First Detection of the Adventive Egg Parasitoid of Halyomorpha halys (Stål) (Hemiptera: Pentatomidae) Trissolcus mitsukurii (Ashmead) (Hymenoptera: Scelionidae) in France
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Field Surveys
- Sentinel egg masses
- Natural egg masses
- Emergence of parasitoids
2.2. Species Identification
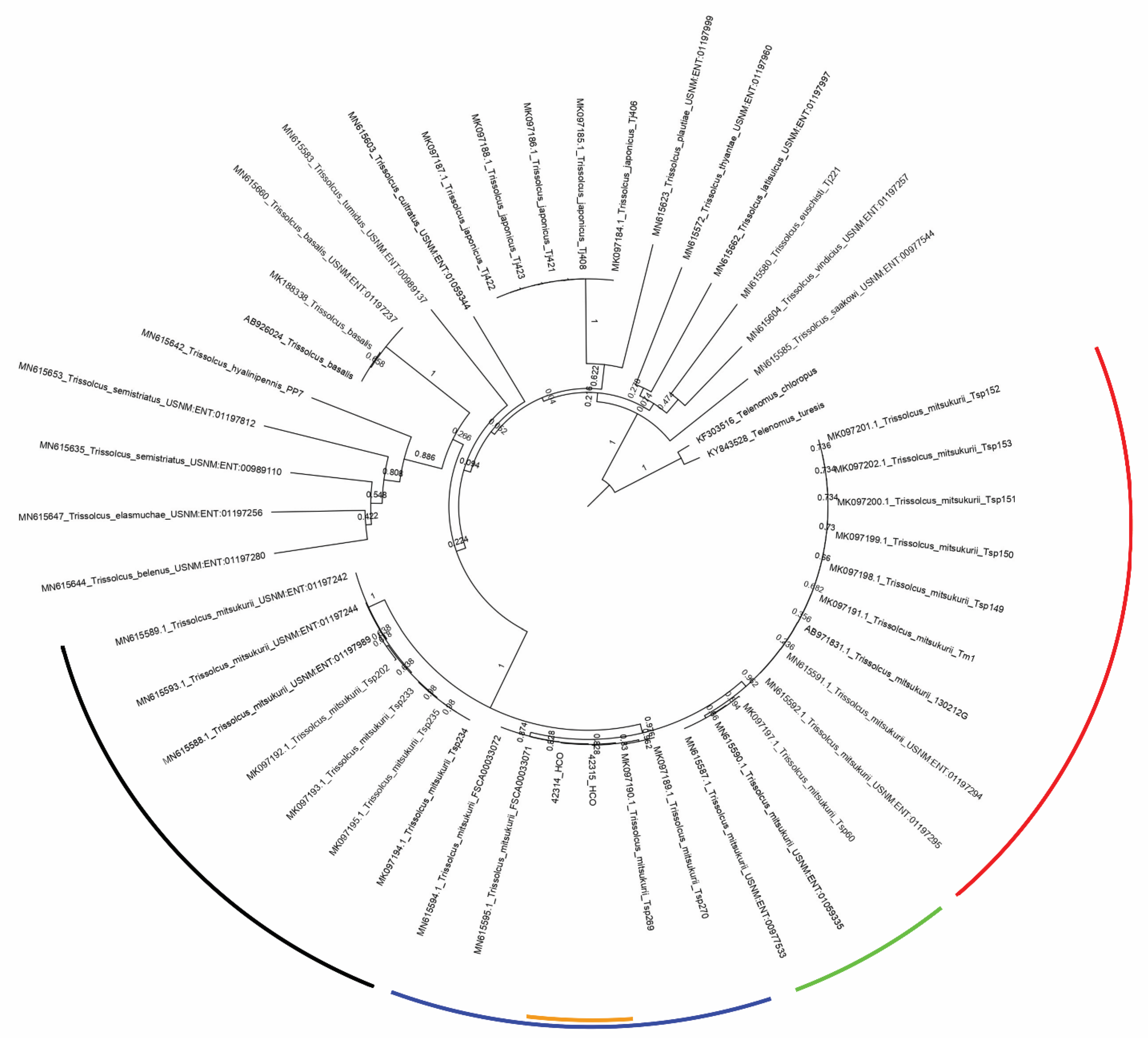
- DNA barcoding
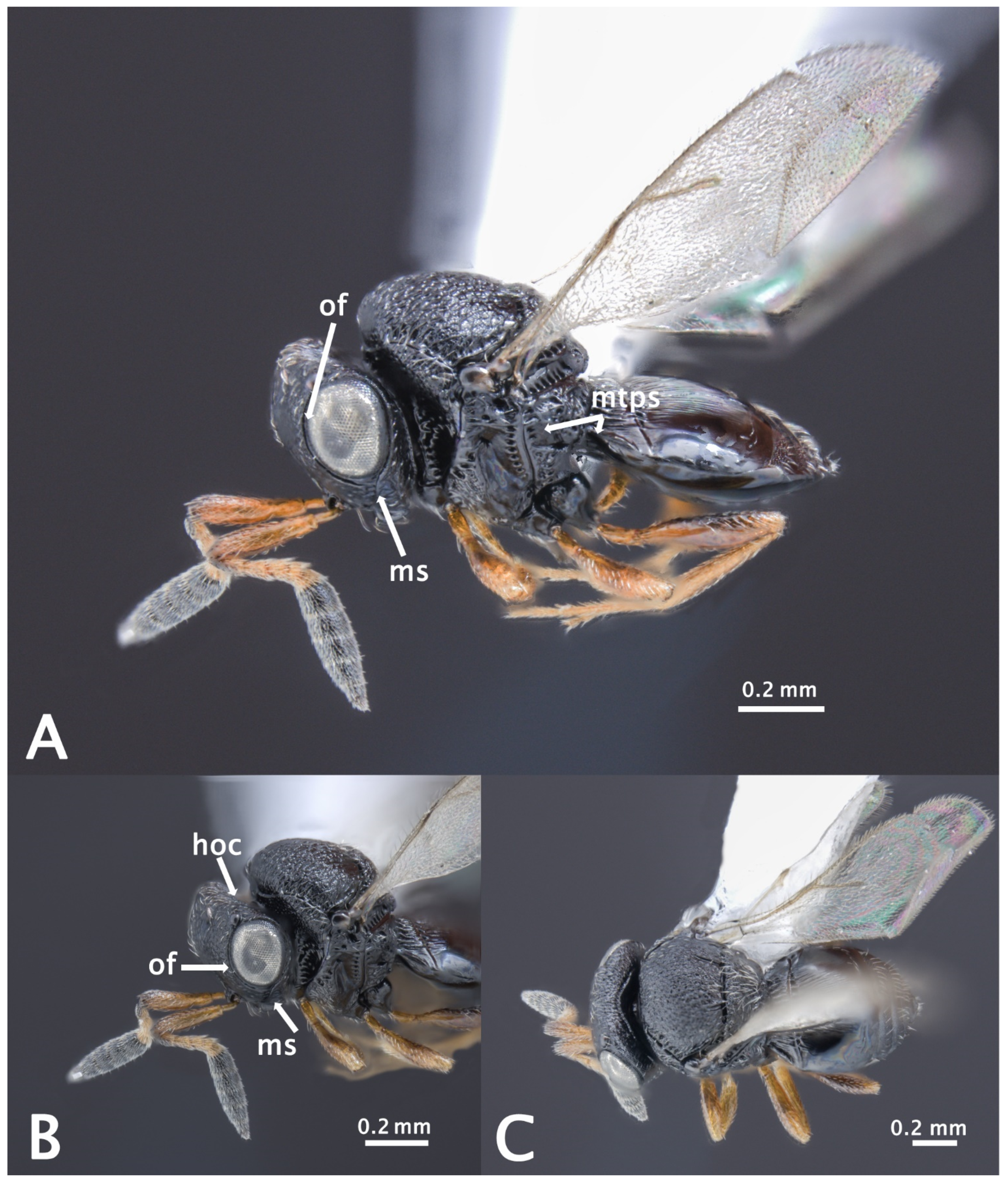
- Morphological identification
3. Results
Trissolcus mitsukurii
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vilà, M.; Hulme, P.E. Impact of Biological Invasions on Ecosystem Services; Springer: New York, NY, USA, 2017. [Google Scholar] [CrossRef]
- Kenis, M.; Auger-Rozenberg, M.-A.; Roques, A.; Timms, L.; Péré, C.; Cock, M.; Settele, J.; Augustin, S.; Lopez-Vaamonde, C. Ecological effects of invasive alien insects. Biol. Invasions 2008, 11, 21–45. [Google Scholar] [CrossRef]
- Kenis, M.; Branco, M. Chapter 5: Impact of alien terrestrial arthropods in Europe. BioRisk 2010, 4, 51–71. [Google Scholar] [CrossRef] [Green Version]
- Vaes-Petitgnat, S.; Nentwig, W. Environmental and economic impact of alien terrestrial arthropods in Europe. NeoBiota 2014, 22, 23–42. [Google Scholar]
- Schindler, S.; Staska, B.; Adam, M.; Rabitsch, W.; Essl, F. Alien species and public health impacts in Eu-rope: A literature review. NeoBiota 2015, 27, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Roy, H.E.; Bacher, S.; Essl, F.; Adriaens, T.; Aldridge, D.C.; Bishop, J.D.D.; Blackburn, T.M.; Branquart, E.; Brodie, J.; Carboneras, C.; et al. Developing a list of invasive alien species likely to threaten biodiversity and ecosystems in the European Union. Glob. Chang. Biol. 2018, 25, 1032–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roques, A.; Shi, J.; Auger-Rozenberg, M.-A.; Ren, L.; Augustin, S.; Luo, Y.-Q. Are Invasive Patterns of Non-native Insects Related to Woody Plants Differing Between Europe and China? Front. For. Glob. Chang. 2020, 2. [Google Scholar] [CrossRef] [Green Version]
