Multiple Mating of Aphelinus asychis Enhance the Number of Female Progeny but Shorten the Longevity
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects and Plant Cultures
2.2. Mating Behaviour Assay
2.3. Influence of the Mating Frequency on the Population Fitness of A. asychis
2.4. Influence of Backcross on the Population Fitness of A. asychis
2.5. Statistical Analysis
3. Result
3.1. Effect of Mating Times on the Female Fitness
3.1.1. Observation of Mating Behavior and Duration
3.1.2. Longevity
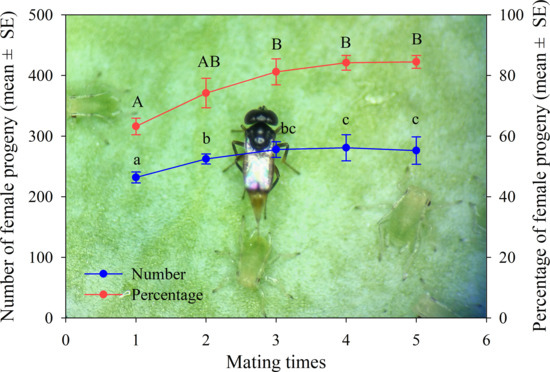
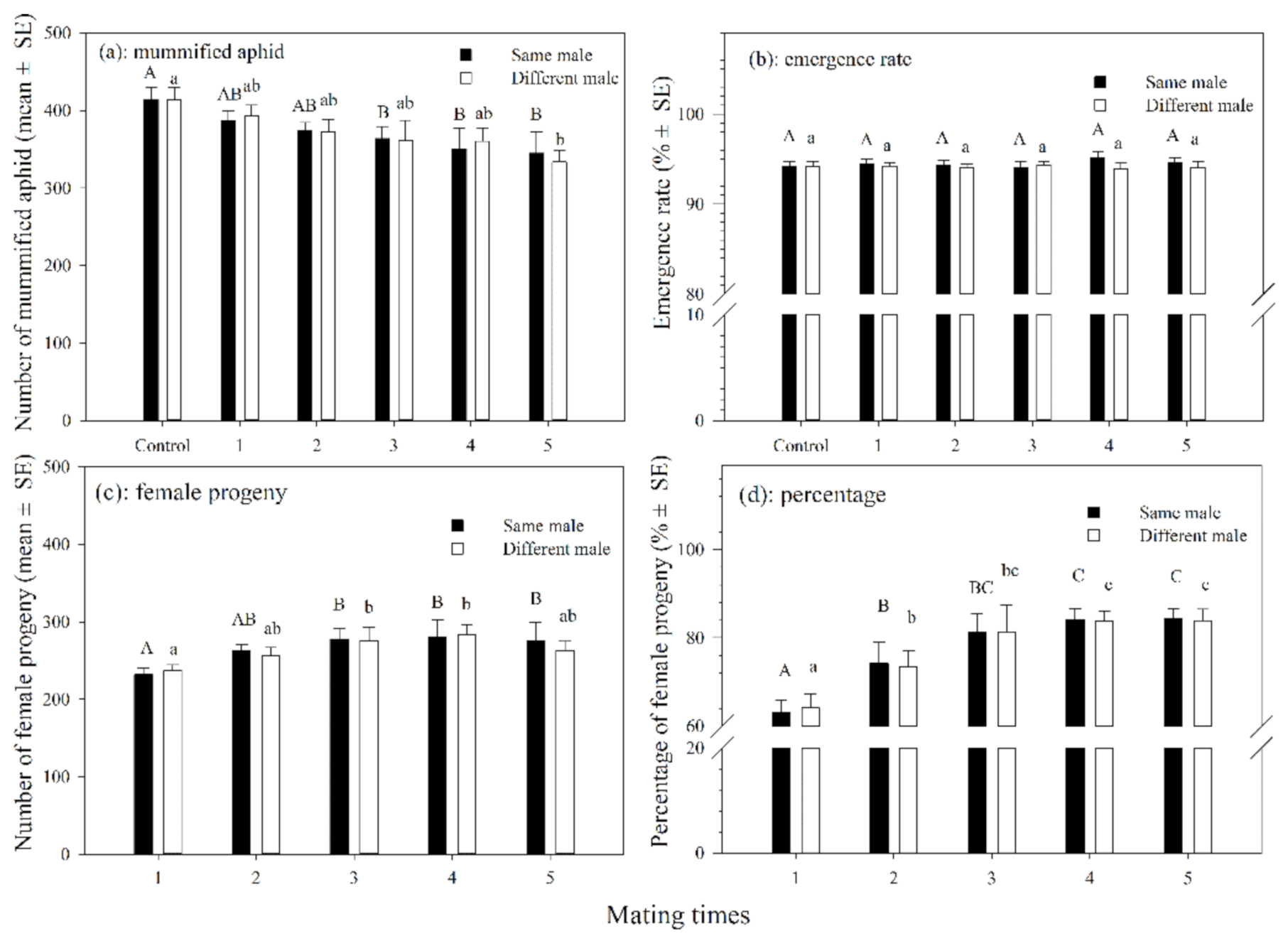
3.1.3. Mummified Aphids and Female Progenies
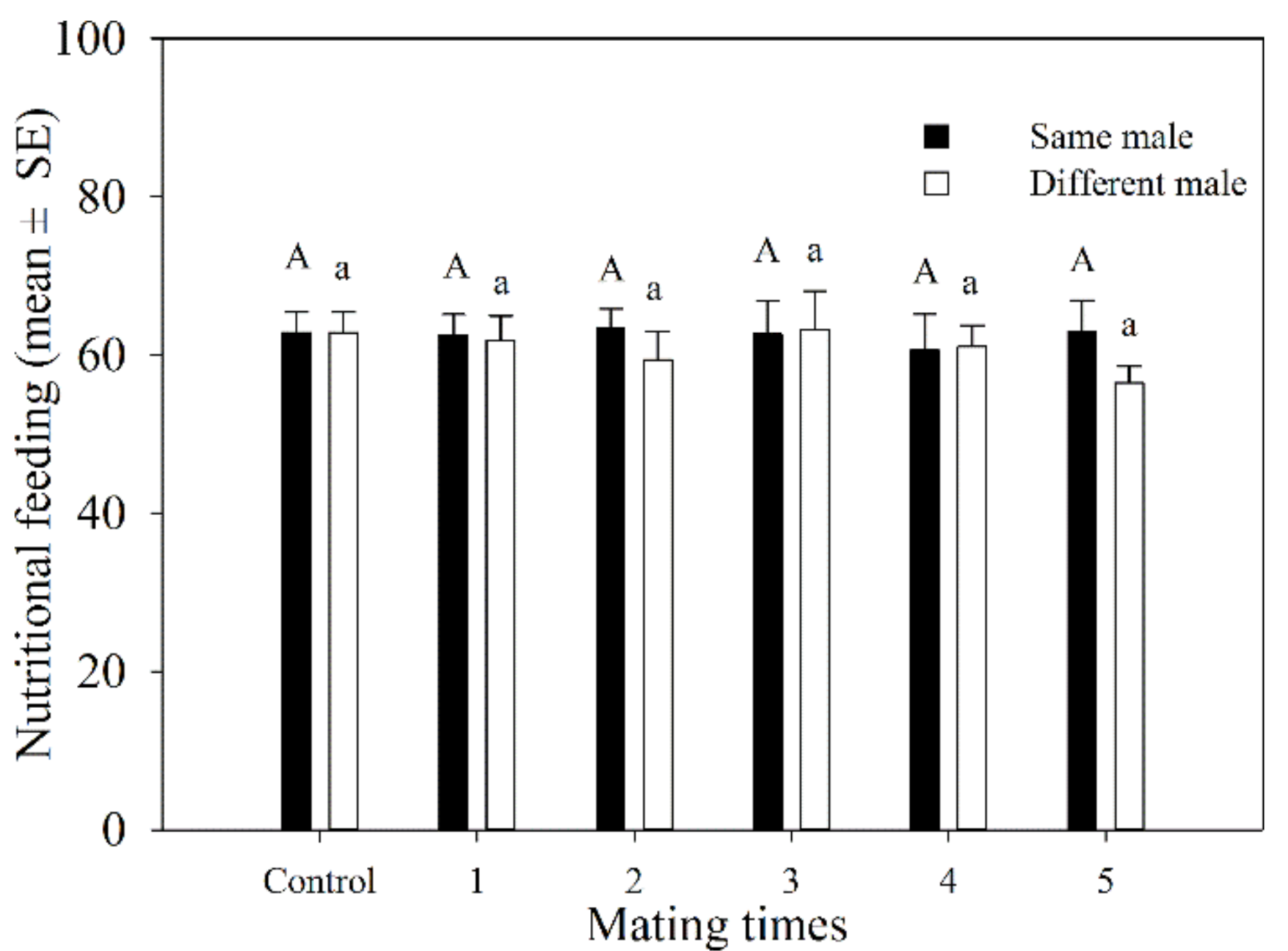
3.1.4. Host Feeding
3.2. Backcross Experiment
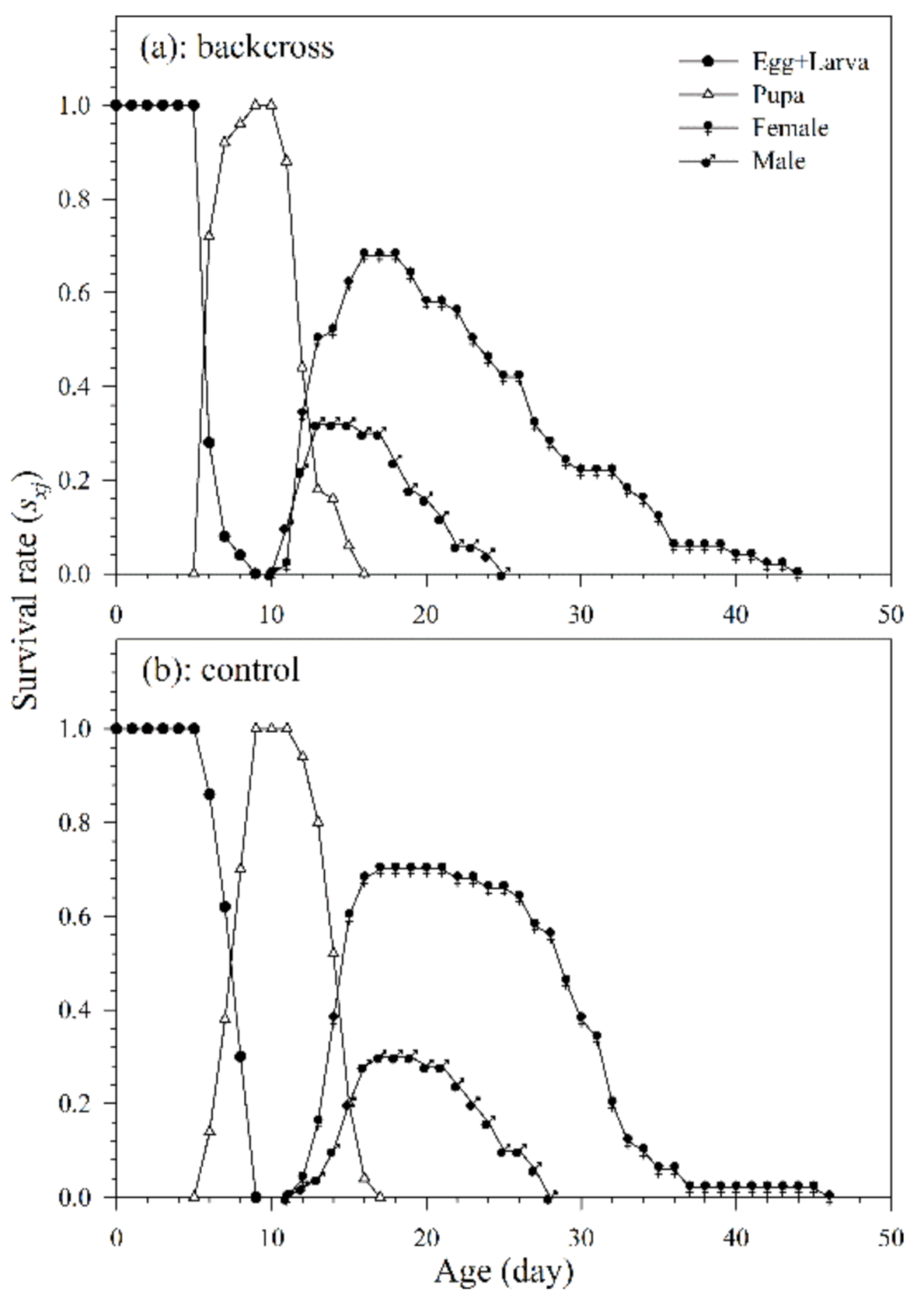
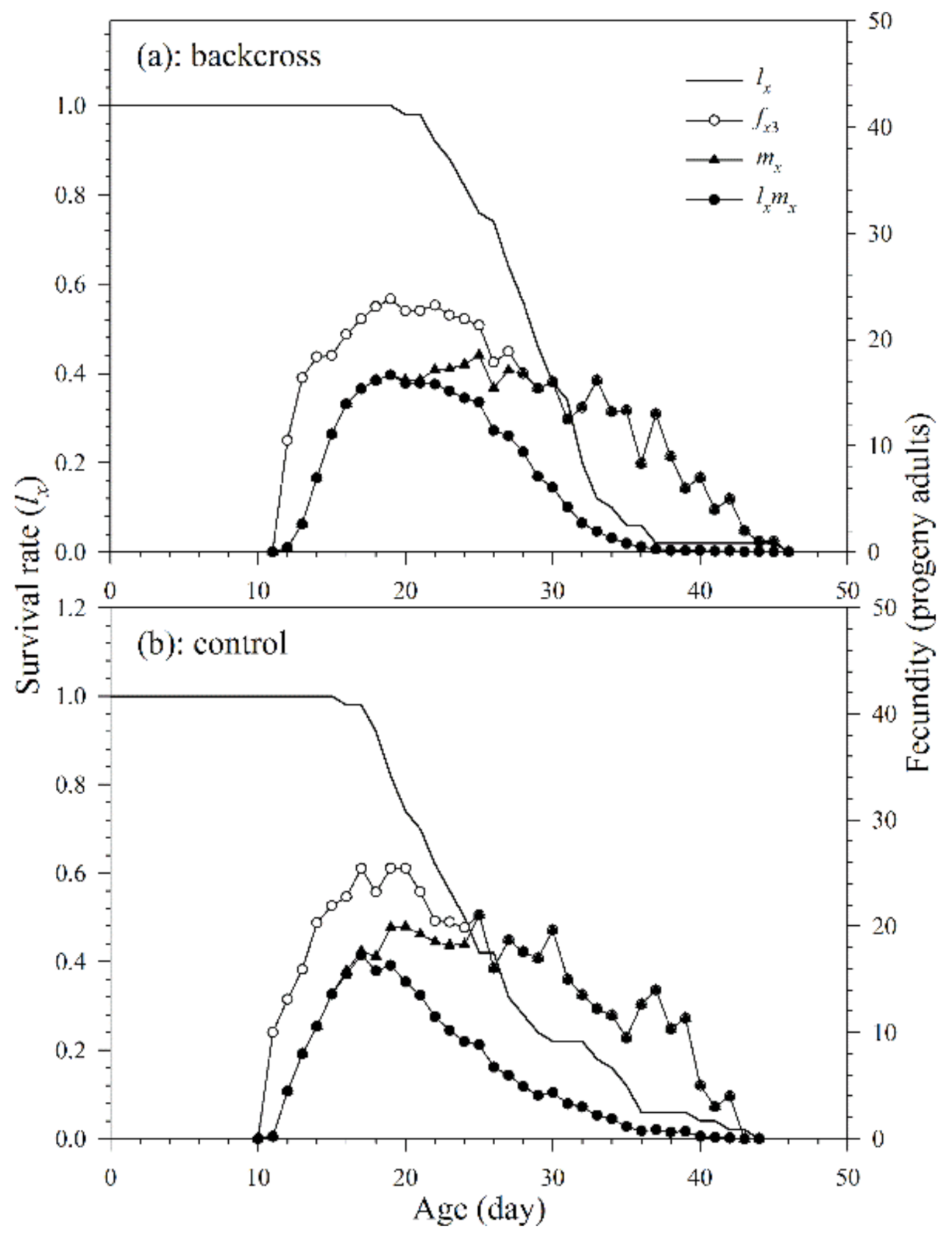
3.2.1. Age-Stage, Two-Sex Life Table
3.2.2. Population Growth Parameters
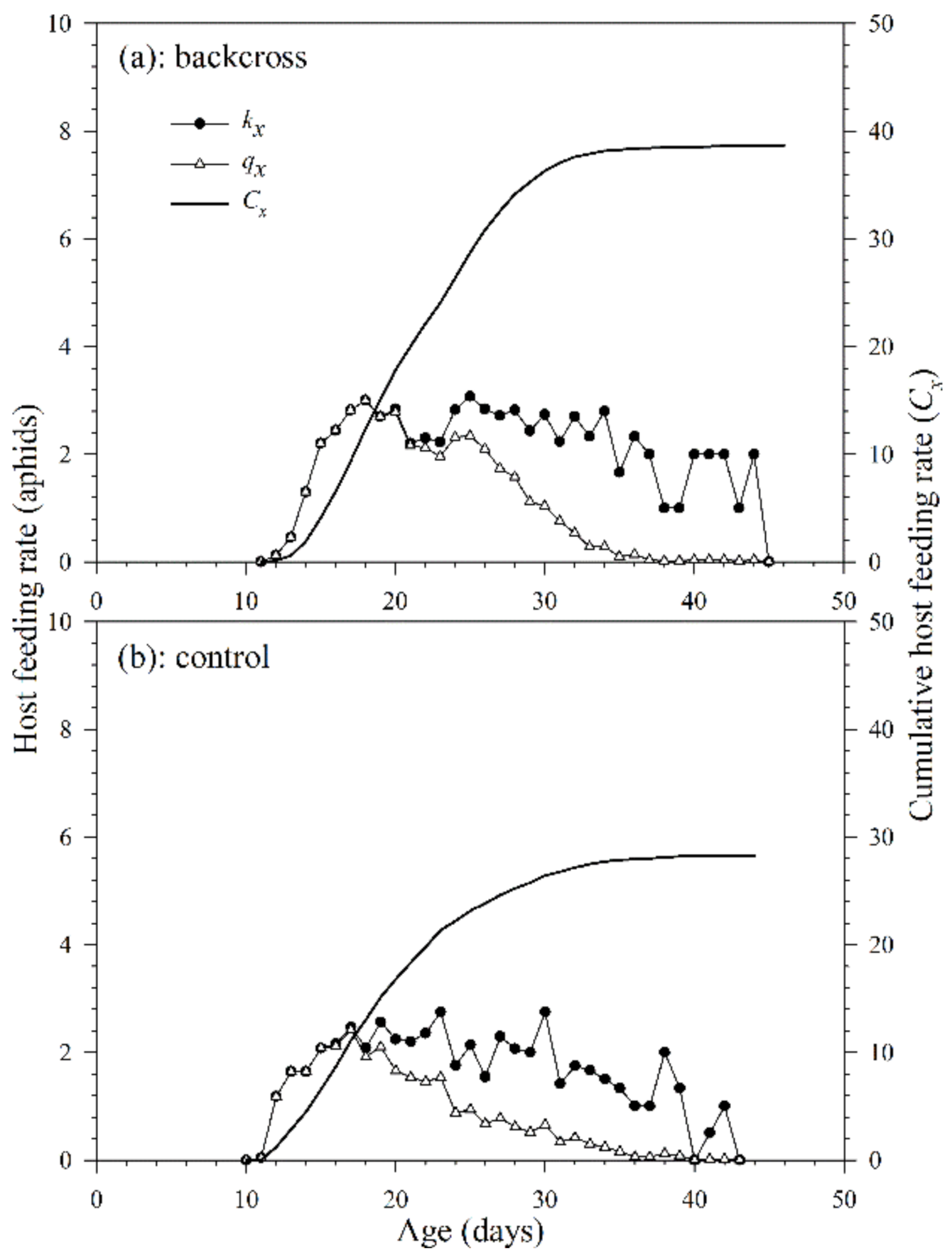
3.2.3. Host Feeding
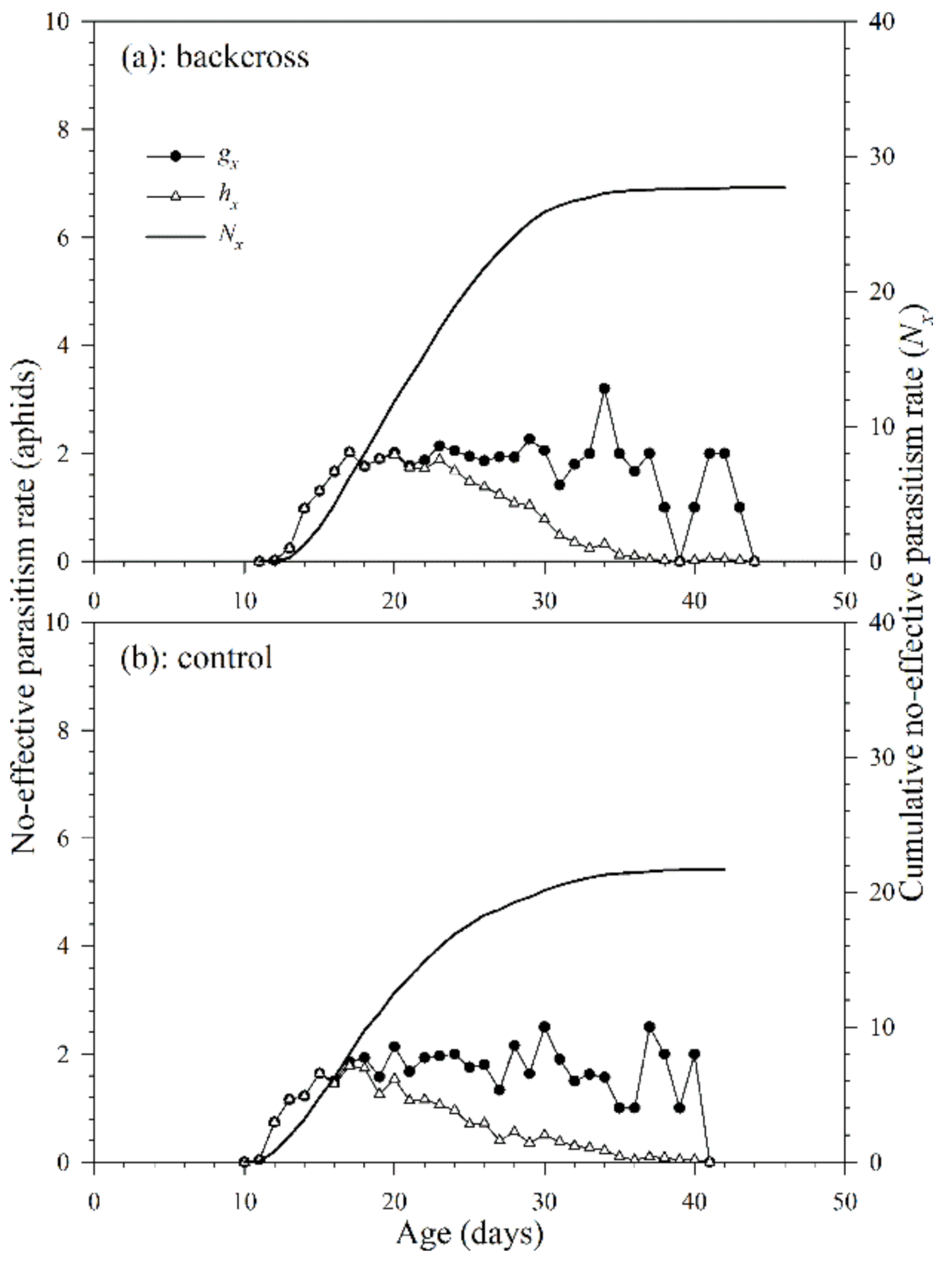
3.2.4. Non-Effective Parasitism Rate
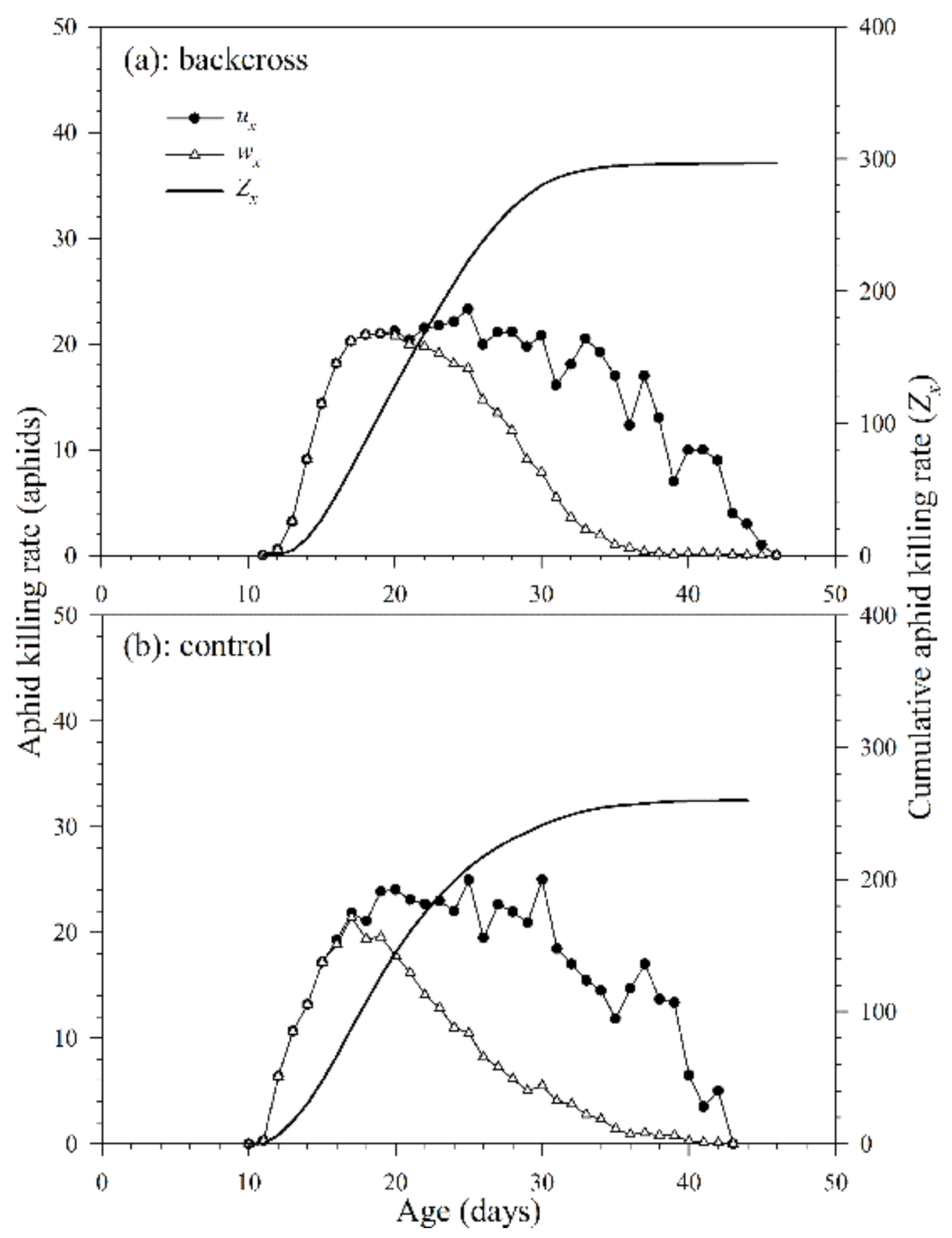
