Simple Summary
In the developing countries of sub-Saharan Africa, there is currently growing interest in the consumption of Lepidoptera larvae by humans, due to the important role they play in food security and poverty alleviation. In order to consider Lepidoptera larvae as sustainable alternative protein food, it is important to study the possibility of breeding the species of food interest. A previous literature review on Lepidoptera larvae consumed in Africa revealed a paucity of information on the bioecology of most species and examples of sustainable edible caterpillar rearing practices. This is not surprising given that most research focuses on aspects related to their consumption and nutritional composition. The aim of this work is to collect data on some aspects on their biology, their food plants and provide a guide to orientate the choice of species to raise. In addition, studies on the bioecology and husbandry of edible caterpillars should be more carried out for a sustainable and rational exploitation.
Abstract
There are 472 edible insect species in sub-Saharan Africa, of which 31% are Lepidoptera. Wild harvesting is still the main source of supply for these prized species to this day, with some harvesting techniques negatively impacting the environment. The successful production of edible caterpillars requires the appropriate and efficient implementation of husbandry techniques and practices. In this review, we present current literature on edible caterpillars. We provide a general overview of their life history, nutritional composition, and availability associated with specific host plants, with emphasis on semi-domestication and rearing practices that should replace wild harvest. Based on the assimilated information, a proposal of potential species for farming is provided, with details on key characteristics of development cycles to promote the establishment and development of sustainable farms of edible caterpillars at small and large scales. Such advances would contribute toward reducing anthropological pressure related to the exploitation of these food resources, as well as the environmental footprint of this widespread practice.
1. Introduction
By 2050, the world’s population is predicted to exceed 9 billion, which would further aggravate the problems of food security in developing countries. To feed this growing population, food production must increase by nearly 70% and, if possible, double in developing countries. This is because demographic growth in these countries will be coupled with increasing urbanization, and a rise in the middle classes [1]. In a context of increasing scarcity of natural resources and agricultural land, the use of alternative and ecologically sustainable protein sources, including insects (e.g., Tenebrio molitor), algae (e.g., Arthrospira platensis), and edible mushrooms (e.g., Psathyrella tuberculate), seems to be vital to facilitate an increase in world food production [2,3,4,5]. The Food and Agriculture Organization of the United Nations has identified edible insects as one key solution to food insecurity [6].
From a nutritional perspective, insects are not inferior to other protein sources, such as fish, chicken, and beef [7]. In parallel, insect production is considered more sustainable compared to domestic animals, because insects have a high food conversion efficiency, excellent potential (for some species) to be raised using organic by-products [8,9,10], high fecundity, and short development cycles [11]. Edible insects also require less space and water in the process of mass production [12,13,14,15]. For example, one gram of edible protein from beef requires eight to 14 times more land and about five times more water than mealworms [16]. Other more advanced arguments in favor of insects include the fact that they are a rich source of antioxidants and are beneficial for the intestinal microbiota of humans [17,18,19].
In addition to being a nutritious food for some families in developing countries, the exploitation of these non-timber forest products (NTFPs) offers employment opportunities and additional income to people that actively collect, produce, process, and market insects [20]. In developing countries, these edible insects are generally collected in the wild using a wide variety of collection methods depending on the behavior of the targeted insects, as well as the cultures and countries. Methods range from simple hand-picking to the use of specific tools (e.g., glue, sticks, nets, and baskets) [7,21]
For example, edible caterpillars (e.g., Emperor moth Cirina forda (See Table 1 for details of all taxa cited in the text) and African moth Imbrasia oyemensis) are usually harvested by hand [22,23]. Termites (Macrotermes natalensis) and grasshoppers (Ruspolia differens) are attracted using light traps. Dragonflies (Orthetrum sabina) are collected using sticky sap from fruit trees or glue spread on the end of long poles [24]. Orthoptera, such as Brachytrupes membranaceus, are easily detected based on the sound they emit, and are captured by hand [7,24].

Table 1.
Classification of the scientific names mentioned in the manuscript.
Although recognized as the primary source of edible insects, natural environments only offer seasonal production, with limited availability. The increasing and continuous demand for edible insects has led to an imbalance in the forest ecosystem, with repercussions on the survival of certain insect species and/or their host plants and natural predators [24,25].
Thus, the development of breeding methods would facilitate a continuous supply of edible insects, with a reduced impact on the environment [7]. In parallel, the controlled production of insects would help reduce health risks associated with their consumption, by avoiding the possible bioaccumulation of substances harmful to the health of consumers from certain toxic plants, polluted areas, or farmed areas containing pesticides [26]. In Africa, very few species are mass produced. Examples of mass produced insects include silkworm caterpillars of Bombyx mori and Gonometa postica palmarum [24]. This almost non-existent production of edible caterpillars should raise interest in developing both caterpillar breeding systems and silvicultural systems aimed at multiplying the host plants that serve as food to insects in the vicinity of homes, villages, and farms. Such practices would involve local communities in the management of these initiatives and protection of targeted species [22,27].
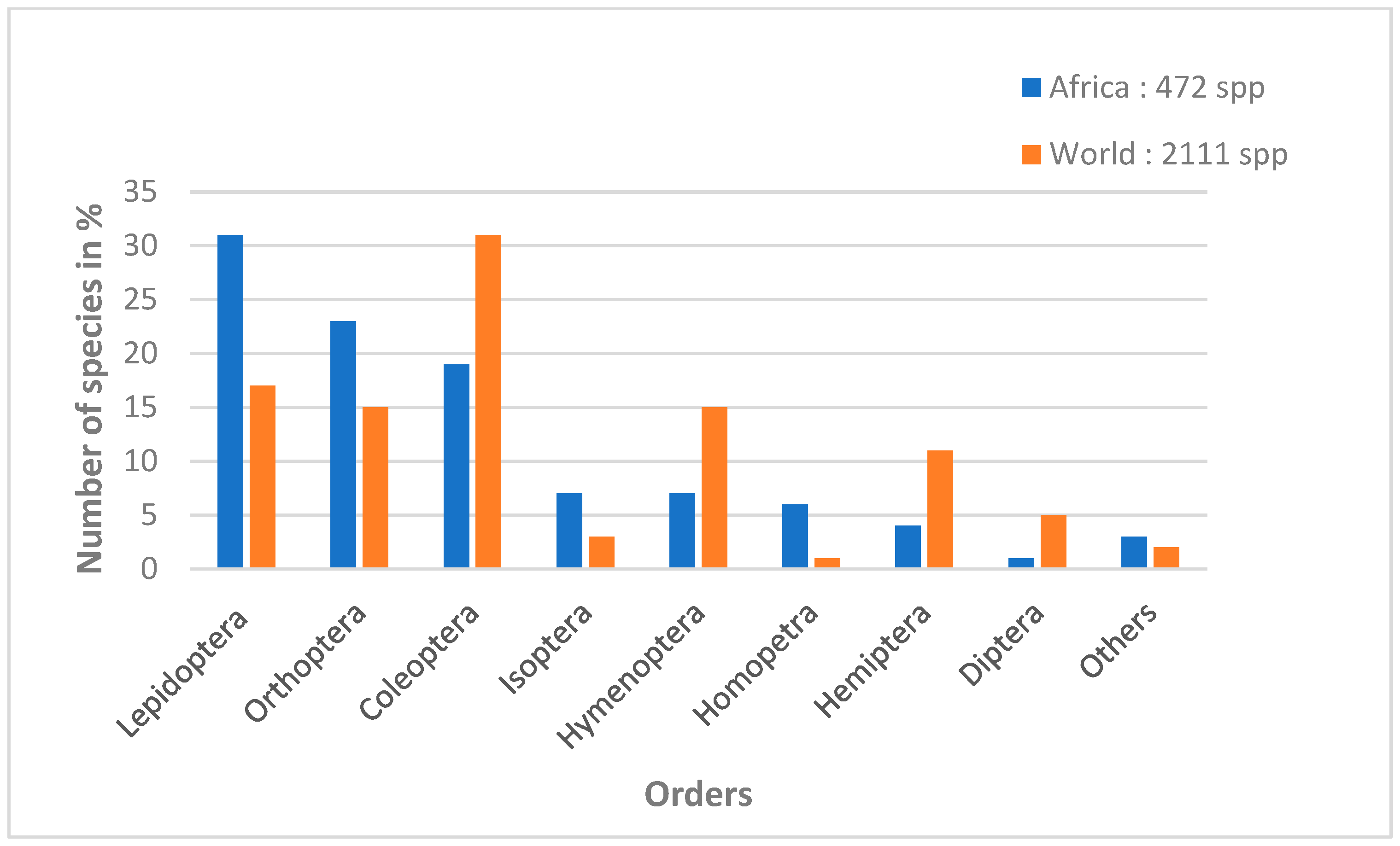
Worldwide, more than 2 billion people consume more than 2000 species of edible insects, occasionally to regularly. Targeted insects mainly belong to eight of the 12 insect orders [11,28,29,30]. In descending order, the most consumed insects worldwide are Coleoptera (beetles), Lepidoptera (caterpillars), Hymenoptera (bees, wasps and ants), Orthoptera (grasshoppers, crickets), Hemiptera (cicadas, leafhoppers, mealybugs and bugs), Isoptera (termites), Odonata (dragonflies), Diptera (flies) and some species of insects belonging to other orders [6]. Among these orders, Lepidoptera have the greatest diversity of species consumed in tropical Africa. Lepidoptera represent 31% of edible species out of 472 identified insect species (Figure 1), belonging to 128 families, of which 36 include species consumed by humans, mainly in the form of caterpillars and, more rarely, chrysalides [31].
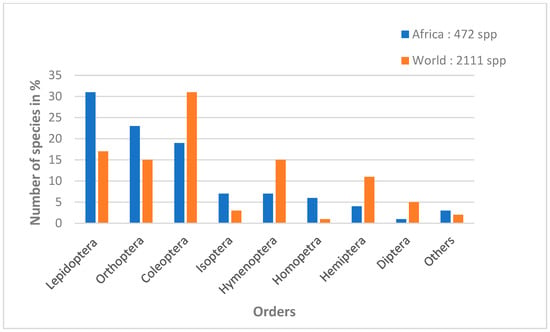
Figure 1.
Percentage of insects consumed by order in Sub-Saharan Africa and worldwide (adapted from Mariod [28]).
Thus, this literature review focuses on demonstrating that caterpillar consumption is a common practice in tropical Africa, and that this traditional food source deserves greater attention. We review information on the biology of edible Lepidoptera, their food value, appreciation by local populations, their food plants, and the environmental risks associated with their exploitation. We apply this information to identify potential avenues for species domestication programs, and potential issues associated with edible caterpillar farming.