- Bigler, F.; Babendreier, D.; Kuhlmann, U. (Eds.) Environmental Impact of Invertebrates for Biological Control of Arthropods: Methods and Risk Assessment; CABI Publishers: Walling-ford, UK, 2006. [Google Scholar] [CrossRef]
- Heimpel, G.E.; Cock, M. Shifting paradigms in the history of classical biological control. BioControl 2017, 63, 27–37. [Google Scholar] [CrossRef]
- Mason, P.G.; Olfert, O.O.; Haye, T.; Gariepy, T.D.; Abram, P.K.; Gillespie, D.R. Risks and benefits of accidental introductions of biological control agents in Canada. In Proceedings of the 5th International Symposium on Biological Control of Arthropods, Langkawi, Malaysia, 11–15 September 2017. [Google Scholar] [CrossRef]
- Masner, L.; Johnson, N.F.; Musetti, L. Calliscelio elegans (Perkins), a tramp species, and a review of the status of the genus Caenoteleia Kieffer (Hymenoptera: Platygastridae). Zootaxa 2009, 2237, 59–66. [Google Scholar] [CrossRef] [Green Version]
- Popovici, O.A.; Masner, L.; Viciriuc, M.; Pintilioaie, A.; Notton, D.G.; Talamas, E. New distribution data for some charismatic tramp species of Platygastroidea (Hymenoptera). Zootaxa 2018, 4370, 1–22. [Google Scholar] [CrossRef]
- Leskey, T.C.; Nielsen, A.L. Impact of the Invasive Brown Marmorated Stink Bug in North America and Europe: History, Biology, Ecology, and Management. Annu. Rev. Èntomol. 2018, 63, 599–618. [Google Scholar] [CrossRef] [Green Version]
- Conti, E.; Avila, G.; Barratt, B.; Cingolani, F.; Colazza, S.; Guarino, S.; Hoelmer, K.; Laumann, R.A.; Maistrello, L.; Martel, G.; et al. Biological control of invasive stink bugs: Review of global state and future prospects. Entomol. Exp. Appl. 2021, 169, 28–51. [Google Scholar] [CrossRef]
- Taylor, M.E.; Bundy, C.S.; McPherson, J.E. Life history and laboratory rearing of Bagrada hilaris (He-miptera: Heteroptera: Pentatomidae) with descriptions of immature stages. Ann. Entomol. Soc. Am. 2015, 108, 536–551. [Google Scholar] [CrossRef]
