Effect of Cry Toxins on Xylotrechus arvicola (Coleoptera: Cerambycidae) Larvae
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. X. arvicola Collection and Rearing
2.2. Cry Proteins Preparation
2.3. Bioassays of Cry Proteins Sprayed on Artificial Diet to Rear X. arvicola Larvae
2.4. Statistical Analysis
3. Results
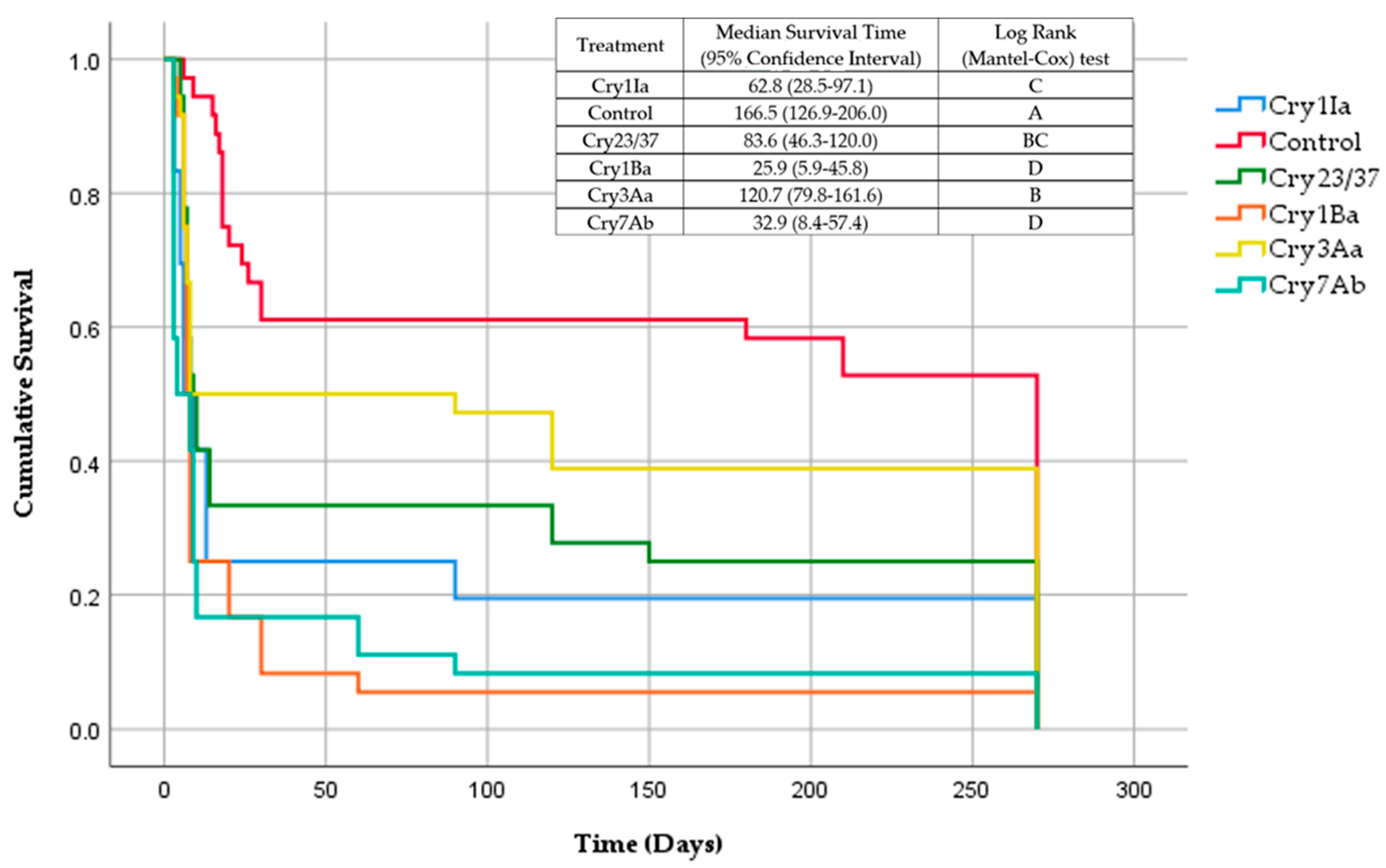
3.1. Development of X. arvicola Larvae Treated with Cry Proteins during 9 Months
3.2. Development Time of Pupal Stages and Adults’ Lifespan in Days after the Cry Protein Treatments
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Monné, M.L.; Monné, M.A.; Wang, Q. General morphology, classification and biology of Cerambycidae. In Cerambycidae of the World; Wang, Q., Ed.; Biology and Pest Management; CRC Press: Boca Raton, FL, USA, 2017; p. 642. [Google Scholar]
- Tavakilian, G.; Chevillotte, H. TITAN: Cerambycidae Database. Available online: https://titan.gbif.fr/ (accessed on 6 May 2020).