3.2.5. Killing Rate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raney, H.G.; Coles, L.W.; Eikenbary, R.D.; Morrison, R.D.; Starks, K.J. Host preference, longevity, developmental period and sex ratio of Aphelinus asychis with 3 sorghum-fed species of aphids held at controlled temperatures. Ann. Entomol. Soc. Am. 1971, 64, 169–176. [Google Scholar] [CrossRef]
- Byeon, Y.W.; Tuda, M.; Takagi, M.; Kim, J.H.; Choi, M.Y. Life history parameters and temperature requirements for development of an aphid parasitoid Aphelinus asychis (Hymenoptera: Aphelinidae). Environ. Entomol. 2011, 40, 431–440. [Google Scholar] [CrossRef]
- Byeon, Y.W.; Tuda, M.; Kimb, J.H.; Choi, M.Y. Functional responses of aphid parasitoids, Aphidius colemani (Hymenoptera: Braconidae) and Aphelinus asychis (Hymenoptera: Aphelinidae). Biocontrol Sci. Technol. 2011, 21, 57–70. [Google Scholar] [CrossRef]
- Wang, S.Y.; Chi, H.; Liu, T.X. Demography and parasitic effectiveness of Aphelinus asychis reared from Sitobion avenae as a biological control agent of Myzus persicae reared on chili pepper and cabbage. Biol. Control 2016, 92, 111–119. [Google Scholar] [CrossRef]
- Wang, S.Y.; Liang, N.N.; Tang, R.; Liu, Y.H.; Liu, T.X. Brief heat stress negatively affects the population fitness and host feeding of Aphelinus asychis (Hymenoptera: Aphelinidae) parasitizing Myzus persicae (Hemiptera: Aphididae). Environ. Entomol. 2016, 3, 1–7. [Google Scholar]
