honey bee workers from oxidative stress. Proc. Natl. Acad. Sci. USA 2006, 103, 962–967. [Google Scholar] [CrossRef] [PubMed]
- Antonio, D.S.M.; Guidugli-Lazzarini, K.R.; Do Nascimento, A.M.; Simões, Z.L.P.; Hartfelder, K. RNAi-mediated silencing of vitellogenin gene function turns honeybee (Apis mellifera) workers into extremely precocious foragers. Sci. Nat. 2008, 95, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Awata, H.; Wakuda, R.; Ishimaru, Y.; Matsuoka, Y.; Terao, K.; Katata, S.; Matsumoto, Y.; Hamanaka, Y.; Noji, S.; Mito, T. Roles of OA1 octopamine receptor and Dop1 dopamine receptor in mediating appetitive and aversive reinforcement revealed by RNAi studies. Sci. Rep. 2016, 6, 29696. [Google Scholar] [CrossRef] [PubMed]
- Cristino, A.S.; Barchuk, A.R.; Freitas, F.C.; Narayanan, R.K.; Biergans, S.D.; Zhao, Z.; Simoes, Z.L.; Reinhard, J.; Claudianos, C. Neuroligin-associated microRNA-932 targets actin and regulates memory in the honeybee. Nat. Commun. 2014, 5, 5529. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.G.; Michel, K.; Bartholomay, L.C.; Siegfried, B.D.; Hunter, W.B.; Smagghe, G.; Zhu, K.Y.; Douglas, A.E. Towards the elements of successful insect RNAi. J. Insect Physiol. 2013, 59, 1212–1221. [Google Scholar] [CrossRef]
- Yang, D.; Xu, X.; Zhao, H.; Yang, S.; Wang, X.; Zhao, D.; Diao, Q.; Hou, C. Diverse factors affecting efficiency of RNAi in honey bee viruses. Front. Genet. 2018, 9, 384. [Google Scholar] [CrossRef]
- Whangbo, J.S.; Hunter, C.P. Environmental RNA interference. Trends Genet. 2008, 24, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Wynant, N.; Duressa, T.F.; Santos, D.; Van Duppen, J.; Proost, P.; Huybrechts, R.; Broeck, J.V. Lipophorins can adhere to dsRNA, bacteria and fungi present in the hemolymph of the desert locust: A role as general scavenger for pathogens in the open body cavity. J. Insect Physiol. 2014, 64, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Evans, J.D.; Huang, Q.; Rodríguez-García, C.; Liu, J.; Hamilton, M.; Grozinger, C.M.; Webster, T.C.; Su, S.; Chen, Y.P. Silencing the honey bee (Apis mellifera) naked cuticle gene (nkd) improves host immune function and reduces Nosema ceranae infections. Appl. Environ. Microbiol. 2016, 82, 6779–6787. [Google Scholar] [CrossRef] [PubMed]
- Maori, E.; Paldi, N.; Shafir, S.; Kalev, H.; Tsur, E.; Glick, E.; Sela, I. IAPV, a bee-affecting virus associated with colony collapse disorder can be silenced by dsRNA ingestion. Insect Mol. Biol. 2009, 18, 55–60. [Google Scholar] [CrossRef]
- Guo, X.; Wang, Y.; Sinakevitch, I.; Lei, H.; Smith, B.H. Comparison of RNAi knockdown effect of tyramine receptor 1 induced by dsRNA and siRNA in brains of the honey bee, Apis mellifera. J. Insect Physiol. 2018, 111, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Sumaria, C.S.; Pradeepkumar, P. Exploring chemical modifications for siRNA therapeutics: A structural and functional outlook. Chemmedchem 2010, 5, 328–349. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Asanuma, S.; Fujiyuki, T.; Kiya, T.; Sasaki, T.; Endo, D.; Morioka, M.; Kubo, T. Differential gene expression in the mandibular glands of queen and worker honeybees, Apis mellifera L.: Implications for caste-selective aldehyde and fatty acid metabolism. Insect Biochem. Mol. Biol. 2009, 39, 661–667. [Google Scholar] [CrossRef]