- Eger, J.E.; Ames, L.M.; Suiter, D.R.; Jenkins, T.M.; Rider, D.A.; Halbert, S.E. Occurrence of the Old World bug Megacopta cribaria (Fabricius) (Heteroptera: Plastaspididae) in Georgia: A serious home invader and potentil legume pest. Insecta Mundi 2010, 121, 1–11. [Google Scholar]
- Gardner, W.A.; Peeler, H.B.; Laforest, J.; Roberts, P.M.; Sparks, A.N.; Greene, J.K.; Reisig, D.; Suiter, D.R.; Bacheler, J.S.; Kidd, K.; et al. Confirmed Distribution and Occurrence of Megacopta cribraria (F.) (Hemiptera: Heteroptera: Plataspidae) in the Southeastern United States. J. Èntomol. Sci. 2013, 48, 118–127. [Google Scholar] [CrossRef] [Green Version]
- Talamas, E.J.; Herlihy, M.V.; Dieckhoff, C.; Hoelmer, K.A.; Buffington, M.; Bon, M.C.; Weber, D.C. Tris-solcus japonicus (Ashmead) (Hymenoptera, Scelionidae) emerges in North America. J. Hymenopt. Res. 2015, 43, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Milnes, J.M.; Wiman, N.G.; Talamas, E.J.; Brunner, J.F.; Hoelmer, K.A.; Buffington, M.L.; Beers, E.H. Discovery of an Exotic Egg Parasitoid of the Brown Marmorated Stink Bug, Halyomorpha halys (Stål) in the Pacific Northwest. Proc. Entomol. Soc. Wash. 2016, 118, 466–470. [Google Scholar] [CrossRef]
- Abram, P.K.; Talamas, E.J.; Acheampong, S.; Mason, P.G.; Gariepy, T.D. First detection of the samurai wasp, Trissolcus japonicus (Ashmead) (Hymenoptera, Scelionidae), in Canada. J. Hymenopt. Res. 2019, 68, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Sabbatini-Peverieri, G.; Talamas, E.; Bon, M.C.; Marianelli, L.; Bernardinelli, I.; Malossini, G.; Benvenuto, L.; Roversi, P.F.; Hoelmer, K. Two Asian egg parasitoids of Halyomorpha halys (Stål) (Hemiptera, Penta-tomidae) emerge in northern Italy: Trissolcus mitsukurii (Ashmead) and Trissolcus japonicus (Ashmead) (Hymenoptera, Scelionidae). J. Hymenopt. Res. 2018, 67, 37–53. [Google Scholar] [CrossRef]
- Stahl, J.; Tortorici, F.; Pontini, M.; Bon, M.C.; Hoelmer, K.; Marazzi, C.; Haye, T. First discovery of ad-ventive populations of Trissolcus japonicus in Europe. J. Pest Sci. 2019, 92, 371–379. [Google Scholar] [CrossRef] [Green Version]