- Wang, S.Y.; Zhang, D.Y.; Liu, T.X. Influence of temperature on the demographics and control efficiency of Aphelinus asychis, a parasitoid of the cabbage pest, Myzus persicae. Phytoparasitica 2020, 48, 767–783. [Google Scholar] [CrossRef]
- Byeon, Y.W.; Tuda, M.; Takagi, M.; Kim, J.H.; Kim, Y.H. Non-reproductive host killing caused by Aphelinus asychis (Hymenoptera: Aphelinidae), a parasitoid of cotton aphid, Aphis gossypii (Homoptera: Aphididae). J. Fac. Agric. Kyushu Univ. 2009, 54, 369–372. [Google Scholar] [CrossRef]
- Logan, J.A.; Wollkind, D.J.; Hoyt, S.C.; Tanigoshi, L.K. An analytic model for description of temperature dependent rate phenomena in arthropods. Environ. Entomol. 1976, 1, 133–140. [Google Scholar] [CrossRef]
- Zhang, S.Z.; Cao, Z.; Zhang, F.; Liu, T.X. Exposing eggs to high temperatures affects the development, survival and reproduction of Harmonia axyridis. J. Thermal Biol. 2014, 39, 40–44. [Google Scholar] [CrossRef]
- Geden, C.J.; Kaufman, P.E. Development of Spalangia cameroni and Muscidifurax raptor (Hymenoptera: Pteromalidae) on live house fly (Diptera: Muscidae) pupae and pupae killed by heat shock, irradiation, and cold. Environ. Entomol. 2007, 36, 34–39. [Google Scholar] [CrossRef] [Green Version]
- Roux, O.; Lann, C.L.; van Alphen, J.J.M.; van Baaren, J. How does heat shock affect the life history traits of adults and progeny of the aphid parasitoid Aphidius avenae (Hymenoptera: Aphidiidae). Bull. Entomol. Res. 2010, 100, 543–549. [Google Scholar] [CrossRef]
- Firake, D.M.; Khan, M.A. Alternating temperatures affect the performance of Trichogramma species. J. Insect Sci. 2014, 14, 41–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Hafez, A.A.; El-Sharkawy, M.A.A.; Karim, A.H. Consequential effects of high temperature on biological characteristics influencing the efficacy of Trichogramma evanescens Westwood and its progeny. Egypt J. Biol. Pest Control 2014, 24, 57–63. [Google Scholar]
- Wang, M.X.; Cui, S.M.; Wang, H.B.; Li, Z.X.; Li, H.T.; Zhang, X.; Hu, B.; Zhang, X.B.; Zhang, X.M. The performance of illumination, temperature and humidity in solar greenhouse. Greenh. Hortic. 2008, 5, 19–22. [Google Scholar]