- Fong, W.-P.; Cheng, C.; Tang, W.-K. Antiquitin, a relatively unexplored member in the superfamily of aldehyde dehydrogenases with diversified physiological functions. Cell. Mol. Life Sci. 2006, 63, 2881–2885. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.-K.; Cheng, C.H.; Fong, W.-P. First purification of the antiquitin protein and demonstration of its enzymatic activity. FEBS Lett. 2002, 516, 183–186. [Google Scholar] [CrossRef]
- Demozay, D.; Rocchi, S.; Mas, J.-C.; Grillo, S.; Pirola, L.; Chavey, C.; Van Obberghen, E. Fatty aldehyde dehydrogenase: Potential role in oxidative stress protection and regulation of its gene expression by insulin. J. Biol. Chem. 2004, 279, 6261–6270. [Google Scholar] [CrossRef]
- Pena, I.A.; Roussel, Y.; Daniel, K.; Mongeon, K.; Johnstone, D.; Weinschutz Mendes, H.; Bosma, M.; Saxena, V.; Lepage, N.; Chakraborty, P. Pyridoxine-dependent epilepsy in zebrafish caused by Aldh7a1 deficiency. Genetics 2017, 207, 1501–1518. [Google Scholar] [CrossRef]
- Mao, W.; Schuler, M.A.; Berenbaum, M.R. A dietary phytochemical alters caste-associated gene expression in honey bees. Sci. Adv. 2015, 1, e1500795. [Google Scholar] [CrossRef]
- Islam, M.T.; Lee, B.-R.; Lee, H.; Jung, W.-J.; Bae, D.-W.; Kim, T.-H. p-Coumaric acid induces jasmonic acid-mediated phenolic accumulation and resistance to black rot disease in Brassica napus. Physiol. Mol. Plant Pathol. 2019, 106, 270–275. [Google Scholar] [CrossRef]
- Stuible, H.-P.; Büttner, D.; Ehlting, J.; Hahlbrock, K.; Kombrink, E. Mutational analysis of 4-coumarate: CoA ligase identifies functionally important amino acids and verifies its close relationship to other adenylate-forming enzymes. FEBS Lett. 2000, 467, 117–122. [Google Scholar] [CrossRef]
- Cukovica, D.; Ehlting, J.; Ziffle, J.A.V.; Douglas, C.J. Structure and evolution of 4-coumarate: Coenzyme A ligase (4CL) gene families. Biol. Chem. 2001, 382, 645–654. [Google Scholar] [CrossRef]
- Mosser, D.D.; Caron, A.W.; Bourget, L.; Denis-Larose, C.; Massie, B. Role of the human heat shock protein hsp70 in protection against stress-induced apoptosis. Mol. Cell. Biol. 1997, 17, 5317–5327. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.B.; Roberts, S.P.; Elekonich, M.M. Age and natural metabolically-intensive behavior affect oxidative stress and antioxidant mechanisms. Exp. Gerontol. 2008, 43, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Morammazi, S.; Shokrollahi, B. The pattern of HSP70 gene expression, flight activity and temperature in Apis mellifera meda Colonies. J. Therm. Biol. 2020, 91, 102647. [Google Scholar] [CrossRef] [PubMed]
- Reitmayer, C.M.; Ryalls, J.M.; Farthing, E.; Jackson, C.W.; Girling, R.D.; Newman, T.A. Acute exposure to diesel exhaust induces central nervous system stress and altered learning and memory in honey bees. Sci. Rep. 2019, 9, 5793. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.P.; Elekonich, M.M. Muscle biochemistry and the ontogeny of flight capacity during behavioral development in the honey bee, Apis mellifera. J. Exp. Biol. 2005, 208, 4193–4198. [Google Scholar] [CrossRef] [PubMed]
- Nunes, F.M.F.; Simões, Z.L.P. A non-invasive method for silencing gene transcription in honeybees maintained under natural conditions. Insect Biochem. Mol. Biol. 2009, 39, 157–160. [Google Scholar] [CrossRef]
- Mittal, V. Improving the efficiency of RNA interference in mammals. Nat. Rev. Genet. 2004, 5, 355–365. [Google Scholar] [CrossRef]