2. Life Cycle of Lepidoptera
Published literature documenting the developmental cycle of tropical Lepidoptera remains limited (Table 2). Most species widely consumed in Africa produce a single generation per year (e.g., C. forda). This phenomenon is thought to be regulated by abiotic factors, such as photoperiod, temperature and host plant availability, which mainly affect pupation [32]. However, species such as the caterpillars Imbrasia belina, Bunea alcinoe and the African moths Gonimbrasia zambesina, Gonimbrasia krucki, Gonimbrasia cocaulti, and Gynanisa nigra can complete two cycles in one-year while others, such as the Eri silkworm complete several cycles in a single year [30,33,34,35].

Table 2.
Life cycle of Lepidoptera for which the larvae are consumed in Africa.
3. Nutritional Composition of Edible Caterpillars
Insects are an alternative food source that has a high content of essential nutrients (proteins, lipids, and minerals) for humans and animals [43].
Malaisse [44] provides a detailed overview of the nutritional values of some edible caterpillars from sub-Saharan Africa, confirming the empirical knowledge of local populations. The nutritional analysis of 24 species of dried edible caterpillars allowed us to determine the average proportion of proteins (63.5%), lipids (15.7%), and energy value (457 kcal/100 g) contained in these insects on a dry matter basis [22,45]. These data revealed clear variations in nutritional composition of different edible caterpillars. This variation is associated with species, stage of development, biotope, diet, method of preparation (e.g., roasted or boiled caterpillars), and analytical method used (Table 3, [46,47]). Edible caterpillars have higher protein levels (28 g/100 g on a fresh matter basis) compared to chicken meat (21 g/100 g of fresh matter protein). The energy intake of caterpillars (370 kcal/100 g) is similar to pork (416 kcal/100 g) [10].
Although a 100 g portion of insects is not enough to ensure the daily vitamin needs for humans (e.g., A and C), they also contribute different vitamins (depending on species) (e.g., thiamine/B1, riboflavine/B2, pyridoxine/B6, pantothenic acid, niacin) and minerals (e.g., K, Ca, Mg, Zn, P, Fe). Their bioavailability provides a means of combating malnutrition in Africa and preventing metabolic diseases [4,22,48,49,50,51,52]. Because of their high nutritional value, caterpillars are sometimes mixed with flour to prepare a porridge at breakfast to combat malnutrition in children, frail people, and pregnant women [4,45,53,54].
Proteins are major nutritional components of insects, providing essential and non-essential amino acids to the human body [43,55]. The digestibility of insect proteins is comparable to that of casein or soy proteins (77–98%) [43]. Some studies have reported that the digestibility of the moth Clanis bilineata was 95.8% compared to casein [51]. Oibiokpa et al. [48] also showed that the moth C. forda has a higher biological value (86.90%) compared to casein (73.45%).


Table 3.
Nutritional composition of edible caterpillars consumed in Africa (dry matter basis).
Table 3.
Nutritional composition of edible caterpillars consumed in Africa (dry matter basis).
| Family | Species | Protein (g/100 g) | Carbohydrates (g/100 g) | Fat (g/100 g) | Ash % | Energy (kcal/100 g) | Fiber % | Location |
|---|---|---|---|---|---|---|---|---|
| Notodontidae | Anaphe infracta | 20 | / | 15.2 | 1.6 | / | 2.4 | Guinea-Conakry, Cameroun, DRC, Equatorial Africa |
| Elaphrodes lactea | 53.6 | 1.4 | 21.9 | 3.9–4.2 | 417 | / | Zambia, DRC | |
| Saturnidae | Bunaea alcinoe | 74.3 | / | 14.1 | 2.9 | / | / | Mali, Burkina Faso, Nigeria, Ghana, Cameroon, South Africa, Zambia, DRC, Congo, RCA, Zimbabwe, Nigeria, Tanzania |
| Cirina butyrospermi | 63 | 13 | 14.5 | 5.1 | 432 | 5 | Togo, Mali, Burkina Faso, Nigeria, Ghana | |
| Cirina forda | 12–74.4 | 1.7–7 | 5.3–20.2 | 1.5–11.5 | 359–410 | 5.2–9.4 | Nigeria, DRC, CAR, Zambia, South Africa, Botswana, Burkina Faso, Mozambique, Namibia, Ghana, Togo, Tchad, Cameroon | |
| Imbrasia epimethea | 57.8–73 | 0.9–5.3 | 12.4–23 | 3.3–7.5 | 419–501 | / | Guinea-Conakry, DRC, Zambia, South Africa, Cameroon, Congo, CAR, Zimbabwe | |
| Imbrasi belina | 35.2–56.9 | 7.8–10.9 | 10–23.3 | 6.9–11.3 | 402 | 27.8 | DCR, Zambia, South Africa, Zimbabwe, Botswana, Malawi | |
| Imbrasia oyemensis | 23.7–61.6 | 1.5–11 | 19.1–57.7 | 2.6–5.5 | 384–477 | Guinea-Conakry, Cameroon, DRC | ||
| Imbrasia obscura | 62.3 | / | 12.2 | / | / | / | DRC, South Africa, Zimbabwe, Botswana, Gabon, Mozambique, Namibia | |
| Imbrasia truncata | 54.5–73 | 1.2–11 | 15.2–27.8 | 2.7–5.5 | 418–499 | / | DRC |
Source: [40,43,49,50,53,56,57,58,59,60,61,62].
However, the digestibility of insect proteins could be improved by eliminating the rigid chitin-rich exoskeleton, which reduces the digestibility of their crude proteins, despite the presence of two chitinases in the human stomach [48,63,64]. Chemical methods can be used to remove the exoskeleton, whereby strong acids and bases are used to dissolve calcium carbonates and proteins, respectively [65]. For example, chitin removal by alkaline extraction increases the digestibility of bee protein from 71.5% to 94.3% [63].
The quality of proteins depends on the amino acid composition [66], with insects being particularly rich in lysine and threonine, but sometimes deficient in methionine and cysteine [67]. However, some amino acids could be limiting depending on insect species (Table 4, [49,68]). Many amino acid sequences have been identified in a wide range of dietary proteins that are generally considered to be sources of bioactive peptides (BAPs), such as the tripeptides valine-proline-proline (VPP) and isoleucine-proline-proline (IPP), and the polypeptides phenylalanine-phenylalanine-valine-alanine-proline- phenylalanine-proline-glutamate-valine-phenylalanineglycine-lysine (FFVAPFPEVFGK) and tyrosine-leucine-glycine-tyrosine-leucine-glutamate-glutamine-leucinearginine (YLGYLEQLLR) [69,70]. These BAPs might have biological functions and hypotensive, antioxidant, antidiabetic, immunomodulatory or mineral-binding properties [69,70]. Among known edible insect species, the biofunctional properties of proteins and peptides from B. mori have been extensively studied. For example, analysis of angiotensin converting enzyme (ACE) revealed the existence of angiotensin I converting enzyme (ACE) inhibitory peptides that reduce blood pressure. For example, peptides identified in the protein of B. mori pupae include tripeptides (KHV and ASL) and the pentapeptide GNPWM [71,72,73].

Table 4.
Amino acid composition of caterpillars consumed in sub-Saharan Africa (in mg/g dry matter basis).
Reference protein intake for health adults is estimated to be 0.83 g protein/kg body weight per day (i.e., 62.3 g for a 75 kg adult). Fogang et al. [74] reported that the consumption of a 100 g portion of Imbrasia truncata or Imbrasia epimethea caterpillars covered 30.6% and 32.3% of the required protein intake of a 75 kg adult, respectively.
Lipids are the most energy-dense group of macronutrients [2]. They store and provide energy, and support and protect the various organs [83]. They are made up of triglycerides, each with a glycerol molecule and three fatty acids that are saturated or unsaturated [2]. Caterpillars are among the most fat-rich insects [84].
Like other edible insects, edible caterpillars are a source of fatty acids [45], with most caterpillars being rich in mono- and, even, polyunsaturated fatty acids (PUFA) [85]. These fatty acids are mainly linolenic (C18:3n3) and linoleic (C18:2n6) acids, commonly known as omega-3 and omega-6 fatty acids, respectively [85]. These PUFAs are not synthesized by the human body, and must be obtained through the diet [65]. Therefore, the ingested amounts and balanced proportions of these fatty acids should be provided sufficiently, as unbalanced ratios are often associated with health problems in humans, such as coronary heart disease, cancer, and autoimmune and inflammatory diseases [86].
Table 5 shows the fatty acid composition of some caterpillar species consumed in sub-Saharan Africa. The fatty acid composition of the lipid content of C. butyrospermi, C. forda and I. belina caterpillars is estimated to contain 37–54% of PUFAs, 32–57% of saturated fatty acids (SFA), and 1–27% of monounsaturated fatty acids (MUFA) [50,52,87]. Other edible lepidopteran larvae (such as confused Emperor Imbrasi ertili and Heliothis zea) are composed of up to about 70% unsaturated fatty acids [88]. There is large variability in the fatty acid profiles of different caterpillar species. These differences in fatty acid composition are attributed to different fat and calorigenic content, along with the diet of caterpillars [45].

Table 5.
Fatty acid composition of caterpillars consumed in sub-Saharan Africa (% on dry matter basis).
The diets of people living in developing countries are generally characterized by micronutrient deficiencies, resulting in major health consequences [7]. Interestingly, the micronutrient content of edible insects is influenced by their diet [91]. Importantly, the consumption of edible insects would provide significant amounts of minerals that are sufficient to meet human needs. Examples include copper (e.g., Usta terpsichore, mealworm adult), iron, and zinc (e.g., B. mori), as well as vitamins (carotene and vitamins B1, B2, B6, D, E, K, and C) [51].
Certain minerals (such as iron and zinc) are of particular interest, because they are often the source of deficiencies in developing countries [7,11]. Fe and Zn deficiency is particularly prevalent in regions with high cereal and low animal food consumption. In fact, both Fe and Zn help prevent malnutrition and early stunting [92]. Mwangi et al. [92] compared the Fe and Zn content in meat from conventionally raised animals (8 mg/100 g Fe and 21 mg/100 g Zn beef, 4 mg/100 g Fe and 6 mg/100 g Zn pork, and 3 mg/100 g Fe and 6 mg/100 g chicken) against three edible insect species (6 mg/100 g Fe and 13 mg/100 g Zn T. molitor, 14 mg/100 g Fe and 21 mg/100 g Zn Acheta domesticus, and 19 mg/100 g Fe and 15 mg/100 g Zn L. migratoria). The authors showed that edible insects contained Fe and Zn levels similar to, or higher than, those of conventional farm animals [92]. Of note, mineral content varies according to insect species, stage of development, and diet [7,43]. Better control of food intake would enable easy modification of insect mineral content [11].