- Ribaut, J.M.; Ragot, M. Marker-assisted selection to improve drought adaptation in maize: The backcross approach, perspectives, limitations, and alternatives. J. Exp. Bot. 2007, 58, 351–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynolds, J.D. Animal breeding systems. Trends Ecol. Evol. 1996, 11, 68–72. [Google Scholar] [CrossRef]
- Arnqvist, G.; Nilsson, T. The evolution of polyandry: Multiple mating and female fitness in insects. Anim. Behav. 2000, 60, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Ridley, M. Clutch size and mating frequency in parasitic hymenoptera. Am. Nat. 1993, 142, 893–910. [Google Scholar] [CrossRef]
- King, B.H.; Bressac, C. No fitness consequence of experimentally induced polyandry in a monandrous wasp. Behaviour 2010, 147, 85–102. [Google Scholar] [CrossRef] [Green Version]
- King, B.H.; Fischer, C.R. Male mating history: Effects on female sexual responsiveness and reproductive success in the parasitoid wasp Spalangia endius. Behav. Ecol. Sociobiol. 2010, 64, 607–615. [Google Scholar] [CrossRef]
- King, B.H.; Saporito, K.B.; Ellison, J.H.; Bratzke, R.M. Unattractiveness of mated females to males in the parasitoid wasp Spalangia endius. Behav. Ecol. Sociobiol. 2005, 57, 350–356. [Google Scholar] [CrossRef]