- Bezark, L.G. Checklist of the Oxypeltidae, Vesperidae, Disteniidae and Cerambycidae, (Coleoptera) of the Western Hemisphere. Available online: https://bezbycids.com/byciddb/wdefault.asp?w=n (accessed on 20 May 2020).
- Hanks, L.M. Influence of the larval host plant on reproductive strategies of cerambycid beetles. Ann. Rev. Entomol. 1999, 44, 483–505. [Google Scholar] [CrossRef]
- Cavey, J.F. Solid Wood Packaging Material from China. Initial Pest Risk Assessment on Certain Wood Boring Beetles Known to Be Associated with Cargo Shipments: Asian Longhorned Beetle (Anoplophora glabripennis), Ceresium, Monochamus, and Hesperophanes; National Identification Service: Riverdale, MD, USA, 1988. [Google Scholar]
- Cocquempot, C. Alien longhorned beetles (Coleoptera Cerambycidae): Original interceptions and introductions in Europe, mainly France, and notes about recently imported species. Redia 2006, 89, 35–50. [Google Scholar]
- Grebennikov, V.V.; Gill, G.D.; Vigneault, R. Trichoferus campestris (Faldermann) (Coleoptera: Cerambycidae), an Asian woodboring beetle recorded in North America. Coleopt. Bull. 2010, 64, 13–20. [Google Scholar] [CrossRef]
- Olmi, M. Un caso Piemontese di gradazione di Vesperus strepens Fabricius in. e reimpiego di una vecchia tecnica insetticida. Studi del Gruppo di Lavoro, C.N.R. per la lotta integrata contro i nemici animali delle Piante. 130—Ann. Della Fac. Di Sci. Agrar. Dell’università Di Torino. Vitis 1974, 9, 347–360. [Google Scholar]
- Jacquelin Du Val, C. Note sur le male du Vesperus xatarti Mulsant. Ann. Soc. Entomol. Fr. 1850, 8, 347–349. [Google Scholar]
- Mendizábal, M. Notas para un Estudio de Las Especies Españolas del Género Vesperus; Publicaciones del Ministerio de Agricultura; Sección de Fitopatología; Instituto de Investigaciones Agronómicas: Madrid, Spain, 1937. [Google Scholar]
- Ruiz Castro, A. Fauna Entomológica De La Vid en España. Estudio Sistemático-Biológico De Las Especies De Mayor Importancia Económica; IV (Coleoptera); CSIC: Madrid, Spain, 1943. [Google Scholar]
- Domínguez García-Tejero, F. Plagas Y Enfermedades De Las Plantas Cultivadas; Mundi-Prensa: Madrid, Spain, 2004. [Google Scholar]
- Baggiolini, M.; Epard, S. Un nouveau ravageur de la vigne, le Clyte (Clytus arietis L.). Agric. Romande 1968, 7–8, 91–92. [Google Scholar]
- Goodwin, S. A new strategy for the chemical control of fig longicorn, Acalolepta vastator (Newman) (Coleoptera: Cerambycidae), infesting grapevine. Aust. J. Entomol. 2005, 44, 170–174. [Google Scholar] [CrossRef]
- Armendáriz, I.; Juárez, J.S.; Campillo, G.; Miranda, L.; Pérez-Sanz, A. Daños mecánicos producidos por Xylotrechus arvicola (Olivier, 1875) (Coleoptera, Cerambycidae). Boletin de Sanidad Vegetal Plagas 2008, 34, 477–483. [Google Scholar]
- Sakai, T.; Nakagawa, J.; Takahashi, J.; Iwabuchi, K.; Ishii, K. Isolation and identification of the male sex pheromone of the grape borer Xylotrechus pyrrhoderus Bates (Coleoptera: Cerambycidae). Chem. Lett. 1984, 13, 263–264. [Google Scholar] [CrossRef]
- Ocete, R.; López-Martínez, M.A.; Prendes, C.; Lorenzo, C.D.; González-Andújar, J.L. Relación entre la infestación de Xylotrechus arvicola (Coleoptera: Cerambycidae) (Olivier) y la presencia de hongos patógenos en un viñedo de la Denominación de Origen “La Mancha”. Boletin de Sanidad Vegetal Plagas 2002, 28, 97–102. [Google Scholar]