The presence of iron in edible caterpillars (Cinabra hyperbius) allows could eliminate iron deficiency anemia in people that consume them, due to the lack of protein necessary to synthesize blood cells, which is an issue some children have in developing countries [45]. The iron content of C. butyrospermi, C. forda, I. epimethea, I. belina and the African moth I. truncata caterpillars ranges from 1.30 to 64 mg/100 g dry weight. These values are higher compared to beef, which is 6 mg per 100 g dry weight. Similarly, Zn levels in C. forda and I. belina caterpillars range from 3.71 to 24.2 mg/100 g dry weight, whereas beef contains an average of 12.5 mg per 100 g dry weight (Table 6, [93]).

Table 6.
Mineral composition (mg/100 g dry matter basis) of caterpillars consumed in Sub-Saharan.
Although 100 g of edible caterpillars does not meet the daily calcium requirement, with the exception of the edible caterpillar (Tagoropsis flavinata) [45]. Mineral requirements could be met by consuming edible caterpillars, meeting recommended nutrient intake for adults (Table 6). Rumpold & Schlüter [43] reported that some caterpillars (U. terpsichore, Imbrasia ertli) have high levels of sodium per 100 g and, in some cases, could exceed the maximum daily intake of 1500 mg. In parallel, insects are sources of vitamins [22]. As with mineral content, the vitamin content of insects could be altered if they are raised on vitamin-rich substrates [91]. Of note, data available on vitamins present in edible insects varies across publications [2]. These differences are likely attributed to the species used, analytical methods, and insect diet [51]. Depending on the species, caterpillars are rich in various water-soluble vitamins (thiamine/B1, riboflavin/B2, pyridoxine/B6, pantothenic acid, niacin). According to Malaisse [44], the daily consumption of 50 g dried caterpillars would meet human needs for riboflavin and pantothenic acid. Furthermore, 30% of niacin requirements would be met [22].
4. Availability, Host Plants, Harvesting, and Storage
Availability and Relationship of Lepidoptera with Host Plant(s)
The seasonal availability of edible caterpillars varies with region and reflects variation in climatic conditions [16,22,30]. In the Central African Republic (CAR), caterpillars are available from mid-June to late September [20], whereas they are available from July to October in Cameroon and from August to January in Congo-Brazzaville. In the Democratic Republic of Congo (DRC), edible caterpillars are available between July and September in the western Kasai region, between June and September in the Kisangani region [20], and from September to December in the Bandundu region [20].
This seasonality is related to the presence of plants on which caterpillar feed at the beginning of the rainy season, because caterpillars specifically feed on one or more host plants that only grow in certain ecosystems [30,94]. Several studies have provided information on the host plants of edible caterpillars in tropical Africa [27,94,95,96,97,98]. These studies show that edible caterpillars are generally polyphagous, associating with several host plants (Table 7). For example, the caterpillar C. forda feeds on Vitellaria paradoxa (Sapotaceae) in West Africa, Autranella congoensis in CAR, and Burkea africana in South Africa. In the DRC, these caterpillars are associated with Crossopteryx febrifuga in Bas-Congo, E. suaveolens in the Kisangani-Tshopo region, Erythrophleum africanum in Bandundu, and Albizia antunesiana in Katanga [99].

Table 7.
Availability of edible caterpillars and their host plants.
Of note, the abundance and availability of edible caterpillars is sometimes affected by the felling of host plants. For example, the woody plants, Sapelli (Entandrophragma cylindricum) and Tali (Erythrophleum suaveolens) are both widely harvested for timber and edible caterpillars (I. oyemensis and C. forda, respectively). Consequently, conflict has risen between one-time timber harvests and the annual harvests of edible caterpillars spanning decades [105]. Forest management approaches should support the production of these wood and non-wood resources to benefit multiple stakeholders in these forests. This would minimize potential conflicts between logging and the needs of local people who consume these wild foods [105].
To improve the management of these resources, it is important to provide information on the yields of edible caterpillars inhabiting timber trees, and how logging affects their availability. For instance Muvatsi et al. [105] quantified the density of Sapelli and Tali trees of different size classes within a 10 km radius of four villages in the Kisangani region (DRC) in 2012, along with the annual cutting areas of two logging concessions. Stumps of these two forest species were identified and measured in 21 five-hectare plots around each village and 20 five-hectare plots in each concession. Around the villages and in the concessions, Sapelli were present at densities of 0.048 ± 0.008 harvestable trees (≥80 cm diameter at breast height [dbh]) ha−1 and 0.135 ± 0.019 precommercial trees ha−1. Harvestable Tali trees (≥60 cm dbh) were seven times more abundant with 0.347 ± 0.032 ha−1, while precommercial Tali trees were present at densities of 0.329 ± 0.033 trees ha−1. Based on estimated tree densities, caterpillar yields were estimated for a 15,700-ha semicircle within a 10 km radius of the villages. Depending on the village, yields were estimated at 11.6–34.5 kg yr−1 for I. oyemensis on Sapelli trees, and 65.8–80.9 kg yr−1 for C. forda on Tali trees, averaging 0.74–2.2 kg ha−1 yr−1 and 4.2–5.2 kg ha−1 yr−1 fresh weight, respectively (0.23–0.68 kg ha−1 yr−1 and 1.3–1.6 kg ha−1 yr−1 dry weight, respectively).
5. Harvest
Edible caterpillars are harvested in forests, savannahs, and other uncultivated lands based on the periods and harvesting sites known to farmers [106,107]. Prized species are identified from accumulation of their droppings at the base of tree trunks where they feed on leaves, their characteristic smell, or consumed leaves [20]. In general, caterpillars are harvested manually by women and children on the ground, trunks, branches, or leaves of plants on which they are found [108,109].To reach caterpillars at the top of large trees, some harvesters hit the trunks with hammers, or use a long bamboo pole to knock the caterpillars off the top of the trees, while some pygmy ethnic groups in the CAR climb trees to harvest them [20]. Caterpillars are also harvested by felling host plants. For example, in the DRC, 65% of respondents stated that they cut down trees to harvest the most commonly consumed caterpillar species (including C. forda, I. epimethea, I. ertli, and I. oyemensis) despite the threat to the ecosystem and targeted caterpillar species [25].
The sanitary quality of caterpillars collected from the natural environment raises some public health concerns because it is difficult to control the environment in which they are found and the level of toxins transferred from the host plants on which they feed, along with the possible risks of microbiological contamination [7,40,110].
The bibliographical syntheses of Rothschild et al. [111] and Nishida [112] exhaustively list the Lepidoptera that potentially accumulate various toxic substances, including cardenolides, aristolochic acids, HCN, histamine, acetylcholine, cardiac glycosides, and pyrrolizidine. Some edible caterpillars contain antinutritional factors, including phytate, tannin, oxalate, hydrocyanide, saponins, and alkaloids. These antinutritional factors affect and inhibit the availability of food nutrients [113,114]. For example, antinutritional analysis of the degutted, boiled, dried, and ground larvae of C. forda collected in the wild revealed the presence of oxalate (4.1 mg/100 g) and phytic acid (1.0 mg/100 g), whereas tannins were not detected [113]. Similarly, antinutritional analysis of B. alcinoe larvae revealed the presence of oxalate (15.47 ± 1.88 mg/100 g), phytate (18.21 ± 2.14 mg/100 g), and cyanide (1.68 ± 0.20 mg/100 g), which were within the permissible limits [115].
Some edible caterpillars (African silkworm Anaphe venata, B. mori) also exhibit thiaminase I activity. This enzyme induces thiamine deficiency or beriberi [116,117]. The thiaminase activity of B. mori caterpillars is less than one-third that of A. venata [51]. In parts of southwestern Nigeria, an acute, seasonal ataxic syndrome characterized by tremors, ataxia, and variable levels of altered consciousness has been observed annually for over 40 years after consuming slow-cooked, high-thiaminase chrysalises or silkworms of African silkworm, A. venata [51,117]. The characteristics of Anaphe thiaminase include high heat resistance, which explains why symptoms of seasonal ataxia appear within hours of eating a carbohydrate meal with a stew containing roasted A. venata larvae [116]. Detoxification of the African silkworm would make these prized larvae a safe source of food for local populations [51,117]. Some studies have also reported the presence of bacterial populations associated with the exoskeleton and intestinal contents of B. alcinoe larvae freshly collected from the wild in Nigeria [40,113]. Specifically, nine bacterial isolates belonging to the genera Staphylococcus, Bacillus, Micrococcus, and Acinetobacter were isolated from the exoskeleton of B. alcinoe. Furthermore, 11 isolates representing the genera Staphylococcus, Bacillus, and Micrococcus were recovered from the intestinal contents of B. alcinoe. Importantly, this study demonstrated that Staphylococcus aureus was present both on the exoskeleton and in the intestinal contents, along with a Bacillus species (Bacillus cereus), both of which produce enterotoxins. However, while S. aureus can be destroyed at high cooking temperatures, B. cereus has the ability to resist such temperatures via the production of endospores. Therefore, post-harvest treatments are essential to prevent any contamination or deterioration of products. Treatments could include screening of marketable insects, development of appropriate treatment standards, and the manipulation and storage of collected insects. Such actions would minimize all associated risks with consuming insects, ensuring the safety of consumers [113,118].
In addition to their role in feeding households, harvested edible caterpillars also contribute toward improving the economy of local communities by marketing part of their harvest [22]. The edible caterpillar trade generates additional income within the households that practice it, and contribute significantly toward improving the living conditions of local populations [109]. Caterpillars can be marketed both directly and indirectly; however, in most cases, sales are completed indirectly. The direct method involves no intermediaries between the producer (collector) and consumer, whereas indirect sales involve intermediaries (wholesalers and resellers) between the collector and consumer [20]. However, the method of selling caterpillars individually is only profitable for producers. For example, in Cameroon, the local population makes significant profits, and earns much more than the salary of a servant, gardener, or even a driver. The sale of edible caterpillars provides wholesalers and retailers with a profit margin of 125% and 21%, respectively [20]. In the DRC, the caterpillar trade provides employment opportunities for the Congolese, providing opportunities to earn profit margins of over 100% [97]. Vantomme et al. [22] reported the import of about 8 t of dried Imbrasia sp. to France and Belgium from the DRC, valued at about US$41,500 in Belgium (average price of US$13.8 per kg). Improving how the caterpillar market is organized would facilitate a move from individual sales to contractual sales, which would benefit all actors in the production chain (i.e., producers, intermediaries, and consumers) [97].