- Damiens, D.; Boivin, G. Male reproductive strategy in Trichogramma evanescens: Sperm production and allocation to females. Physiol. Entomol. 2005, 30, 241–247. [Google Scholar] [CrossRef]
- Charnov, E.L. Parent-offspring conflict over reproductive effort. Am. Nat. 1982, 119, 736–737. [Google Scholar] [CrossRef]
- Godfray, H.C.J. Parasitoids: Behavioral and Evolutionary Ecology; Princeton University Press: Princeton, NJ, USA, 1994. [Google Scholar]
- Cournault, L.; Aron, S. Diploid males, diploid sperm production, and triploid females in the ant Tapinoma erraticum. Naturwissenschaften 2009, 96, 393–400. [Google Scholar] [CrossRef] [Green Version]
- Li, C.D.; Byeon, Y.W.; Choi, B.R. An Aphelinid species, Aphelinus asychis Walker (Hymenoptera: Aphelinidae) new to Korea. J, Asia-Pac. Entomol. 2007, 10, 13–15. [Google Scholar] [CrossRef]
- Chi, H. TWOSEX-MSChart: Computer Program for Age Stage, Two-Sex Life Table Analysis. 2017. Available online: http://140.120.197.173/ecology/ (accessed on 2 September 2021).
- Chi, H. CONSUME-MSChart: Computer Program for Consumption Rate Analysis Based on the Age Stage, Two-Sex Life Table. 2017. Available online: http://140.120.197.173/ecology/ (accessed on 2 September 2021).