- Dieckhoff, C.; Wenz, S.; Renninger, M.; Reißig, A.; Rauleder, H.; Zebitz, C.; Reetz, J.; Zimmermann, O. Add Germany to the List—Adventive Population of Trissolcus japonicus (Ashmead) (Hymenoptera: Scelionidae) Emerges in Germany. Insects 2021, 12, 414. [Google Scholar] [CrossRef]
- Scaccini, D.; Falagiarda, M.; Tortorici, F.; Martinez-Sañudo, I.; Tirello, P.; Reyes-Domínguez, Y.; Gallmetzer, A.; Tavella, L.; Zandigiacomo, P.; Duso, C.; et al. An insight into the role of Trissolcus mitsuku-rii as biological control agent of Halyomorpha halys in Northeastern Italy. Insects 2020, 11, 306. [Google Scholar] [CrossRef] [PubMed]
- Rot, M.; Maistrello, L.; Costi, E.; Bernardinelli, I.; Malossini, G.; Benvenuto, L.; Trdan, S. Native and Non-Native Egg Parasitoids Associated with Brown Marmorated Stink Bug (Halyomorpha halys [Stål, 1855]; Hemiptera: Pentatomidae) in Western Slovenia. Insects 2021, 12, 505. [Google Scholar] [CrossRef] [PubMed]
- Ganjisaffar, F.; Talamas, E.J.; Bon, M.-C.; Brown, B.V.; Gonzalez, L.; Perring, T.M. Trissolcus hyalinipennis Rajmohana & Narendran (Hymenoptera, Scelionidae), a parasitoid of Bagrada hilaris (Burmeister) (Hemiptera, Pentatomidae), emerges in North America. J. Hymenopt. Res. 2018, 65, 111–130. [Google Scholar] [CrossRef] [Green Version]
- Gardner, W.A.; Blount, J.L.; Golec, J.R.; Jones, W.A.; Hu, X.P.; Talamas, E.; Evans, R.M.; Dong, X.; Ray, C.H.; Buntin, G.D.; et al. Discovery of Paratelenomus saccharalis (Dodd) (Hymenoptera: Platygastridae), an Egg Parasitoid of Megacopta cribraria F. (Hemiptera: Plataspidae) in its Expanded North American Range. J. Èntomol. Sci. 2013, 48, 355–359. [Google Scholar] [CrossRef] [Green Version]
- Haye, T.; Hoelmer, K.A.; Rossi, J.P.; Streito, J.C.; et Tassus, X. Analyse de risque phytosanitaire express Halyomorpha halys—la punaise diabolique. In Avis de l’Anses, Rapport D’expertise Collective; Edition Scientifique, Maisons Alfort; 2014; Available online: http://www.anses.fr/sites/default/files/documents/SVEG2013sa0093Ra.pdf (accessed on 11 March 2021).