In the past, the harvesting of edible caterpillars was regulated by: (a) monitoring host plant abundance and possible changes to the ecosystem; (b) protecting vulnerable life stages and specific habitats (e.g., timing of forest fires to reduce the destruction of host plants and/or eggs and caterpillars); and (c) determining a harvesting schedule. Unfortunately, population pressure, poverty, and increased demand for caterpillars from outside buyers have led to changes in human behavior [16]. Certain edible caterpillar species have been overexploited, including the mopane caterpillar (I. belina) in the Miombo forests of southern Africa [25] and Sapelli caterpillars (I. oyemensis) in Central Africa. The excessive collection of these caterpillars has adverse effects on future harvests [22]. The availability of edible caterpillars might also be affected by logging, as the host trees of some edible caterpillar are also used as valuable timber (e.g., E. cylindricum) or artisanal charcoal production [105,119,120]. Deforestation destroys the natural habitat of edible caterpillars and their host plants by reducing the amount of foliage used to feed them [121]. Conversely, overexploitation, forest fires, and overgrazing have caused the extinction of many caterpillar species, threatening the well-being of local human populations, especially highly vulnerable rural populations [20,122,123]. Finally, edible insects are also part of the food chain of other animals, including birds and other small vertebrates. Therefore, any decrease in edible caterpillars would also affect the stocks of their predators, threatening species biodiversity [22].
Conversely, the harvesting of edible caterpillars positively feeds back on bushfire frequency and forest management [22,124,125]. Fires set at the end of the dry season, when the weather is too hot and trees start to produce new leaves, causes widespread damage by killing trees, reducing regrowth, and increasing erosion [124]. Some studies show that late fires are almost absent in areas where edible caterpillars are harvested by villagers, inducing favorable impacts on forest management [22]. Harvesting caterpillars encourages people to burn early, which protects the caterpillars and promotes forest regeneration [124]. Therefore, it is more beneficial to have early fires, which helps avoid damage caused by late fires [125]. Likewise, Leleup and Daems [126] identified the relationship between brush burning dates and the seasonal occurrence of different developmental stages of certain edible caterpillar species in the DRC. Based on this information, these authors provided several recommendations on optimal periods for burning savanna and open forest [22].
Since most edible caterpillars are harvested from the wild, the development and adoption of harvesting methods appears to be the simplest conservation management option [107]. Evaluation of each species should include the determination of biological factors, including distribution, host plant (or habitat) specificity, range of host plant(s), various life history parameters (number of generations, developmental time), dispersal ability, and abundance. The suitability for the semi-domestication or breeding of these species should also be investigated. Emphasis should be placed on establishing sustainable harvesting protocols, including the possibility of using simple semi-domestication procedures to breed local species [20,107]. In addition, to contributing to the sustainable production of edible caterpillars, silvicultural systems that integrate forest management by supporting the multiplication of tree species (e.g., Entandrophragma spp., Isoberlinia spp., and Colophospermum spp.) associated with caterpillars of high food value could be developed [127]. Thus, local communities could benefit from both caterpillars and wood production, while providing an opportunity for harvesters to better protect and manage the habitats from which these insects are harvested [22,27]. For example, in Central Kongo Province (DRC), Acacia auriculiformis is planted for firewood, but also supports five species of edible native caterpillars [99].
6. Conservation
After harvesting, some caterpillars can be kept alive for about three days, or dried for a shelf life of several months. In the latter case, the preservation treatment consists of exposing caterpillars to the sun or simply smoking them [20]. Larvae are dried in the sun on wooden grids or corrugated sheets, with a long period of sunlight being required. This technique is less practiced than smoking, because the shelf life is shorter. Before exposing caterpillars to the sun, they are eviscerated shortly after they have been removed from the foliage of host plants, roasted in hot ashes from wood fires to remove their hair until they harden, and, finally, they are exposed to the sun until they become crispy and dry [20,95]. A smoking step extends the preservation of caterpillars to a period of three months [20]. It involves spreading the insects on a grate (wooden or other substrates) placed over a heat source. The caterpillars are frequently smoked after being boiled for 30 to 45 min [20]. Therefore, the food safety of insects harvested in the wild and the method of preservation requires evaluation.
7. Semi-Domestication and Farming
Wild harvesting, semi-domestication, and farming are the three possibilities for obtaining edible insects. Of the recognized edible insect species, 92% are harvested from the wild, 6% are considered semi-domesticated, and only 2% are considered farmed [107]. Despite the small percentage of insects that are produced or semi-domesticated, these production methods represent huge potential for the sustainable supply of edible insects, which would reduce the negative impacts of wild harvesting on the natural environment [107,128].
7.1. Semi-Domestication
The domestication of edible insects appears to be a relatively simple and economical method of production. However, to date, only silkworms (B. mori) and honeybees (Apis melifera) are considered fully domesticated [129].
For an edible insect species to be targeted for domestication, a number of criteria should be met, including a short reproductive cycle, gregarious behavior, high reproduction and survival rates, modifiable diet, and high nutritional value [124,128]. For this purpose, S. ricini, would be a suitable candidate. It is a multivoltine species with at least six to seven generations per year. It has a total life cycle duration of 53 and 57 days for females and males, respectively. It has a hatching rate of 97%, with highly fecund females (360 eggs on average) [130].
Very few examples of semi-domestication of edible caterpillars are available. Semi-domestication involves the transfer of insect specimens to other breeding sites or the manipulation/creation of habitats [27]. Some first instar caterpillars of the edible Saturnidae (e.g., Lobobunaea phaedusa, I. obscura, I. eblis, and I. epimethea) are transferred from wild trees in forests to domestic trees near to the dwellings of villagers [21]. This action allows larvae to continue their development in an environment where they are kept safe from drought, heat, and predation, all of which contribute to their mortality in the wild [6,21]. In Bas-Congo, the caterpillars I. obscura, I. ertli, and C. forda are introduced to villages by placing them on trees close to the houses of farmers and on their lands [99]. Malaisse [44] also recorded that branches with many caterpillars are transferred to the same tree species closer to villages. This system allows villagers to monitor the caterpillars and improve the harvesting schedule. If the area is protected from fire during this phase, the moths of some species lay their eggs on the tree beneath which they previously pupated, or on nearby trees. One farmer followed this facilitation system by introducing C. forda caterpillars to a savanna area abundant in the host tree species C. febrifuga, and subsequently harvested caterpillars regularly following introduction [131]. Other authors, such as Ngoka et al. [132], reported cases of semi-domestication with wild African silkworms Gonometa postica. However, it is often difficult to semi-domesticate the species of Gonometa spp., with failures being recorded for Gonometa rufobrunnea [132,133]. This failure has been attributed to several relevant questions remaining unanswered. These include the factors that induce or cause diapause, conditions required for successful mating, development of effective methods for seeding populations, and the conditions required for the successful mating of butterflies. Therefore, further studies are needed to determine the appropriate captive rearing and breeding conditions for this species to enable sustainable production [133]. For instance, an initial population of G. postica live pupae in cocoons were reared under semi-captivity after collection from host plants in the study area. Oviposition occurred on the branches of its two host plants (Acacia Mearnsii and Acacia hockii), which were protected by netting to protect the larvae from predators and parasitoids. This rearing technique allowed the development cycle of this species to be characterized (i.e., egg incubation of 14 to 18 days, larval development of 73 to 99 days, and pupal development of 48 to 72 days).
Ghazoul [134] reported the potential of rearing a population of I. belina in captivity for three years. However, the long-term viability of continuous production of fresh mopane worms needs to be ascertained, along with the control of viral or parasitoid disease accumulation, particularly when worms are unavailable for long periods. High mortality might occur as a result of unprotected natural conditions, exposing worms to disease, parasitoid attack, predation, heat, and drought. However, such mortality could be controlled and mitigated by introducing various approaches, such as the use of protective sleeves around branches and the construction of shade houses. Larvae can be successfully reared in shade cloth bags draped over small trees, especially until the end of the second or third instar [134].
Under normal environmental conditions, larvae can be effectively released from bags at the end of the second instar (or from the greenhouse during the third instar) on exposed trees with fresh leaves. After this point, mortality is relatively low; thus, worm production could be increased, without needing to invest effort in protecting large 4th and 5th instar larvae. In contrast, under persistent hot and dry conditions, larvae placed in shade cloth bags overheat and dry out. In such cases, larvae could be reared successfully in larger shade bags under dry conditions; however, the risk of disease remains, which appears to accumulate over the season. Unfortunately, under both conditions, the risk of increasing viral load remains a major issue. This risk is attributed to the fact that larvae are maintained for long periods in the same area over multiple years. Thus, viral infection appears to be the main factor limiting mopane worm production, along with pupal diapause [134].
7.2. Farming
Although insect farming for human consumption has been introduced in both tropical and temperate countries, it remains a relatively rarely used method of producing edible insects [128]. In practice, insect farming is not very common [128].
A major challenged face by breeders in rearing edible caterpillars is the lifting of pupal diapause and ensuring the sufficient production of food plants for caterpillars. For example, in Zimbabwe, the second generation of mopane worms undergoes a long diapause of six to seven months. This presents an opportunity for scientists to break the diapause to allow the year-round supply of mopane worms. The physiological processes that I. belina undergoes during diapause, however, are not yet fully understood or documented, with only general information. For most insects, these processes are primarily regulated by abiotic factors, such as photoperiod and temperature. It is important to understand how photoperiod and temperature affect these processes to predict the initiation and termination of diapause in the field [32]. Two successful pupation methods include boxes and pits. In the first method, plywood boxes with hinged lids were constructed with dimensions of 26 × 52 × 32 cm. To evaluate both the suitability of substrate and survival of larvae in boxes, different types of soil were used, filling up to half or three-quarters of the height of boxes. Then, different numbers of caterpillars at the prepupal stage were placed in the boxes. Some mopane leaves were included in case some larvae were still feeding. After about one month, the boxes were emptied to count the number of live, deformed, dead, and parasitized pupae. In the second method, open pits were constructed by digging a 2.5 × 1.0 m area to 20 cm depth. Sheets of plywood were placed along the sides of the pit. The pit was then covered with shade cloth. Each pit was filled with soil to a depth of about 20 cm to hold the plywood sheets in place, which extended about 25 cm above ground level. Then, the whole structure was covered with a shade cloth. Finally, pre-pupating larvae were placed on the surface of the pit with some fresh mopane leaves [134].
Both methods could be used; however, the pit method is less labor intensive and less amenable to experimentation and monitoring. Pupae remain viable for at least 2.5 years, and artificial cooling prolongs adult hatching. To continue mopane worm rearing, the conditions under which dormancy could be extended or adult emergence artificially induced require investigation [134].