- Peláez, H.; Hernández, J.M.; Martín, M.C.; Moreno, C.M.; Santiago, Y. Determinación De Las Características Del Huevo de Xylotrechus arvicola (Coleoptera: Cerambycidae, Olivier, 1795). In Libro de Actas del X Congreso Ibérico de Entomología; de Diputación, Z., Ed.; Castilla y León: Zamora, Spain, 2002; p. 52. [Google Scholar]
- Rodríguez-González, A.; Peláez, H.J.; Mayo, S.; González-López, O.; Casquero, P.A. Biometric traits of Xylotrechus arvicola adults from laboratory and grape field. Vitis 2016, 55, 73–78. [Google Scholar]
- Rodríguez-González, A.; Peláez, H.J.; González-López, O.; Mayo, S.; Casquero, P.A. Reproductive patterns of Xylotrechus arvicola (Coleoptera: Cerambycidae), an emerging pest of grapevines, under laboratory conditions. J. Econ. Entomol. 2016, 109, 1226–1230. [Google Scholar] [CrossRef]
- Moreno, C.M. Xylotrechus arvicola (Olivier 1795) (Coleóptera: Cerambycidae): Descripción Morfológica, Ciclo Biológico, Incidencia y Daños en El Cultivo De La Vid. Ph.D. Thesis, Publicaciones del Instituto Tecnológico Agrario de Castilla y León (ITACYL), Valladolid, Spain, 2005. [Google Scholar]
- Rodríguez-González, A.; Peláez, H.J.; González-Núñez, M.; Casquero, P.A. Control on egg and neonate larvae of Xylotrechus arvicola (Coleoptera: Cerambycidae), a new vineyard pest, under laboratory conditions. Aust. J. Grape Wine Res. 2017, 23, 112–119. [Google Scholar] [CrossRef]
- García-Ruiz, E.; Marco, V.; Pérez-Moreno, I. Laboratory rearing and life history of an emerging grape pest, Xylotrechus arvicola (Coleoptera: Cerambycidae). Bull. Entomol. Res. 2012, 102, 89–96. [Google Scholar] [CrossRef]
- García-Ruiz, E. Contribución al Manejo de Plagas en vid: Xylotrechus arvicola y Lobesia botrana Denis & Schiffermüller (Lepidoptera: Tortricidae). Ph.D. Thesis, University of La Rioja, Logroño, Spain, 2009. [Google Scholar]
- Soria, F.J.; López, M.A.; Pérez, M.A.; Maistrello, L.; Armendáriz, I.; Ocete, R. Predictive model for the emergence of Xylotrechus arvicola (Coleoptera: Cerambycidae) in La Rioja vineyards (Spain). Vitis 2013, 52, 91–96. [Google Scholar]
- Biurrun, R.; Yanguas, R.; Garnica, I.; Benito, A. Xylotrechus arvicola. El taladro del endrino. Navar. Agrar. 2007, 164, 47–51. [Google Scholar]
- Rodríguez-González, A.; Casquero, P.A.; García-González, J.; Rodríguez-Robles, D.; Morán-Del Pozo, J.M.; Juan-Valdés, A. Analysis of the mechanical properties of wood attacked by Xylotrechus arvicola larvae, and its influence on the structural properties of the plant. Vitis 2019, 58, 105–112. [Google Scholar]
- Rodríguez-González, A.; Peláez, H.J.; Mayo, S.; González-López, O.; Casquero, P.A. Egg development and toxicity of insecticides to eggs, neonate larvae and adults of Xylotrechus arvicola, a pest in Iberian grapevines. Vitis 2016, 55, 83–93. [Google Scholar]
- Rodríguez-González, A.; Mayo, S.; González-López, O.; Peláez, H.J.; Casquero, P.A. Response to rearing in laboratory of the xylophagous grape pest, Xylotrechus arvicola (Coleoptera: Cerambycidae). Entomol. Res. 2017, 47, 235–242. [Google Scholar] [CrossRef]