- Leskey, T.C.; Short, B.D.; Butler, B.R.; Wright, S.E. Impact of the Invasive Brown Marmorated Stink Bug, Halyomorpha halys(Stål), in Mid-Atlantic Tree Fruit Orchards in the United States: Case Studies of Commercial Management. Psyche A J. Èntomol. 2012, 2012, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Bosco, L.; Moraglio, S.T.; Tavella, L. Halyomorpha halys, a serious threat for hazelnut in newly invaded areas. J. Pest Sci. 2017, 91, 661–670. [Google Scholar] [CrossRef]
- Qiu, L.; Yang, Z.; Tao, W. Biology and Population Dynamics of Trissolcus halyomorphae. Sci. Silvae Sin. 2007, 43, 62–65. [Google Scholar] [CrossRef]
- Yang, Z.-Q.; Yao, Y.-X.; Qiu, L.-F.; Li, Z.-X. A New Species of Trissolcus (Hymenoptera: Scelionidae) Parasitizing Eggs of Halyomorpha halys (Heteroptera: Pentatomidae) in China with Comments on Its Biol-ogy. Annu. Entomol. Soc. Am. 2009, 102, 39–47. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, F.; Gariepy, T.; Mason, P.; Gillespie, D.; Talamas, E.; Haye, T. Seasonal parasitism and host specificity of Trissolcus japonicus in northern China. J. Pest Sci. 2017, 90, 1127–1141. [Google Scholar] [CrossRef] [Green Version]
- Hedstrom, C.; Lowenstein, D.; Andrews, H.; Bai, B.; Wiman, N. Pentatomid host suitability and the dis-covery of introduced populations of Trissolcus japonicus in Oregon. J. Pest Sci. 2017, 90, 1169–1179. [Google Scholar] [CrossRef]
- Charles, J.G.; Avila, G.A.; Hoelmer, K.A.; Hunt, S.; Gardner-Gee, R.; MacDonald, F.; Davis, V. Experi-mental assessment of the biosafety of Trissolcus japonicus in New Zealand, prior to the anticipated arrival of the invasive pest Halyomorpha halys. Biocontrol 2019, 64, 367–379. [Google Scholar] [CrossRef]
- Haye, T.; Moraglio, S.T.; Stahl, J.; Visentin, S.; Gregorio, T.; Tavella, L. Fundamental host range of Tris-solcus japonicus in Europe. J. Pest Sci. 2020, 93, 171–182. [Google Scholar] [CrossRef] [Green Version]
- Jarrett, B.J.M.; Pote, J.; Talamas, E.; Gut, L.; Szucs, M. The Discovery of Trissolcus japonicus (Hymenop-tera: Scelionidae) in Michigan. Great Lakes Entomol. 2019, 52, 8. [Google Scholar]
- Holthouse, M.C.; Schumm, Z.R.; Talamas, E.J.; Spears, L.R.; Alston, D.G. Surveys in northern Utah for egg parasitoids of Halyomorpha halys (Stål) (Hemiptera: Pentatomidae) detect Trissolcus japonicus (Ashmead) (Hymenoptera: Scelionidae). Biodivers. Data J. 2020, 8, e53363. [Google Scholar] [CrossRef] [PubMed]
- Moraglio, S.T.; Tortorici, F.; Pansa, M.G.; Castelli, G.; Pontini, M.; Scovero, S.; Visentin, S.; Tavella, L. A 3-year survey on parasitism of Halyomorpha halys by egg parasitoids in northern Italy. J. Pest Sci. 2019, 93, 183–194. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochon-drial cytochrome c oxydase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [Green Version]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Talamas, E.J.; Johnson, N.F.; Buffington, M. Key to Nearctic species of Trissolcus Ashmead (Hymenoptera, Scelionidae), natural enemies of native and invasive stink bugs (Hemiptera, Pentatomidae). J. Hymenopt. Res. 2015, 43, 45–110. [Google Scholar] [CrossRef] [Green Version]
- Talamas, E.; Buffington, M.; Hoelmer, K. Revision of Palearctic Trissolcus Ashmead (Hymenoptera, Scelionidae). J. Hymenopt. Res. 2017, 56, 79–261. [Google Scholar] [CrossRef] [Green Version]