There are very few cases of edible caterpillar breeding because breeding requires suitable mastery of the ecology and biology of targeted species, both animal and host plant. However, various laboratory rearing trials have been conducted in Africa, starting with emblematic caterpillars, including C. forda and C. butyrospermi [33,37]. For example, for C. butyrospermi, rearing involved feeding caterpillars individually in Petri dishes with young shea leaves given ad libitum, and then the different biological parameters were measured. A developmental time from egg to adult of approximately 398 days was recorded, with egg incubation of 30 days, larval development of 33 days, and a very long pupal stage of 335 days. In contrast, adults lived just 5 days [37]. In the Lwiro region of the DRC, the production of A. infracta fed ad libitum with the leaves of their host plant Bridelia micrantha resulted in the production of 9.32 kg caterpillars based on 10 pairs of adults over a 49-day feeding period [39]. For this species, the pupation time was relatively short (77 days), which is an advantage for the mass production of caterpillars. In addition, in the DRC, Muya et al. [42] described the development cycle of the caterpillar Aegocera rectilinea, locally called “Mikombidila”. Again, the caterpillars were fed ad libitum with the leaves of their host plant, Boerhavia diffusa, with the development cycle from egg to adult lasting about 36 days. Due to this short cycle, it would be possible to achieve about 10 cycles per year under controlled conditions for this multivoltine species. Thus, the profitability and feasibility of Lepidoptera rearing would benefit by focusing on multivoltine species. To produce edible caterpillars effectively, short-cycle species (e.g., A. rectilinea) should be targeted, until the issue of long pupal diapause, which seems to be a characteristic of most edible caterpillars in the family Saturnidae, is solved (Table 2).
8. Conclusions
This review consolidated available data on edible caterpillars in sub-Saharan Africa regarding both ecology and rearing. First, information was assimilated on their life cycle, nutritional composition, availability, and relationship with the host plant(s). Then, methods and issues were considered with respect to promoting breeding by optimally controlling their development cycle and the production of their food plants to obtain food and nutritional security. Emphasis was placed on identifying caterpillar species with a high potential for breeding via mass production to ensure a sustainable supply.
To date, most research on edible caterpillars has focused on their diversity, collection, preparation, consumption, nutritional composition, and host plant diversity. However, there is a paucity of studies on the life history and rearing techniques of prized species.
More research is needed to elucidate the bioecology of Lepidopteran life cycles. Experimental rearing could be used to determine various aspects related to their biological parameters. For instance, it is necessary to measure the duration of each stage of the development cycle (egg, larva or caterpillar, nymph or chrysalis, adult or butterfly), type of pupation (underground and/or aerial), the longevity of adults, rate of fecundity, reproduction and hatching, survival rate of larvae and chrysalises, optimal conditions for reproduction, and embryonic and larval development.
Ultimately, to reap the benefits of edible caterpillars at scale, rearing technologies must be intensified rather than depending on wild harvesting. This step, in advance, requires the selection of potential species for farming. Criteria that should be considered include their suitability for mass production, biomass supply, nutritional content, and environmental implications. Thus, it is important to invest in the research and development of breeding techniques for edible caterpillars. These caterpillars have high biological and nutritional value as a potential source of protein, in addition to providing various socio-economic benefits to local communities in Africa. We suggest that future research begins with identifying Lepidoptera species of which the larvae and caterpillars are consumed, but for which there is limited information on their bioecology. Based on Table 2, we propose five caterpillars (A. infracta, A. rectilinea, C. forda, C. butyrospermi, E. lactea, and I. belina) that should be investigated to integrate into production systems, as they are among the most consumed and valued caterpillar species. Among these species, the caterpillars A. infracta, A. rectilinea, and I. belina have more advantages in terms of characteristics related to seasonality and length of the development cycle (Table 2). Table 2 also shows that information on the life cycle of certain edible caterpillar species (B. alcinoe, I. obscura) is extremely limited. By collecting these data, future studies could help characterize the life cycle of these species and the phenology of associated host plants. Future studies should also explore how to develop edible caterpillar rearing programs by integrating socio-economic aspects. The commercial potential of edible caterpillar farming has already been demonstrated in terms of the exceptional opportunities it offers. However, the profitability of caterpillar farming is far from being confirmed. We look forward to future research focusing on elucidating and quantifying the costs and benefits that will accrue from edible caterpillar farms.
Finally, in the face of malnutrition and food shortages (current and predicted), which mainly characterize developing countries, the breeding of edible caterpillars could constitute a new source of sustainable food by addressing various expectations connected to the food security of local populations, thus contributing to the objectives of sustainable development.
Author Contributions
Writing—original draft, G.M.N.M.; resources, B.K.M.; supervision, B.K.M. and R.C.M.; funding acquisition, writing—review & editing, J.B. and B.K.M.; validation, F.F.; conceptualization, writing—review & editing, R.C.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Academy for Research and Higher Education (ARES)/Federation of French-speaking higher education establishments in Belgium/Projet de recherche pour le développement: PRD/Insectes.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
The study did not report any data.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank the Academy for Research and Higher Education (ARES) for providing us with the necessary financial resources to carry out our research. At the same time, the authors also thank the various members of the Functional and Evolutionary Entomology Unit of Gembloux Agro-Bio Tech of the University of Liège and those of the Department of Zootechnics of the University of Kinshasa for their support and encouragement.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Naumovski, N. Plenary lecture: Technology and food safety: Facts and myths of genetically modified food and organic food. In Proceedings of the Annual Scientific Sessions of the Nutrition Society of Sri Lanka, Colombo, Sri Lanka, 22–23 January 2022; pp. 40–42. [Google Scholar]
- Caparros Megido, R.; Alabi, T.; Larreché, S.; Alexandra, L.; Haubruge, E.; Francis, F. Risques et valorisation Des insectes dans l’alimentation humaine et animale. Ann. Société Entomol. De Fr. 2015, 51, 215–258. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D.; Waterhouse, G.I.N.; You, L.; Zhang, J.; Liu, Y.; Ma, L.; Gao, J.; Dong, Y. Transforming insect biomass into consumer wellntienchess foods: A Review. Food Res. Int. 2016, 89, 129–151. [Google Scholar] [CrossRef] [PubMed]
- Tiencheu, B.; Womeni, H.M. Entomophagy: Insects as food. In Insect Physiology and Ecology; InTech: London, UK, 2017; pp. 267–322. [Google Scholar]
- Kouame, K.; Koko, A.; Diomande, M.; Konate, I.; Assidjo, N. Caractérisation physicochimique de trois espèces de champignons sauvages comestibles couramment rencontrées dans la région du Haut-Sassandra (Côte d’Ivoire). J. Appl. Biosci. 2018, 121, 12110–12120. [Google Scholar] [CrossRef]
- Van Huis, A.; Van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Insectes Comestibles Perspectives Pour la Sécurité Alimentaire et L’alimentation Animale; FAO Forestry Paper; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013; ISBN 978-92-5-107595-1. [Google Scholar]
- van Huis, A. Insects as food in Sub-Saharan Africa. Insect Sci. Its Appl. 2003, 23, 163–185. [Google Scholar] [CrossRef]
- Gustafsson, J.; Cederberg, C.; Sonesson, U.; Emanuelsson, A. The Methodology of the FAO Study: “Global Food Losses and Food Waste—Extent, Causes and Prevention”; FAO: Rome, Italy, 2013; ISBN 978-91-7290-3234. [Google Scholar]
- Belluco, S.; Losasso, C.; Maggioletti, M.; Alonzi, C.C.; Paoletti, M.G.; Ricci, A. Edible Insects in a food safety and nutritional Perspective: A Critical Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 296–313. [Google Scholar] [CrossRef]
- Lavalette, M. Les Insectes: Une Nouvelle Ressource en Protéines Pour L’alimentation Humaine. Ph.D. Thesis, Université de Lorraine, Nancy, France, 2013. [Google Scholar]