- Hannay, C.L. Crystalline inclusions in aerobic spore forming Bacteria. Nature 1953, 172, 1004. [Google Scholar] [CrossRef]
- Wei, J.Z.; Hale, K.; Carta, L.; Platzer, E.; Wong, C.; Fang, S.C.; Aroian, R.V. Bacillus thuringiensis cristal proteins that target nematodes. Proc. Natl. Acad. Sci. USA 2003, 100, 2760–2765. [Google Scholar] [CrossRef]
- Palma, L.; Muñoz, D.; Berry, C.; Murillo, J.; Caballero, P. Bacillus thuringiensis toxins: An overview of their Biocidal activity. Toxins 2014, 6, 3296–3325. [Google Scholar] [CrossRef]
- Iatsenko, I.; Nikolov, A.; Sommer, R.J. Identification of distinct Bacillus thuringiensis 4A4 nematicidal factors using the model nematodes Pristionchus pacificus and Caenorhabditis elegans. Toxins 2014, 6, 2050–2063. [Google Scholar] [CrossRef]
- Andrews, R.E.; Bibilos, M.M.; Bulla, L.A. Protease activation of the entomocidal protoxin of Bacillus thuringiensis subsp. kurstaki. Appl. Environ. Microbiol. 1985, 50, 737–742. [Google Scholar] [CrossRef]
- Caccia, S.; Chakroun, M.; Vinokurov, K.; Ferré, J. Proteolytic processing of Bacillus thuringiensis Vip3A proteins by two Spodoptera species. J. Invertebr. Pathol. 2014, 67, 76–84. [Google Scholar] [CrossRef]
- Knowles, B.H. Mechanism of action of Bacillus thuringiensis insecticidal endotoxins. Adv. Insect Physiol. 1994, 24, 275–308. [Google Scholar]
- Bravo, A.; Gómez, I.; Conde, J.; Munoz-Garay, C.; Sanchez, J.; Miranda, R.; Zhuang, M.; Gill, S.S.; Soberón, M. Oligomerization triggers binding of a Bacillus thuringiensis Cry1Ab pore-forming toxin to aminopeptidase N receptor leading to insertion into membrane microdomains. Biochim. Biophys. Acta 2004, 1667, 38–46. [Google Scholar] [CrossRef]
- Bravo, A.; Gill, S.S.; Soberón, M. Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon 2007, 49, 423–435. [Google Scholar] [CrossRef]
- Gómez, I.; Pardo-López, L.; Muñoz-Garay, C.; Fernández, L.E.; Pérez, C.; Sánchez, J.; Soberón, M.; Bravo, A. Role of receptor interaction in the mode of action of insecticidal Cry and Cyt toxins produced by Bacillus thuringiensis. Peptides 2007, 28, 169–173. [Google Scholar] [CrossRef]
- Pardo-López, L.; Soberón, M.; Bravo, A. Bacillus thuringiensis insecticidal three domain Cry toxins: Mode of action, insect resistance and consequences for crop protection. FEMS Microbiol. Rev. 2013, 37, 3–22. [Google Scholar] [CrossRef]
- Gomis-Cebolla, J.; Ruiz de Escudero, I.; Vera-Velasco, N.M.; Hernández-Martínez, P.; Hernández-Rodríguez, C.S.; Ceballos, T.; Palma, L.; Escriche, B.; Caballero, P.; Ferré, J. Insecticidal spectrum and mode of action of the Bacillus thuringiensis Vip3Ca insecticidal protein. J. Invertebr. Pathol. 2017, 142, 60–67. [Google Scholar] [CrossRef]
- Yu, C.G.; Mullins, M.A.; Warren, G.W.; Koziel, M.G.; Estruch, J.J. The Bacillus thuringiensis vegetative insecticidal protein Vip3A lyses midgut epithelium cells of susceptible insects. Appl. Environ. Microbiol. 1997, 63, 532–536. [Google Scholar] [CrossRef]
- Caccia, S.; Di Lelio, I.; La Storia, A.; Marinelli, A.; Varricchio, P.; Franzetti, E.; Banyuls, N.; Tettamanti, G.; Casartelli, M.; Giordana, B. Midgut microbiota and host immunocompetence underlie Bacillus thuringiensis killing mechanism. Proc. Natl. Acad. Sci. USA 2016, 113, 9486–9491. [Google Scholar] [CrossRef]