- Efron, B.; Tibshirani, R.J. An Introduction to the Bootstrap; Chapman and Hall: New York, NY, USA, 1993. [Google Scholar]
- Akköprü, E.; Atlıhan, R.; Okut, H.; Chi, H. Demographic assessment of plant cultivar resistance to insect pests: A case study of the dusky-veined walnut aphid (Hemiptera: Callaphididae) on five walnut cultivars. J. Econ. Entomol. 2015, 108, 378–387. [Google Scholar] [CrossRef]
- Gillott, C. Male accessory gland secretions: Modulators of female reproductive physiology and behavior. Annu. Rev. Entomol. 2003, 48, 163–184. [Google Scholar] [CrossRef]
- Chapman, T.; Liddle, L.F.; Kalb, J.M.; Wolfner, M.F.; Partridge, L. Cost of mating in Drosophila melanogaster females is mediated by male accessory gland products. Nature 1995, 373, 241–244. [Google Scholar] [CrossRef]
- Wang, X.P.; Fang, Y.L.; Zhang, Z.N. Effect of male and female multiple mating on the fecundity, fertility, and longevity of diamondback moth, Plutella xylostella (L.). J. Appl. Entomol. 2005, 129, 39–42. [Google Scholar] [CrossRef]
- Rutowski, R.L. Mate choice and Lepidoptera mating behavior. Fla. Entomol. 1982, 65, 72–82. [Google Scholar] [CrossRef]
- Savalli, U.M.; Fox, C.W. The effect of male mating history on paternal investment, fecundity and female remating in the seed beetle Callosobruchus maculates. Funct. Ecol. 1999, 13, 169–177. [Google Scholar] [CrossRef]
- Haughes, L.; Chang, B.S.W.; Wangner, D.; Pierce, N.E. Effects of mating history on ejaculate size, fecundity, longevity, and copulation duration in the ant-tended lycaenid butterfly, Jalmenus evagoras. Behav. Ecol. Sociobiol. 2000, 47, 119–128. [Google Scholar] [CrossRef]
- Kotiaho, J.S.; Simmons, L.W. Longevity cost of reproduction for males but no longevity cost of mating or courtship for females in the male-dimorphic dung beetle Onthophagus binodis. J. Insect Physiol. 2003, 49, 817–822. [Google Scholar] [CrossRef]
- Paukku, S.; Kotiaho, J.S. Cost of reproduction in Callosobruchus maculatus: Effects of mating on male longevity and the effect of male mating status on female longevity. J. Insect Physiol. 2005, 51, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Jacob, H.S.; Evans, E.W. Influence of carbohydrate foods and mating on longevity of the parasitoid Bathyplectes curculionis (Hymenoptera: Ichneumonidae). Environ. Entomol. 2000, 5, 1008–1095. [Google Scholar]
- Fauvergue, K.; Hopper, K.R.; Antolin, M.F.; Kazmer, D.J. Does time until mating affect progeny sex ratio? A manipulative experiment with the parasitoid wasp Aphelinus asychis. J. Evolution. Biol. 1998, 11, 611–622. [Google Scholar]
- Jackson, H.B.; Eikenbary, R.D. Bionomics of Aphelinus asychis (Hymenoptera: Eulophidae) an introduced parasite of the sorghum greenbug. Ann. Entomol. Soc. Am. 1971, 64, 81–85. [Google Scholar] [CrossRef]
- Kang, E.J.; Byeon, Y.W.; Kim, J.H.; Choi, M.Y.; Choi, Y.S. The effect of temperatures on the biological characteristics of two aphid parasitoids Aphelinusasychis (Walker) and Aphelinus varipes (Forster) (Hymenoptera: Aphelinidae) on two aphid hosts. Korean J. Appl. Entomol. 2012, 51, 397–403. [Google Scholar] [CrossRef]
- Sengonca, C.; Schirmer, S.; Blaeser, P. Life table of the aphid parasitoid Aphelinus asychis (Walker) (Hymenoptera, Aphelinidae) parasitizing different age groups of Aphis gossypii Glover (Homoptera, Aphididae). J. Plant Dis. Protect. 2008, 115, 122–128. [Google Scholar] [CrossRef]
- Bernal, J.; Gonzalez, D. Temperature requirements of four parasites of the Russian wheat aphid Diuraphis noxia. Entomol. Exp. Appl. 1993, 69, 173–182. [Google Scholar] [CrossRef]
- Lee, J.H.; Elliott, N.C. Comparison of developmental responses to temperature in Aphelinus asychis (Walker) from two different geographic regions. Southwest. Entomol. 1998, 23, 77–82. [Google Scholar]
- Wang, S.Y.; Wang, L.B.; Yan, G.L.; Liu, Y.H.; Zhang, D.Y.; Liu, T.X. Temperature-dependent demographic characteristics and control potential of Aphelinus asychis reared from Sitobion avenae as a biological control agent for Myzus persicae on chili peppers. Insects 2020, 11, 475. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, E.; Takada, H. Evaluation of Aphelinus asychis and Aphelinus albipodus (Hym., Aphelinidae) as biological control agents against three pest aphids. Appl. Entomol. Zool. 2005, 40, 379–385. [Google Scholar] [CrossRef] [Green Version]
- Chi, H.; Mou, D.F.; Allahyari, H.; Yu, J.Z.; Huang, Y.B.; Yang, T.C.; Farhadi, R.; Gholizadeh, M. Finite predation rate: A novel parameter for the quantitative measurement of predation potential of predator at population level. Nat. Preced. Available online: https://www.nature.com/articles/npre.2011.6651.1 (accessed on 2 September 2021).

| Parameter | Definition | Formula |
|---|---|---|
| sxj | Age-stage-specific survival rate | |
| lx | Age-specific survival rate | |
| mx | Age-specific fecundity | |
| r | Intrinsic rate of increase | |
| R0 | Net reproductive rate | |
| T | Mean generation time | |
| kx | Age-specific host feeding rate | |
| qx | Age-specific net host feeding rate | |
| C0 | Net host feeding rate | |
| ψ | Stable host feeding rate | |
| ω | Finite host feeding rate | |
| gx | Age-specific non-effective parasitism rate | |