- Rumpold, B.A.; Schlüter, O.K. Potential and challenges of insects as an innovative source for food and feed production. Innov. Food Sci. Emerg. Technol. 2013, 17, 1–11. [Google Scholar] [CrossRef]
- Enwemiwe, V.N.; Popoola, K.O.K. Edible Insects: Rearing methods and incorporation into commercial food products–A Critical Review. Int. J. Adv. Res. Publ. 2018, 2, 9. [Google Scholar]
- Baiano, A. Edible Insects: An overview on nutritional characteristics, safety, farming, production technologies, regulatory framework, and socio-economic and ethical implications. Trends Food Sci. Technol. 2020, 100, 35–50. [Google Scholar] [CrossRef]
- Musundire, R.; Ngonyama, D.; Chemura, A.; Ngadze, R.T.; Jackson, J.; Matanda, M.J.; Tarakini, T.; Langton, M.; Chiwona-Karltun, L. Stewardship of wild and farmed edible insects as food and feed in Sub-Saharan Africa: A perspective. Front. Vet. Sci. 2021, 8, 601386. [Google Scholar] [CrossRef]
- Skotnicka, M.; Karwowska, K.; Kłobukowski, F.; Borkowska, A.; Pieszko, M. Possibilities of the development of edible insect-based foods in Europe. Foods 2021, 10, 766. [Google Scholar] [CrossRef]
- Van Huis, A.; Oonincx, D.G.A.B. The Environmental sustainability of insects as food and feed. A Review. Agron. Sustain. Dev. 2017, 37, 43. [Google Scholar] [CrossRef]
- Raubenheimer, D.; Rothman, J.M. Nutritional ecology of entomophagy in humans and other primates. Annu. Rev. Entomol. 2013, 58, 141–160. [Google Scholar] [CrossRef] [PubMed]
- Stull, V.J.; Finer, E.; Bergmans, R.S.; Febvre, H.P.; Longhurst, C.; Manter, D.K.; Patz, J.A.; Weir, T.L. Impact of edible cricket consumption on gut microbiota in healthy adults, a double-blind, randomized crossover trial. Sci. Rep. 2018, 8, 10762. [Google Scholar] [CrossRef] [PubMed]
- Babarinde, S.A.; Mvumi, B.M.; Babarinde, G.O.; Manditsera, F.A.; Akande, T.O.; Adepoju, A.A. Insects in food and feed systems in Sub-Saharan Africa: The untapped potentials. Int. J. Trop. Insect Sci. 2021, 41, 1923–1951. [Google Scholar] [CrossRef]
- Balinga, M.P.; Mapunzu, P.M.; Moussa, J.B.; N’gasse, G. Contribution des Insectes de la Forêt à la Sécurité Alimentaire: L’exemple des Chenilles d’Afrique Centrale; FAO: Rome, Italy, 2004; pp. 66–81. [Google Scholar]
- Niassy, S.; Fiaboe, K.K.M.; Affognon, H.D.; Akutse, K.S.; Tanga, M.C.; Ekesi, S. African indigenous knowledge on edible insects to guide research and policy. J. Insects Food Feed 2016, 2, 161–170. [Google Scholar] [CrossRef]
- Vantomme, P.; Göhler, D.; Deckere-ziangba, F.N. Contribution of forest insects to food security and forest conservation: The example of caterpillars in central Africa. Wildl. Policy Brief. Number 2004, 3, 1–4. [Google Scholar]
- Gahukar, R.T. Edible Insects Collected from Forests for Family Livelihood and Wellness of Rural Communities: A Review. Glob. Food Secur. 2020, 25, 100348. [Google Scholar] [CrossRef]
- Raheem, D.; Carrascosa, C.; Oluwole, O.B.; Nieuwland, M.; Saraiva, A.; Millán, R.; Raposo, A. Traditional consumption of and rearing edible insects in Africa, Asia and Europe. Crit. Rev. Food Sci. Nutr. 2019, 59, 2169–2188. [Google Scholar] [CrossRef]
- Vantomme, P. Way forward to bring insects in the human food chain. J. Insects Food Feed 2015, 1, 121–129. [Google Scholar] [CrossRef]
- Spiegel, M.V.D.; Noordam, M.Y. Safety of novel protein sources (Insects, microalgae, seaweed, duckweed, and rapeseed) and legislative aspects for their application in food and feed production. Compr. Rev. Food Sci. Food Saf. 2013, 12, 662–678. [Google Scholar] [CrossRef]
- Ngute, A.S.K.; Dongmo, M.A.K.; Effa, J.A.M.; Ambombo Onguene, E.M.; Fomekong Lontchi, J.; Cuni-Sanchez, A. Edible caterpillars in central Cameroon: Host plants, value, harvesting, and availability. For. Trees Livelihoods 2020, 29, 16–33. [Google Scholar] [CrossRef]
- Mariod, A.A. African Edible Insects as Alternative Source of Food, Oil, Protein and Bioactive Components; Springer: Cham, Switzerland, 2020; ISBN 978-3-030-32952-5. [Google Scholar]
- Ramos-elorduy, J. Anthropo-Entomophagy: Cultures, evolution and sustainability. Entomol. Res. 2009, 39, 271–288. [Google Scholar] [CrossRef]
- Kusia, E.S.; Borgemeister, C.; Khamis, F.M.; Copeland, R.S.; Tanga, C.M.; Ombura, F.L.; Subramanian, S. Diversity, host plants and potential distribution of edible Saturniid caterpillars in Kenya. Insects 2021, 12, 600. [Google Scholar] [CrossRef] [PubMed]
- Malaisse, F.; Latham, P. Human consumption of Lepidoptera in Africa: An updated chronological list of references (370 Quoted!) with their ethnozoological analysis. Geo-Eco-Trop 2014, 38, 339–372. [Google Scholar]
- Bara, G.T.; Sithole, R.; Macheka, L. The mopane worm (Gonimbrasia Belina Westwood): A review of its biology, ecology and utilisation in Zimbabwe. J. Insects Food Feed 2022, 1–14. [Google Scholar] [CrossRef]
- Ande, A.T.; Fasoranti, J.O. Some aspects of the biology, foraging and defensive behaviour of the emperor moth caterpillar, Cirina Forda (Westwood). Int. J. Trop. Insect Sci. 1998, 18, 177–181. [Google Scholar] [CrossRef]
- Wongsorn, D.; Sirimungkararat, S.; Saksirirat, W. Improvement of Eri silkworm (Samia Ricini, D.) tolerance to high temperature and low humidity conditions by discontinuous regime. Songklanakarin J. Sci. Technol. 2015, 37, 401–408. [Google Scholar]
- Sekonya, J.G.; McClure, N.J.; Wynberg, R.P. New pressures, old foodways: Governance and access to edible mopane caterpillars, Imbrasia (Gonimbrasia) Belina, in the context of commercialization and environmental change in South Africa. Int. J. Commons 2020, 14, 139–153. [Google Scholar] [CrossRef]
- Cloutier, J. Insectes Comestibles En Afrique: Introduction à la Collecte, au Mode de Preparation et à la Consommation des Insectes; Agromisa, CTA Editions: Wageningen, Belgium, 2015; ISBN 978-90-8573-147-4. [Google Scholar]
- Rémy, D.A.; Hervé, B.B.; Sylvain, O.N. Study of some biological parameters of Cirina Butyrospermi Vuillet (Lepidoptera, Attacidae), an edible insect and shea Caterpillar (Butyrospermum Paradoxum Gaertn. F.) in a Context of climate change in Burkina Faso. Adv. Entomol. 2017, 6, 1–8. [Google Scholar] [CrossRef]
- Shuey, J.A.; Butte, J.V.C.; Ohio, O. Life history notes for the pallid emperor moth, Cirina Forda (Saturniidae) in Nigeria. J. Lejiidopterists Soc. 1997, 51, 269–273. [Google Scholar]
- Munyuli, T. Etude préliminaire orientée vers la production des chenilles consommables par l’élevage Des Papillons Anaphe Infracta (Thaumetopoeidae) à Lwiro, Sud-Kivu, République Démocratique Du Congo. Tropicultura 2000, 18, 208–211. [Google Scholar]
- Amadi, E.; Kiin Kabari, D. Nutritional composition and microbiology of some edible insects commonly eaten in Africa, hurdles and future prospects: A Critical Review. J. Food Microbiol. Saf. Hyg. 2016, 1, 1–7. [Google Scholar] [CrossRef]
- Mabossy-Mobouna, G.; Malaisse, F.; Richel, A.; Maesen, P.; Latham, P.; Roulon-Doko, P.; Madamo, F.; Lognay, G. Imbrasia Obscura, an edible caterpillar of tropical Africa: Chemical composition and Nutritional Value. Tropicultura 2018, 36, 798–811. [Google Scholar]
- Muya, G.M.N.; Kambashi, B.M.; Bindelle, J.; Frédéric, F.; Megido, R.C. Description of the development Cycle of Aegocera Rectilinea (Lepidoptera: Noctuidae), a caterpillar consumed in western Democratic Republic of Congo. J. Insects Food Feed 2021, 8, 439–446. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schlüter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef] [PubMed]
- Malaisse, F. Se Nourrir en forêt claire africaine. Approche écologique et nutritionnelle. In Nature Sciences Société; CTA Editions: Gembloux, Belgium, 1997; Volume 88, p. 1007. [Google Scholar]
- Womeni, H.M.; Tiencheu, B.; Mbiapo, F.T.; Linder, M.; Fanni, J.; Parmentier, M.; Villeneuve, P. Oils of insects and larvae consumed in Africa: Potential sources of polyunsaturated fatty acids. OCL Ol. Corps Gras Lipides 2009, 16, 230–235. [Google Scholar] [CrossRef]
- Chen, X.; Feng, Y.; Chen, Z. Common edible insects and their utilization in China. Entomol. Res. 2009, 39, 299–303. [Google Scholar] [CrossRef]
- Bukkens, S.G.F. The Nutritional value of Edible Insects. Ecol. Food Nutr. 1997, 36, 287–319. [Google Scholar] [CrossRef]
- Oibiokpa, F.I.; Akanya, H.O.; Jigam, A.A.; Saidu, A.N.; Egwim, E.C. Protein quality of four indigenous edible insect species in Nigeria. Food Sci. Hum. Wellness 2018, 7, 175–183. [Google Scholar] [CrossRef]
- Moyo, S.; Masika, P.J.; Muchenje, V. The potential of Imbrasia belina worm as a poultry and fish feed. A Review. J. Anim. Feed Sci. 2019, 28, 209–219. [Google Scholar] [CrossRef]
- Akinnawo, O.; Ketiku, A.O. Chemical Composition and Fatty Acid Profile of Edible Larva of Cirina Forda (Westwood). Afr. J. Biomed. Res. 2000, 3, 93–96. [Google Scholar] [CrossRef]
- Mlcek, J.; Rop, O.; Borkovcova, M.; Bednarova, M. A Comprehensive look at the possibilities of edible insects as food in Europe—A Review. Pol. J. Food Nutr. Sci. 2014, 64, 147–157. [Google Scholar] [CrossRef]
- Anvo, P.M.; Toguyéni, A.; Otchoumou, A.K.; Zoungrana-Kaboré, C.Y.; Koumelan, E.P. Nutritional qualities of edible caterpillars Cirina butyrospermi in southwestern of Burkina Faso. Int. J. Innov. Appl. Stud. 2016, 18, 639–645. [Google Scholar]
- Langrenez, L.; Messier, J. Insectes Comestibles; Privateley Published: Paris, France, 2017. [Google Scholar]