- Domínguez-Arrizabalaga, M.; Villanueva, M.; Escriche, B.; Ancín-Azpilicueta, C.; Caballero, P. Insecticidal Activity of Bacillus thuringiensis Proteins Against Coleopteran Pests. Toxins 2020, 29, 430. [Google Scholar] [CrossRef]
- Schnepf, E.; Crickmore, N.; Van Rie, J.; Lereclus, D.; Baum, J.; Feitelson, J.; Zeigler, D.R.; Dean, D.H. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 1998, 62, 775–806. [Google Scholar] [CrossRef]
- Bel, Y.; Sheets, J.J.; Tan, S.Y.; Narva, K.E.; Escriche, B. Toxicity and binding studies of Bacillus thuringiensis Cry1Ac, Cry1F, Cry1C, and Cry2A proteins in the soybean pests Anticarsia gemmatalis and Chrysodeixis (Pseudoplusia) includens. Appl. Environ. Microbiol. 2017, 83, 11. [Google Scholar] [CrossRef]
- Yang, J.; Quan, Y.; Sivaprasath, P.; Shabbir, M.Z.; Wang, Z.; Ferré, J.; He, K. Insecticidal activity and synergistic combinations of ten different Bt Toxins against Mythimna separata (Walker). Toxins 2018, 10, 454. [Google Scholar] [CrossRef]
- Hernández-Martínez, P.; Hernández-Rodríguez, C.S.; Rie, J.V.; Escriche, B.; Ferré, J. Insecticidal activity of Vip3Aa, Vip3Ad, Vip3Ae, and Vip3Af from Bacillus thuringiensis against lepidopteran corn pests. J. Invertebr. Pathol. 2013, 113, 78–81. [Google Scholar] [CrossRef]
- Crava, C.M.; Bel, Y.; Ferré, J.; Escriche, B. Susceptibility to Cry proteins of a Spanish Ostrinia nubilalis glasshouse population repeatedly sprayed with Bacillus thuringiensis formulations. J. Appl. Entomol. 2014, 138, 78–86. [Google Scholar] [CrossRef]
- Calles-Torrez, V.; Knodel, J.J.; Boetel, M.A.; Doetkott, C.D.; Podliska, K.K.; Ransom, J.K.; Beauzay, P.; French, B.W.; Fuller, B.W. Transgenic Bt Corn, soil insecticide, and insecticidal seed treatment effects on corn rootworm (Coleoptera: Chrysomelidae) beetle emergence, larval feeding injury, and corn yield in North Dakota. J. Econ. Entomol. 2018, 111, 348–360. [Google Scholar] [CrossRef]
- Rodríguez-González, Á.; Porteous-Álvarez, A.J.; del Val, M.; Casquero, P.A.; Escriche, B. Toxicity of five Cry proteins against the insect pest Acanthoscelides obtectus (Coleoptera: Chrisomelidae: Bruchinae). J. Invertebr. Pathol. 2020, 169, 107295. [Google Scholar] [CrossRef] [PubMed]
- van Frankenhuyzen, K. Insecticidal activity of Bacillus thuringiensis crystal proteins. J. Invertebr. Pathol. 2009, 101, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Schnepf, E. Bacillus thurigiensis toxins-regulation, activities and diversity. Curr. Opin. Biotechnol. 1995, 6, 305–312. [Google Scholar] [CrossRef]
- Frankenhuyzen, K.V. Cross-order and cross-phylum activity of Bacillus thuringiensis pesticidal proteins. J. Invertebr. Pathol. 2013, 114, 76–85. [Google Scholar] [CrossRef]
- Walters, F.S.; Stacy, C.M.; Lee, M.K.; Palekar, N.; Chen, J.S. An engineered chymotrypsin/cathepsin G site in domain I renders Bacillus thuringiensis Cry3A active against Western corn rootworm larvae. Appl. Environ. Microbiol. 2008, 74, 367–374. [Google Scholar] [CrossRef]