| hx | Age-specific net non-effective parasitism rate | |
| N0 | Net non-effective parasitism rate | |
| γ | Stable non-effective parasitism rate | |
| ε | Finite non-effective parasitism rate | |
| ux | Age-specific aphid killing rate | |
| wx | Age-specific net aphid killing rate | |
| Z0 | Net aphid killing rate | |
| ϑ | Stable aphid killing rate | |
| θ | Finite aphid killing rate | |
| Qp | Transformation rate |
| Mating Times | 1 | 2 | 3 | 4 | 5 | df | F | p |
|---|---|---|---|---|---|---|---|---|
| Same male—Courtship | 61.3 ± 5.4 A | 162.2 ± 9.9 B * | 192.6 ± 13.0 B * | 280.2 ± 29.0 C * | 464.4 ± 24.9 D * | 4128 | 76.509 | <0.001 |
| Different males—Courtship | 59.3 ± 3.4 a | 66.8 ± 3.0 ab | 90.2 ±6.5 bc | 109.5 ± 8.4 c | 233.5 ± 22.8 d | 4128 | 60.195 | <0.001 |
| df | 78 | 69 | 58 | 42 | 25 | |||
| F | 3.449 | 9.527 | 7.350 | 6.331 | 6.819 | |||
| p | 0.135 | <0.001 | <0.001 | <0.001 | <0.001 | |||
| Same male—Precopulatory | 63.1 ± 3.2 A | 64.1 ± 3.5 A | 75.8 ± 3.6 A | 64.5 ± 6.2 A | 72.8 ± 5.0 A | 4128 | 2.080 | 0.087 |
| Different males—Precopulatory | 61.7 ± 3.2 a | 59.0 ± 2.9 a | 67.2 ± 3.0 a | 66.6 ± 3.8 a | 68.2 ± 3.7 a | 4128 | 1.651 | 0.165 |
| df | 78 | 69 | 58 | 42 | 25 | |||
| t | 0.313 | 1.132 | 4.021 | 0.303 | 0.756 | |||
| p | 0.755 | 0.262 | 0.653 | 0.764 | 0.457 | |||
| Same male—Copulation | 6.9 ± 0.6 A | 7.1 ± 0.6 A | 8.1 ± 0.8 A | 7.8 ± 1.1 A | 5.5 ± 0.5 A | 4128 | 1.119 | 0.350 |
| Different males—Copulation | 6.5 ± 0.5 a | 6.7 ± 0.7 a | 6.8 ± 0.8 a | 6.8 ± 0.9 a | 6.7 ± 0.8 a | 4128 | 0.047 | 0.996 |
| df | 78 | 69 | 57 | 42 | 25 | |||
| F | 0.46 | 0.404 | 1.054 | 0.684 | 1.292 | |||
| p | 0.647 | 0.688 | 0.296 | 0.497 | 0.208 | |||
| Same male—Post copulatory | 181.4 ± 7.8 A | 171.5 ± 10.1 A | 199.3 ± 11.8 A | 179.3 ± 9.3 A | 183.9 ± 13.0 A | 4103 | 1.005 | 0.408 |
| Different males—Post copulatory | 166.3 ± 6.2 a | 179.9 ± 7.4 a | 178.3 ± 5.6 a | 183.3 ± 7.6 a | 178.2 ± 8.7 a | 4122 | 1.015 | 0.402 |
| df | 67 | 57 | 45 | 33 | 23 | |||
| F | 1.518 | 0.685 | 1.752 | 0.333 | 0.37 | |||
| p | 0.134 | 0.496 | 0.087 | 0.741 | 0.715 |
| Stage | Backcross | Control | t | p | ||
|---|---|---|---|---|---|---|
| n | Mean ± SE | n | Mean ± SE | |||
| Egg + larva (d) | 50 | 7.8 ± 0.2 a | 50 | 6.4 ± 0.1 b | 24.571 | <0.001 |
| Pupa (d) | 50 | 6.7 ± 0.2 a | 50 | 6.3 ± 0.1 b | 19.284 | 0.003 |
| Preadult (d) | 50 | 14.5 ± 0.2 a | 50 | 12.7 ± 0.2 b | 26.335 | <0.001 |
| Female longevity (d) | 35 | 31.1 ± 0.7 a | 34 | 28.5 ± 1.2 b | 28.214 | <0.001 |
| Female adult longevity (d) | 35 | 16.7 ± 0.7 a | 34 | 15.5 ± 1.1 a | 2.137 | 0.162 |
| Male longevity (d) | 15 | 24.7 ± 0.6 a | 16 | 20.6 ± 0.7 b | 14.262 | 0.005 |
| Male adult longevity (d) | 15 | 9.9 ± 0.7 a | 16 | 8.6 ± 0.7 a | 2.864 | 0.157 |
| Reproduction period (d) | 35 | 16.7 ± 0.7 a | 34 | 15.2 ± 1.1 a | 1.935 | 0.219 |
| Progeny (egg/female) | 35 | 331.3 ± 13.6 a | 34 | 309.4 ± 21.8 a | 2.094 | 0.131 |
| Parameters | Backcross | Control | t | p |
|---|---|---|---|---|
| Intrinsic rate of increase, r (d−1) | 0.2687 ± 0.0060 a | 0.2858 ± 0.0070 b | 29.672 | <0.001 |
| Finite rate of increase, λ (d−1) | 1.3082 ± 0.0078 a | 1.3308 ± 0.0093 b | 23.251 | 0.002 |
| Net reproductive rate, R0 (offspring) | 231.9 ± 23.4 a | 210.4 ± 25.2 a | 1.567 | 0.232 |
| Mean generation time, T (d) | 20.3 ± 0.3 a | 18.7 ± 0.3 b | 28.232 | <0.001 |
| Net host feeding rate, C0 (aphids) | 38.7 ± 4.0 a | 23.2 ± 3.0 b | 35.817 | <0.001 |
| Stable host feeding rate, ψ | 0.0549 ± 0.0050 a | 0.0397 ± 0.0045 b | 42.890 | <0.001 |
| Finite host feeding rate, ω | 0.0718 ± 0.0069 a | 0.0528 ± 0.0063 b | 33.795 | <0.001 |
| Net non-effective parasitism rate, N0 (aphids) | 27.7 ± 2.9 a | 28.2 ± 3.3 a | 2.134 | 0.195 |
| Stable non-effective parasitism rate, ε | 0.0366 ± 0.0033 a | 0.0540 ± 0.0053 b | 24.059 | 0.006 |
| Finite non-effective parasitism rate, γ | 0.0478 ± 0.0046 a | 0.0719 ± 0.0075 b | 25.770 | <0.001 |
| Net killing rate, Z0 (aphids) | 298.3 ± 30.0 a | 261.8 ± 31.1 a | 2.184 | 0.178 |
| Stable killing rate, ϑ | 0.3999 ± 0.0332 a | 0.4251 ± 0.0399 a | 1.319 | 0.224 |
| Finite killing rate, θ | 0.5232 ± 0.0464 a | 0.5657 ± 0.0570 a | 2.953 | 0.194 |
| Transformation rate, Qp | 1.2864 ± 0.0068 a | 1.2443 ± 0.0059 b | 27.033 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Wang, L.; Liu, J.; Zhang, D.; Liu, T. Multiple Mating of Aphelinus asychis Enhance the Number of Female Progeny but Shorten the Longevity. Insects 2021, 12, 823. https://doi.org/10.3390/insects12090823
Wang S, Wang L, Liu J, Zhang D, Liu T. Multiple Mating of Aphelinus asychis Enhance the Number of Female Progeny but Shorten the Longevity. Insects. 2021; 12(9):823. https://doi.org/10.3390/insects12090823
Chicago/Turabian StyleWang, Shengyin, Libo Wang, Jiawen Liu, Dayu Zhang, and Tongxian Liu. 2021. "Multiple Mating of Aphelinus asychis Enhance the Number of Female Progeny but Shorten the Longevity" Insects 12, no. 9: 823. https://doi.org/10.3390/insects12090823
APA StyleWang, S., Wang, L., Liu, J., Zhang, D., & Liu, T. (2021). Multiple Mating of Aphelinus asychis Enhance the Number of Female Progeny but Shorten the Longevity. Insects, 12(9), 823. https://doi.org/10.3390/insects12090823