- Nantanga, K.K.M.; Amakali, T. Diversification of mopane caterpillars (Gonimbrasia Belina) Edible forms for improved livelihoods and food security. J. Arid Environ. 2020, 177, 104148. [Google Scholar] [CrossRef]
- Millward, D.J.; Layman, D.K.; Tomé, D.; Schaafsma, G. Protein quality assessment: Impact of expanding understanding of protein and amino acid needs for optimal health. Am. J. Clin. Nutr. 2008, 87, 1576S–1581S. [Google Scholar] [CrossRef] [PubMed]
- Banjo, A.D.; Lawal, O.A.; Songonuga, E.A. The nutritional value of fourteen species of edible insects in Southwestern Nigeria. Afr. J. Biotechnol. 2006, 5, 298–301. [Google Scholar] [CrossRef]
- Okangola, E.; Solomo, E.; Tchatchambe, W.B.; Mate, M.; Upoki, A.; Dudu, A.; Asimonyio, J.A.; Bongo, G.N.; Pius, T. Valeurs nutritionnelles des chenilles comestibles de la ville de Kisangani et ses environs (Province de la Tshopo, République Démocratique Du Congo). Int. J. Inov. Sci. Res. 2016, 25, 10. [Google Scholar]
- Lautenschläger, T.; Neinhuis, C.; Kikongo, E.; Henle, T.; Förster, A. Impact of different Preparations on the Nutritional value of the edible caterpillar Imbrasia Epimethea from northern Angola. Eur. Food Res. Technol. 2017, 243, 1–10. [Google Scholar] [CrossRef]
- Kanga-Kanga, M.R.; Mulungu-Lungu, N.D.; Mpanda, G.N.; L’kisaten, H.M.; Kiyula, F.M.; Kalaka, C.; Kasumpa, D.B.; Tshovu, D.N.; Sumba, J.K. Valeur nutritionnelle des chenilles comestibles de la ville de Lubumbashi (Province du Haut-Katanga, R.D.C.). Int. J. Innov. Appl. Stud. 2018, 24, 6. [Google Scholar]
- Justin, B. Nutritional quality evaluation of complementary foods flour based on edible caterpillars: Bunaeopsis aurantiaca, Imbrasia oyemensis and Cirina forda eaten in south Kivu province, eastern D.R. Ccongo. Anal. Food Sci. Technol. 2019, 20, 18. [Google Scholar]
- Kim, T.K.; Yong, H.I.; Kim, Y.B.; Kim, H.W.; Choi, Y.S. Edible insects as a protein source: A review of public perception, processing technology, and research trends. Food Sci. Anim. Resour. 2019, 39, 521–540. [Google Scholar] [CrossRef] [PubMed]
- Hlongwane, Z.T.; Slotow, R.; Munyai, T.C. Nutritional Composition of Edible Insects Consumed in Africa: A Systematic Review. Nutrients 2020, 12, 2786. [Google Scholar] [CrossRef] [PubMed]
- Ozimek, L.; Sauer, W.C.; Kozikowski, V.; Ryan, J.K.; Jørgensen, H.; Jelen, P. Nutritive value of protein extracted from honey Bees. J. Food Sci. 1985, 50, 1327–1329. [Google Scholar] [CrossRef]
- Dušková, J.; Tishchenko, G.; Ponomareva, E.; Šimůnek, J.; Koppová, I.; Skálová, T.; Štěpánková, A.; Hašek, J.; Dohnálek, J. Chitinolytic enzymes from bacterium inhabiting human gastrointestinal Tract—Critical parameters of protein isolation from anaerobic culture. Acta Biochim. Pol. 2011, 58, 2275. [Google Scholar] [CrossRef]
- Jantzen da Silva Lucas, A.; Menegon de Oliveira, L.; da Rocha, M.; Prentice, C. Edible Insects: An alternative of nutritional, functional and bioactive compounds. Food Chem. 2020, 311, 126022. [Google Scholar] [CrossRef]
- Ladrón de Guevara, O.; Padilla, P.; García, L.; Pino, J.M.; Ramos-Elorduy, J. Amino acid determination in some edible mexican insects. Amino Acids 1995, 9, 161–173. [Google Scholar] [CrossRef]
- DeFoliart, G.R. Insects as Human Food: Gene DeFoliart discusses some nutritional and economic aspects. Crop Prot. 1992, 11, 395–399. [Google Scholar] [CrossRef]
- Moyo, S.; Jaja, I.F.; Mopipi, K.; Masika, P.; Muchenje, V. Effect of graded levels of Imbrasia belina meal on blood lipid profile, bone morphometric and mineral content of broiler chickens. Anim. Feed Sci. Technol. 2021, 271, 114736. [Google Scholar] [CrossRef]
- Li-Chan, E.C. Bioactive peptides and protein hydrolysates: Research trends and challenges for application as nutraceuticals and functional food ingredients. Curr. Opin. Food Sci. 2015, 1, 28–37. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Colletti, A. Potential role of bioactive peptides in prevention and treatment of chronic diseases: A narrative review: Bioactive peptides effects. Br. J. Pharmacol. 2017, 174, 1378–1394. [Google Scholar] [CrossRef]
- Vercruysse, L.; Smagghe, G.; Beckers, T.; Camp, J.V. Antioxidative and ACE inhibitory activities in enzymatic hydrolysates of the cotton leafworm, Spodoptera littoralis. Food Chem. 2009, 114, 38–43. [Google Scholar] [CrossRef]
- Lemes, A.; Sala, L.; Ores, J.; Braga, A.; Egea, M.; Fernandes, K. A Review of the latest advances in encrypted bioactive peptides from protein-rich Waste. Int. J. Mol. Sci. 2016, 17, 950. [Google Scholar] [CrossRef] [PubMed]
- Jakubczyk, A.; Karaś, M.; Rybczyńska-Tkaczyk, K.; Zielińska, E.; Zieliński, D. Current trends of bioactive peptides—new sources and therapeutic effect. Foods 2020, 9, 846. [Google Scholar] [CrossRef] [PubMed]
- Fogang Mba, A.R.; Kansci, G.; Viau, M.; Rougerie, R.; Genot, C. Edible Caterpillars of Imbrasia truncata and Imbrasia epimethea contain lipids and proteins of high potential for nutrition. J. Food Compos. Anal. 2019, 79, 70–79. [Google Scholar] [CrossRef]
- Mabossy-Mobouna, G.; Kinkela, T.; Lenga, A.; Malaisse, F. Imbrasia truncata Aurivillius (Saturniidae): Importance en afrique centrale, commercialisation et valorisation à Brazzaville. Geo-Eco-Trop 2013, 37, 313–330. [Google Scholar]
- Atowa, C.O.; Okoro, B.C.; Umego, E.C.; Atowa, A.O.; Emmanuel, O.; Ude, V.C.; Ugbogu, E.A. Nutritional values of Zonocerus variegatus, Macrotermes bellicosus and Cirina Forda insects: Mineral composition, fatty acids and amino acid profiles. Sci. Afr. 2021, 12, e00798. [Google Scholar] [CrossRef]
- Rapatsa, M.M.; Moyo, N.A.G. Evaluation of Imbrasia belina meal as a fishmeal substitute in Oreochromis mossambicus diets: Growth performance, histological analysis and enzyme activity. Aquac. Rep. 2017, 5, 18–26. [Google Scholar] [CrossRef]
- Foua Bi, F.; Meite, A.; Dally, T.; Ouattara, H.; Kouame, K.; Kati-Coulibaly, S. Étude de la qualité biochimique et nutritionnelle de la poudre séchée d’Embrasai oyemensis, chenilles consommées au Centre-Ouest de la Côte d’Ivoire. J. App. Biosci. 2015, 96, 9039. [Google Scholar] [CrossRef]
- Anvo, M.P.M.; Aboua, B.R.D.; Compaoré, I.; Sissao, R.; Zoungrana-Kaboré, C.Y.; Kouamelan, E.P.; Toguyéni, A. Fish meal replacement by Cirina butyrospermi caterpillar’s meal in practical diets for Clarias Gariepinus Fingerlings. Aquac. Res. 2017, 48, 5243–5250. [Google Scholar] [CrossRef]
- Dooshima Igbabul, B. Nutritional and microbial quality of dried larva of Cirina forda. Int. J. Nutr. Food Sci. 2014, 3, 602. [Google Scholar] [CrossRef]
- Olaleye, A.A. Amino acid profiles of five commonly consumed insects in southwestern Nigeria. Carpathian J. Food Sci. Technol. 2021, 12, 42–51. [Google Scholar] [CrossRef]
- Séré, A.; Bougma, A.; Bazié, B.S.R.; Traoré, E.; Parkouda, C.; Gnankiné, O.; Bassolé, I.H.N. Chemical composition, energy and nutritional values, digestibility and functional properties of defatted flour, protein concentrates and isolates from Carbula marginella (Hemiptera: Pentatomidae) and Cirina Butyrospermi (Lepidoptera: Saturniidae). BMC Chem. 2021, 15, 46. [Google Scholar] [CrossRef]
- Durst, P.B.; Johnson, D.V.; Leslie, R.N.; Shono, K. Forest Insects as Food: Humans Bite Back; RAP Publication: Rockville, MD, USA, 2010; ISBN 9789251064887. [Google Scholar]
- Kouřimská, L.; Adámková, A. Nutritional and sensory quality of edible insects. NFS J. 2016, 4, 22–26. [Google Scholar] [CrossRef]
- Nowakowski, A.C.; Miller, A.C.; Miller, M.E.; Xiao, H.; Wu, X. Potential health benefits of edible insects. Crit. Rev. Food Sci. Nutr. 2021, 62, 3499–3508. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; Laurent, S.; Veenenbos, M.E.; van Loon, J.J.A. Dietary enrichment of edible insects with omega 3 fatty acids. Insect Sci. 2019, 27, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Motshegwe, S.M.; Holmback, J.; Yeboah, S.O. General properties and the fatty acid composition of the oil from the mophane caterpillar, Imbrasia Belina. JAOCS J. Am. Oil Chem. Soc. 1998, 75, 725–728. [Google Scholar] [CrossRef]
- Zinzombe, M.; Georges, S. Larval lipid quality of lepidoptera: Gonimbrasia Belina. Botsw. Notes Rec. 1994, 26, 167–173. [Google Scholar]
- Aiko, Y.B.; Jacob, J.O.; Salihu, S.O.; Dauda, B.E.N.; Suleiman, M.A.T.; Akanya, H.O. Fatty acid and amino acid profile of emperor moth caterpillar (Cirina Forda) in Paikoro Local Government Area of Niger State, Nigeria. Am. J. Biochem. 2014, 2014, 29–34. [Google Scholar] [CrossRef]
- Oriolowo, O.B.; Abubakar, D.S.; Bidda, R.D.; Masa’udu, S. Nutritional comparison of the pallid emperor moth, Cirina Forda and the atlantic mackerel, scombrus scomber. Acta Entomol. Zool. 2021, 2, 24–31. [Google Scholar] [CrossRef]
- Pennino, M.; Dierenfeld, E.S.; Behler, J.L. Retinol, α-Tocopherol and proximate nutrient composition of invertebrates used as feed. Int. Zoo Yearb. 1991, 30, 143–149. [Google Scholar] [CrossRef]
- Mwangi, M.N.; Oonincx, D.G.A.B.; Stouten, T.; Veenenbos, M.; Melse-Boonstra, A.; Dicke, M.; van Loon, J.J.A. Insects as sources of iron and zinc in human nutrition. Nutr. Res. Rev. 2018, 31, 248–255. [Google Scholar] [CrossRef] [PubMed]
- van Huis, A. Potential of insects as food and feed in assuring food security. Annu. Rev. Entomol. 2013, 58, 563–583. [Google Scholar] [CrossRef] [PubMed]