- Jurat-Fuentes, J.L.; Crickmore, N. Specificity determinants for Cry insecticidal proteins: Insights from their mode of action. J. Invertebr. Pathol. 2017, 142, 5–10. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X.P.; Lu, M.Z.; Du, M.F.; Yin, X.M. Selection of Bacillus thuringiensis strain against the longhorned beetles and preliminary characterization of its insecticidal gene. Scientia Silvae Sinicae 2004, 40, 138–142. [Google Scholar]
- Latham, J.R.; Love, M.; Hilbeck, A. The distinct properties of natural and GM cry insecticidal proteins. Biotechnol. Genet. Eng. Rev. 2017, 33, 62–96. [Google Scholar] [CrossRef]
- Hilbeck, A.; Schmidt, J.E.U. Another view on Bt proteins—How specific are they and what else might they do? Biopestic. Int. 2006, 2, 1–50. [Google Scholar]
- Rodríguez-González, A.; Sánchez-Maíllo, E.; Peláez, H.J.; Mayo, S.; González-López, O.; Carro-Huerga, G.; Casquero, P.A. Evaluation of commercial and prototype traps for Xylotrechus arvicola (Coleoptera: Cerambycidae), an insect pest in Spanish vineyards. Aust. J. Grape Wine Res. 2018, 24, 190–196. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochemm. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ferré, J.; Real, M.D.; Van, R.J.; Jansens, S.; Peferoen, M. Resistance to the Bacillus thuringiensis bioinsecticide in a field population of Plutella xylostella is due to a change in a midgut membrane receptor. Proc. Natl. Acad. Sci. USA 1991, 88, 5119–5123. [Google Scholar] [CrossRef]
- Iglesias, C.; Notario, A.; Baragaño, J.R. Evaluación de las condiciones de cría y datos bionómicos de coleópteros lignícolas de tocón de pino. Boletin de Sanidad Vegetal Plagas 1989, 15, 9–16. [Google Scholar]
- Visitpanich, J. The biology and survival rate of the coffee stem borer, Xylotrechus quadripes Chevrolat (Coleoptera: Cerambycidae). Jpn. J. Entomol. 1994, 62, 731–745. [Google Scholar]
- Lee, C.Y.; Lo, K.C. Rearing of Anoplophora macularia (Thomson) (Coleoptera: Cerambycidae) on artificial diets. Appl. Entomol. Zool. 1998, 33, 105–109. [Google Scholar]
- Kosaka, H.; Ogura, N. Rearing of the Japanese pine sawyer, Monochamus alternatus (Coleoptera: Cerambycidae) on artificial diets. Appl. Entomol. Zool. 1990, 25, 532–534. [Google Scholar] [CrossRef][Green Version]
- van der Berg, J.; Hilbeck, A.; Bøhn, T. Pest resistance to Cry1Ab Bt maize: Field resistance, contributing factors and lessons from South Africa. Crop. Prot. 2013, 54, 154–160. [Google Scholar] [CrossRef]
- Székács, A.; Darvas, B. Comparative aspects of Cry toxin usage in insect control. In Advanced Technologies for Managing Insect Pests; Ishaaya, I., Palli, S.R., Horowitz, A.R., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 195–230. [Google Scholar]
- Marvier, M.; McCreedy, C.; Regetz, J.; Kareiva, P. A meta-analysis of effects of Bt cotton and maize on nontarget invertebrates. Science 2007, 316, 1475–1477. [Google Scholar] [CrossRef]
- Lang, A.; Vojtech, E. The effects of pollen consumption of transgenic Bt maize on the common swallowtail, Papilio machaon L. (Lepidoptera, Papilionidae). Basic Appl. Ecol. 2006, 7, 296–306. [Google Scholar] [CrossRef]
- Gassmann, A.J.; Petzold-Maxwell, J.L.; Keweshan, R.S.; Dunbar, M.W. Field-evolved resistance to Bt maize by Western corn rootworm. PLoS ONE 2011, 6, e22629. [Google Scholar] [CrossRef]