- Krautz-Peterson, G.; Radwanska, M.; Ndegwa, D.; Shoemaker, C.B.; Skelly, P.J. Optimizing gene suppression in schistosomes using RNA interference. Mol. Biochem. Parasitol. 2007, 153, 194–202. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, F.; Liu, Y.; Zheng, M.; Wang, Y.; Zhang, D.; Anraku, Y.; Zou, Y.; Li, J.; Wu, H. Blood-brain barrier–penetrating siRNA nanomedicine for Alzheimer’s disease therapy. Sci. Adv. 2020, 6, eabc7031. [Google Scholar] [CrossRef]
- Pardridge, W.M. shRNA and siRNA delivery to the brain. Adv. Drug Deliv. Rev. 2007, 59, 141–152. [Google Scholar] [CrossRef]
- Kohno, H.; Kubo, T. Genetics in the honey bee: Achievements and prospects toward the functional analysis of molecular and neural mechanisms underlying social behaviors. Insects 2019, 10, 348. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Martínez, F.C.; Guerra, J.; Posadas, I.; Ceña, V. Barriers to non-viral vector-mediated gene delivery in the nervous system. Pharm. Res. 2011, 28, 1843–1858. [Google Scholar] [CrossRef]
- Huang, R.; Ma, H.; Guo, Y.; Liu, S.; Kuang, Y.; Shao, K.; Li, J.; Liu, Y.; Han, L.; Huang, S. Angiopep-conjugated nanoparticles for targeted long-term gene therapy of Parkinson’s disease. Pharm. Res. 2013, 30, 2549–2559. [Google Scholar] [CrossRef]
- Yin, Y.; Hu, B.; Yuan, X.; Cai, L.; Gao, H.; Yang, Q. Nanogel: A versatile nano-delivery system for biomedical applications. Pharmaceutics 2020, 12, 290. [Google Scholar] [CrossRef]
- Jaubert-Possamai, S.; Le Trionnaire, G.; Bonhomme, J.; Christophides, G.K.; Rispe, C.; Tagu, D. Gene knockdown by RNAi in the pea aphid Acyrthosiphon pisum. BMC Biotechnol. 2007, 7, 63. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.S.; Chun, J.; Schwartz, A.; Nelson, S.; Paskewitz, S.M. Induction of mosquito hemolymph proteins in response to immune challenge and wounding. Dev. Comp. Immunol. 1999, 23, 553–562. [Google Scholar] [CrossRef]
- Yu, N.; Christiaens, O.; Liu, J.; Niu, J.; Cappelle, K.; Caccia, S.; Huvenne, H.; Smagghe, G. Delivery of dsRNA for RNAi in insects: An overview and future directions. Insect Sci. 2013, 20, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, D.; Yao, Q.; Zhang, J.; Dong, X.; Tian, H.; Chen, J.; Zhang, W. Feeding-based RNA interference of a trehalose phosphate synthase gene in the brown planthopper, Nilaparvata lugens. Insect Mol. Biol. 2010, 19, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Peng, H.; Yao, Q.; Chen, H.; Xie, Q.; Tang, B.; Zhang, W. Developmental control of a lepidopteran pest Spodoptera exigua by ingestion of bacteria expressing dsRNA of a non-midgut gene. PLoS ONE 2009, 4, e6225. [Google Scholar] [CrossRef]
- Araujo, R.; Santos, A.; Pinto, F.; Gontijo, N.; Lehane, M.; Pereira, M. RNA interference of the salivary gland nitrophorin 2 in the triatomine bug Rhodnius prolixus (Hemiptera: Reduviidae) by dsRNA ingestion or injection. Insect Biochem. Mol. Biol. 2006, 36, 683–693. [Google Scholar] [CrossRef]
- Rajagopal, R.; Sivakumar, S.; Agrawal, N.; Malhotra, P.; Bhatnagar, R.K. Silencing of Midgut Aminopeptidase N of Spodoptera litura by Double-stranded RNA Establishes Its Role asBacillus thuringiensis Toxin Receptor. J. Biol. Chem. 2002, 277, 46849–46851. [Google Scholar] [CrossRef] [PubMed]
- Hunter, C.P. Genetics: A touch of elegance with RNAi. Curr. Biol. 1999, 9, R440–R442. [Google Scholar] [CrossRef][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).