- Mabossy-Mobouna, G.; Bouyer, T.; Latham, P.; Roulondoko, P.; Konda Ku Mbuta, A.; Malaisse, F. Preliminary knowledge for breeding edible caterpillars in congo-brazzaville. Geo-Eco-Trop 2016, 40, 145–174. [Google Scholar]
- Mbata, K.J.; Chidumayo, E.N.; Lwatula, C.M. Traditional regulation of edible caterpillar exploitation in the kopa area of mpika district in northern zambia. J. Insect Conserv. 2002, 6, 16. [Google Scholar] [CrossRef]
- Lisingo, J.; Wetsi, J.L.; Ntahobavuka, H. Enquête sur les chenilles comestibles et les divers usages de leurs plantes hôtes dans les districts de Kisangani et de la Tshopo ( R.D.Congo ). Geo-Eco-Trop J. 2010, 34, 139–146. [Google Scholar]
- Benneker, C.; Assumani, D.M.; Maindo, A.; Kimbuani, G.; Lescuyer, G.; Esuka, J.C.; Kasongo, E. Exploitation artisanale de bois d’oeuvre En RD Congo: Secteur porteur d’espoir pour le développement des petites et moyennes entreprises; Tropenbos International RD Congo: Kisangani, Democratic Republic of the Congo, 2012; ISBN 978-90-5113-109-3. [Google Scholar]
- Bomolo, O.; Niassy, S.; Chocha, A.; Longanza, B.; Bugeme, D.M.; Ekesi, S.; Tanga, C.M. Ecological diversity of edible insects and their potential contribution to household food security in Haut-Katanga province, Democratic Republic of Congo. Afr. J. Ecol. 2017, 55, 640–653. [Google Scholar] [CrossRef]
- Latham, P. Les Chenilles Comestibles et Leurs Plantes Nourricières dans la Province du Bas-Congo, 3rd ed.; Privateley Publised: Paris, France, 2015; ISBN 9780955420863. [Google Scholar]
- Gowdey, C.C. On the utilisation of an indigenous african silk-worm ( Anaphe Infracta , Wlsm.) in Uganda. Bull. Entomol. Res. 1912, 3, 269–274. [Google Scholar] [CrossRef]
- Tchibozo, S.; Malaisse, F.; Mergen, P. Insectes consommés par l’homme en Afrique Occidentale Francophone. Geo-Eco-Trop 2016, 40, 105–114. [Google Scholar]
- Agbidye, F.S.; Nongo, N.N. Harvesting and processing techniques for the larvae of the pallid emperor moth, Cirina Forda Westwood (Lepidoptera: Saturniidae), among the Tiv people of Benue State, Nigeria. J. Res. For. Wildl. Environ. 2009, 1, 123–132. [Google Scholar]
- Bocquet, E.; Maniacky, J.; Vermeulen, C.; Malaisse, F. A Propos de quelques chenilles consommées par les mongo en province de l’Équateur (République Démocratique Du Congo). Geo-Eco-Trop 2020, 44, 109–130. [Google Scholar]
- Payne, C.; Badolo, A.; Sagnon, B.; Cox, S.; Pearson, S.; Sanon, A.; Bationo, F.; Balmford, A. Effects of defoliation by the edible caterpillar “Chitoumou” (Cirina Butyrospermi) on harvests of shea (Vitellaria Paradoxa) and growth of maize (Zea Mays). Agrofor. Syst. 2020, 94, 231–240. [Google Scholar] [CrossRef]
- Muvatsi, P.; Kahindo, J.M.; Snook, L.K. Can the production of wild forest foods be sustained in timber concessions? logging and the availability of edible caterpillars hosted by Sapelli (Entandrophragma Cylindricum) and Tali (Erythrophleum Suaveolens) trees in the Democratic Republic of Congo. For. Ecol. Manag. 2018, 410, 56–65. [Google Scholar] [CrossRef]
- Ramos-Elorduy, J.; Moreno, J.M.P.; Vázquez, A.I.; Landero, I.; Oliva-Rivera, H.; Camacho, V.H.M. Edible Lepidoptera in Mexico: Geographic distribution, ethnicity, economic and nutritional importance for rural people. J. Ethnobiol. Ethnomed. 2011, 7, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Yen, A.L. Insects as food and feed in the asia pacific region: Current perspectives and future directions. J. Insects Food Feed 2015, 1, 33–55. [Google Scholar] [CrossRef]
- Akpalu, W.; Muchapondwa, E.; Zikhali, P. Can the restrictive harvest period policy conserve mopane worms in southern Africa? A bioeconomic modelling approach. Environ. Dev. Econ. 2009, 14, 587–600. [Google Scholar] [CrossRef]
- Illgner, P.; Nel, E. The geography of edible insects in Sub-Saharan Africa: A study of the mopane caterpillar. Geogr. J. 2000, 166, 336–351. [Google Scholar] [CrossRef]
- Vega, F.; Kaya, H. Insect Pathology; Academic Press: London, UK, 2012. [Google Scholar]
- Rothschild, M.; Reichstein, T.; Von Euw, J.; Aplin, R.; Harman, R.R.M. Toxic Lepidoptera. Toxicon 1970, 8, 293–296. [Google Scholar] [CrossRef]
- Nishida, R. Sequestration of defensive substances from plants by Lepidoptera. Annu. Rev. Entomol. 2002, 47, 57–92. [Google Scholar] [CrossRef]
- Mutungi, C.; Irungu, F.G.; Nduko, J.; Mutua, F.; Affognon, H.; Nakimbugwe, D.; Ekesi, S.; Fiaboe, K.K.M. Postharvest Processes of Edible Insects in Africa: A review of processing methods, and the implications for nutrition, safety and new products development. Crit. Rev. Food Sci. Nutr. 2019, 59, 276–298. [Google Scholar] [CrossRef]
- Omotoso, O.T. Nutritional quality, functional properties and anti-nutrient compositions of the larva of Cirina Forda (Westwood) (Lepidoptera: Saturniidae). J. Zhejiang Univ. Sci. B 2006, 7, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Dauda, B. Nutritive and anti-nutritive Composition of locust bean tree emperor moth larvae Bunaea Alcinoe (Lepidoptera-Saturniidae Stoll 1780) from Gurara Local Government Area, Niger State, Nigeria. JSRR 2014, 3, 1771–1779. [Google Scholar] [CrossRef] [PubMed]
- Nishimune, T.; Watanabe, Y.; Okazaki, H.; Akai, H. Thiamin is decomposed due to anaphe spp. entomophagy in seasonal ataxia patients in Nigeria. J. Nutr. 2000, 130, 1625–1628. [Google Scholar] [CrossRef] [PubMed]
- Gordon, E. Investigations of the Source, Distribution, Expression and Physiological Function of Thiaminase I; Cornell University: Ithaca, NY, USA, 2012. [Google Scholar]
- Zhou, J.; Han, D. Safety evaluation of protein of silkworm (Antheraea Pernyi) pupae. Food Chem. Toxicol. 2006, 44, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Amisi, M.F.; Héritier, U.S.; Paul, M.; Georges, A.L.; Innocent, B.K.; Pascal, I.M. Valorisation de la chenille comestible Bunaeopsis aurantiaca dans la gestion communautaire des forêts du Sud-Kivu (République Démocratique du Congo). VertigO Rev. Électronique Sci. L’environ. 2013. [Google Scholar] [CrossRef]
- Noutcheu, R.; Snook, L.K.; Tchatat, M.; Taedoumg, H.; Tchingsabe, O.; Tieguhong, J.C. Do Logging concessions decrease the availability to villagers of foods from timber trees? A quantitative analysis for moabi (Baillonella Toxisperma), Sapelli (Entandrophragma Cylindricum) and Tali (Erythrophleum Suaveolens) in Cameroon. For. Ecol. Manag. 2016, 381, 279–288. [Google Scholar] [CrossRef]
- McGregor, J. Gathered produce in zimbabwe’s communal areas changing resource availability and use. Ecol. Food Nutr. 1995, 33, 163–193. [Google Scholar] [CrossRef]
- Kenis, M.; Sileshi, G.; Mbata, K.; Chidumayo, E.; Meke, G.; Muatinte, B. Towards conservation and sustainable utilization of edible caterpillars of the Miombo. In Presentation to the SIL Annual Conference on Trees for Poverty Alleviation; Cabi: Paris, France, 2006; Volume 9. [Google Scholar]
- Eckebil, P.P.T.; Verheggen, F.; Doucet, J.-L.; Malaisse, F.; Daïnou, K.; Cerutti, P.O.; Vermeulen, C. Entandrophragma cylindricum (Sprague) Sprague (Meliaceae), une espèce ligneuse concurrentielle en Afrique centrale (synthèse bibliographique). Biotechnol. Agron. Soc. Environ. 2017, 18. [Google Scholar] [CrossRef]
- De Foliart, G.R. Edible Insects as minilivestock. Biodivers. Conserv. 1995, 4, 306–321. [Google Scholar] [CrossRef]
- De Foliart, G.R. An overview of the role of edible insects in preserving biodiversity. Ecol. Food Nutr. 1997, 36, 109–132. [Google Scholar] [CrossRef]
- Leleup, N.; Daems, H. Les chenilles alimentaires du Kwango. Causes de leur Raréfaction et mesures préconisées pour y remédier. J. D’agric. Trop. Bot. Appliquée 2014, 16, 1–21. [Google Scholar] [CrossRef]
- Syampungani, S.; Chirwa, P.W.; Akinnifesi, F.K.; Sileshi, G.; Ajayi, O.C. The Miombo woodlands at the cross roads: Potential threats, sustainable livelihoods, policy gaps and challenges. Nat. Resour. Forum 2009, 33, 150–159. [Google Scholar] [CrossRef]
- Govorushko, S. Global Status of insects as food and feed source: A review. Trends Food Sci. Technol. 2019, 91, 436–445. [Google Scholar] [CrossRef]
- Melgar-Lalanne, G.; Hernández-Álvarez, A.-J.; Salinas-Castro, A. Edible insects processing: Traditional and innovative technologies. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1166–1191. [Google Scholar] [CrossRef] [PubMed]
- Birari, V.V.; Siddhapara, M.R.; Patel, D.H. Biology of eri silkworm, Samia Ricini ( Donovan ) on castor, Ricinus Communis L. Entomon 2019, 44, 229–234. [Google Scholar] [CrossRef]
- Van Itterbeeck, J.; van Huis, A. Environmental manipulation for edible insect procurement: A historical perspective. J Ethnobiol. Ethnomedicine 2012, 8, 3. [Google Scholar] [CrossRef]
- Ngoka, B.M.; Kioko, E.N.; Raina, S.K.; Mueke, J.M.; Kimbu, D.M. Semi-captive rearing of the african wild silkmoth Gonometa Postica (Lepidoptera: Lasiocampidae) on an indigenous and a non-indigenous host plant in Kenya. JTI 2007, 27, 183. [Google Scholar] [CrossRef]
- Hartland-Rowe, R. The biology of the wild silk moth Gonometa Rufobrunnea Aurivillius (Lasiocampidae) in northeastern Botswana, with some comments on its potential as a source of wild silk. Botsw. Notes Rec. 1992, 24, 11. [Google Scholar]
- Ghazoul, J. Mopane Woodlands and the Mopane Worm: Enhancing Rural Livelihoods and Resource Sustainability. Wild. Fish. Manag. 2006. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).