- Zhao, J.H.; Ho, P.; Azadi, H. Benefits of Bt cotton counterbalanced by secondary pests? Perceptions of ecological change in China. Environ. Monit. Assess. 2010, 173, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wu, K.; Jiang, Y.; Xia, B.; Li, P.; Feng, H.; Wyckhuys, K.; Guo, Y. Mirid bug outbreaks in multiple crops correlated with wide-scale adoption of Bt cotton in China. Science 2010, 328, 1151–1154. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Shi, G.L.; Song, D.P.; Rogers, D.J.; Davis, L.K.; Chen, X. Development, survival, body weight, longevity, and reproductive potential of Oemona hirta (Coleoptera: Cerambycidae) under different rearing conditions. J. Econ. Entomol. 2002, 95, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, L.M. Rearing wood-boring beetles (Cerambycidae) on artificial diet. Can. Entomol. 1970, 102, 113–117. [Google Scholar] [CrossRef]
- Galford, J.R. Enaphalodes rufulus. In Handbook of Insect Rearing I; Singh, P., Moore, R.F., Eds.; Handbook of Insect Rearing I; Elsevier: New York, NY, USA, 1985; pp. 255–264. [Google Scholar]
- Dubois, T.; Hajek, A.E.; Smith, S. Methods for rearing the Asian longhorned beetle (Coleoptera: Cerambycidae) on artificial diet. Ann. Entomol. Soc. Am. 2002, 95, 223–230. [Google Scholar] [CrossRef]
| Treatments | Lifespan (Mean ± SE) | Adults’ Lifespan (Mean ± SE) | ||||||
|---|---|---|---|---|---|---|---|---|
| Prepupa | Pupa | Total | Male | Female | F | df | p | |
| Control | 10.8 ± 2.2 A (19) a | 13.8 ± 0.5 A (18) a | 36.2 ± 2.9 A (17) b | 37.9 ± 4.1 Aa (9) b | 34.2 ± 4.33 Aa (8) b | 0.375 | 1.15 | 0.550 |
| Cry3Aa | 7.6 ± 0.3 A (14) | 13.9 ± 0.5 A (12) | 39.6 ± 3.2 A (11) | 39.0 ± 5.0 Aa (7) | 40.7 ± 2.9 Aa (4) | 0.060 | 1.9 | 0.811 |
| Cry23/37 | 8.5 ± 0.7 A (9) | 13.0 ± 0.7 A (9) | 45.4 ± 4.0 A (8) | 48.7 ± 4.3 Aa (4) | 42.0 ± 7.0 Aa (4) | 0.678 | 1.6 | 0.442 |
| Cry1Ia | 8.9 ± 1.4 A (7) | 12.1 ± 1.4 A (7) | 22.6 ± 3.9 B (5) | - (3) | -(2) | - | - | - |
| Cry7Ab | -(3) | - (3) | -(3) | - (1) | -(2) | - | - | - |
| Cry1Ba | -(2) | -(2) | -(2) | -(1) | -(1) | - | - | - |
| F | 0.703 | 1.065 | 3.789 | 1.303 | 0.768 | |||
| df | 3.45 | 3.42 | 5.48 | 2.17 | 2.17 | |||
| p | 0.555 | 0.374 | 0.007 | 0.325 | 0.484 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-González, Á.; Porteous-Álvarez, A.J.; Guerra, M.; González-López, Ó.; Casquero, P.A.; Escriche, B. Effect of Cry Toxins on Xylotrechus arvicola (Coleoptera: Cerambycidae) Larvae. Insects 2022, 13, 27. https://doi.org/10.3390/insects13010027
Rodríguez-González Á, Porteous-Álvarez AJ, Guerra M, González-López Ó, Casquero PA, Escriche B. Effect of Cry Toxins on Xylotrechus arvicola (Coleoptera: Cerambycidae) Larvae. Insects. 2022; 13(1):27. https://doi.org/10.3390/insects13010027
Chicago/Turabian StyleRodríguez-González, Álvaro, Alejandra J. Porteous-Álvarez, Marcos Guerra, Óscar González-López, Pedro A. Casquero, and Baltasar Escriche. 2022. "Effect of Cry Toxins on Xylotrechus arvicola (Coleoptera: Cerambycidae) Larvae" Insects 13, no. 1: 27. https://doi.org/10.3390/insects13010027
APA StyleRodríguez-González, Á., Porteous-Álvarez, A. J., Guerra, M., González-López, Ó., Casquero, P. A., & Escriche, B. (2022). Effect of Cry Toxins on Xylotrechus arvicola (Coleoptera: Cerambycidae) Larvae. Insects, 13(1), 27. https://doi.org/10.3390/insects13010027