- Zapponi, L.; Tortorici, F.; Anfora, G.; Bardella, S.; Bariselli, M.; Benvenuto, L.; Bernardinelli, I.; Butturini, A.; Caruso, S.; Colla, R.; et al. Assessing the Distribution of Exotic Egg Parasitoids of Halyomorpha halys in Europe with a Large-Scale Monitoring Program. Insects 2021, 12, 316. [Google Scholar] [CrossRef] [PubMed]
- Avila, G.A.; Charles, J.G. Modelling the potential geographic distribution of Trissolcus japonicus: A biologi-cal control agent of the brown marmorated stink bug, Halyomorpha halys. Biocontrol 2018, 63, 505–518. [Google Scholar] [CrossRef] [Green Version]
- Yonow, T.; Kriticos, D.; Ota, N.; Avila, G.; Hoelmer, K.; Chen, H.; Caron, V. Modelling the Potential Geographic Distribution of Two Trissolcus Species for the Brown Marmorated Stink Bug, Halyomorpha halys. Insects 2021, 12, 491. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, L.; Sabbatini-Peverieri, G.; Marianelli, L.; Rondoni, G.; Conti, E.; Roversi, P.F. Physiological host range of Trissolcus mitsukurii, a candidate biological control agent of Halyomorpha halys in Europe. J. Pest Sci. 2021, 102, 1–14. [Google Scholar] [CrossRef]
| Period | Area | Type of Sampling | Number of Egg Masses | Number of Parasitized Egg Masses | % of Parasitized Egg Masses |
|---|---|---|---|---|---|
| 2018–2019 | National-focus PACA | Sentinel-frozen | 1020 | 223 | 21.82 |
| Sentinel-fresh | 582 | 10 | 17.18 | ||
| 2020 | National-focus PACA | Natural | 129 | 60 | 46.51 |
| May–September 2020 | southwestern France | Sentinel-frozen | 234 | 13 | 5.55 |
| Sentinel-fresh | 15 | 2 | 13.33 | ||
| May–September 2020 | southwestern France | Natural | 289 | 79 | 27.33 |
| Species | Collection Code | Department Country | Year of Collection | GPS Coordinates (DMS) | Host Species | GenBank Accession Number |
|---|---|---|---|---|---|---|
| Trissolcus mitsukurii | 42314_HCO | Lot, France | 2020 | 44°50′8.21″, 0°29′48.534″ | Halyomorpha halys | MZ343334 |
| Trissolcus mitsukurii | 42315_HCO | Lot, France | 2020 | 44°50′8.21″, 0°29′48.534 | Halyomorpha halys | MZ343335 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bout, A.; Tortorici, F.; Hamidi, R.; Warot, S.; Tavella, L.; Thomas, M. First Detection of the Adventive Egg Parasitoid of Halyomorpha halys (Stål) (Hemiptera: Pentatomidae) Trissolcus mitsukurii (Ashmead) (Hymenoptera: Scelionidae) in France. Insects 2021, 12, 761. https://doi.org/10.3390/insects12090761
Bout A, Tortorici F, Hamidi R, Warot S, Tavella L, Thomas M. First Detection of the Adventive Egg Parasitoid of Halyomorpha halys (Stål) (Hemiptera: Pentatomidae) Trissolcus mitsukurii (Ashmead) (Hymenoptera: Scelionidae) in France. Insects. 2021; 12(9):761. https://doi.org/10.3390/insects12090761
Chicago/Turabian StyleBout, Alexandre, Francesco Tortorici, Rachid Hamidi, Sylvie Warot, Luciana Tavella, and Maud Thomas. 2021. "First Detection of the Adventive Egg Parasitoid of Halyomorpha halys (Stål) (Hemiptera: Pentatomidae) Trissolcus mitsukurii (Ashmead) (Hymenoptera: Scelionidae) in France" Insects 12, no. 9: 761. https://doi.org/10.3390/insects12090761
APA StyleBout, A., Tortorici, F., Hamidi, R., Warot, S., Tavella, L., & Thomas, M. (2021). First Detection of the Adventive Egg Parasitoid of Halyomorpha halys (Stål) (Hemiptera: Pentatomidae) Trissolcus mitsukurii (Ashmead) (Hymenoptera: Scelionidae) in France. Insects, 12(9), 761. https://doi.org/10.3390/insects12090761







