Comparative Transcriptome Analysis Reveals bmo-miR-6497-3p Regulate Circadian Clock Genes during the Embryonic Diapause Induction Process in Bivoltine Silkworm
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. mRNA and sRNA Library Construction and Sequencing
2.3. miRNA Identification
2.4. Screening of Differentially Expressed miRNAs
2.5. GO and KEGG Enrichment Analysis of Predicted Target Genes of the DEmiRs
2.6. Construction of miRNA Overexpression Vectors
2.7. Quantitative Real-Time PCR (qRT-PCR)
2.8. Dual Luciferase Reporter (DLR) Assay
3. Results
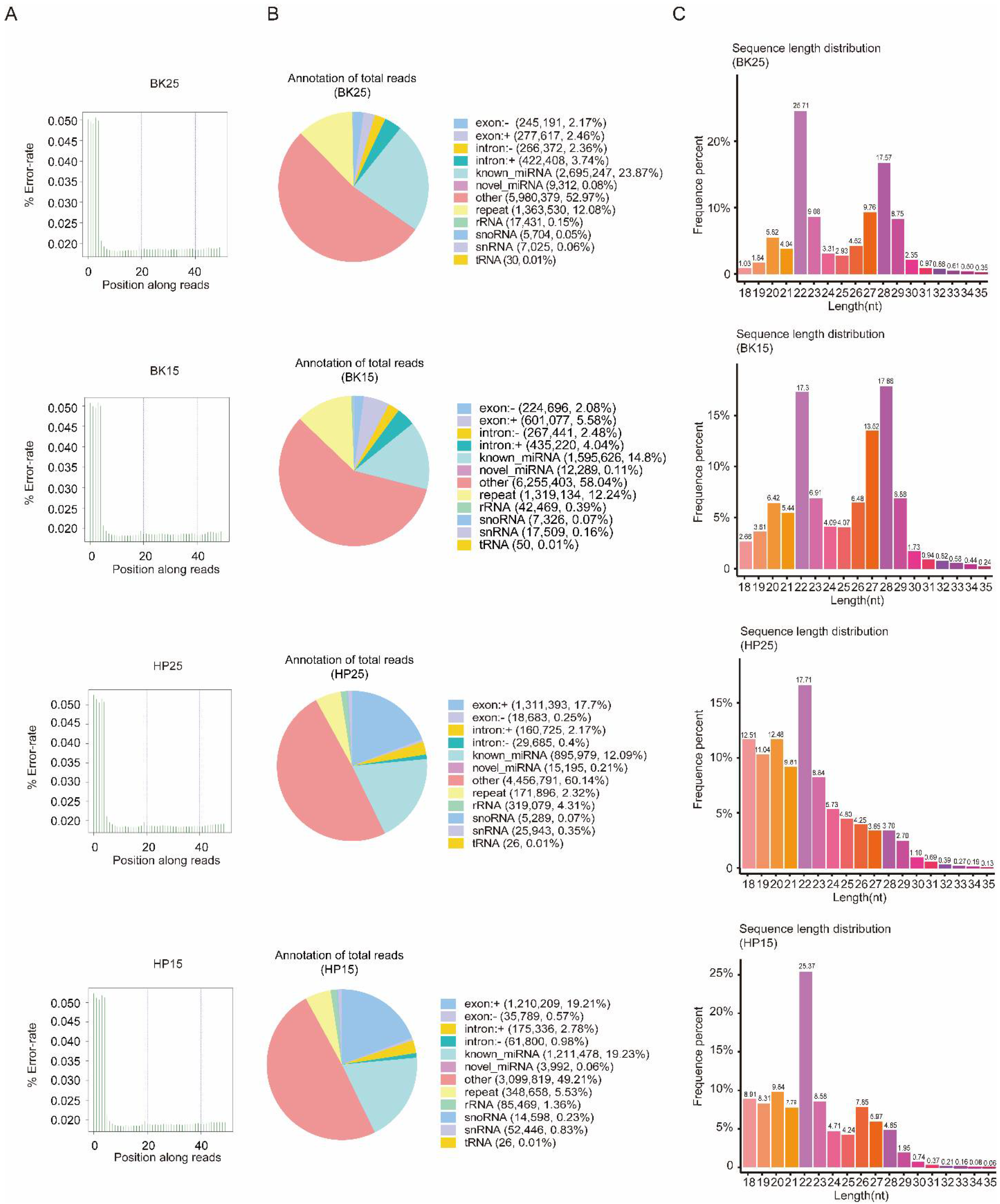
3.1. Quality Control and miRNA Identification
3.2. Differential Expression Analysis of miRNAs
3.3. The Identification of Potential Target Genes of DEmiRs
3.4. Functional Analysis of DECT Genes of DEmiRs
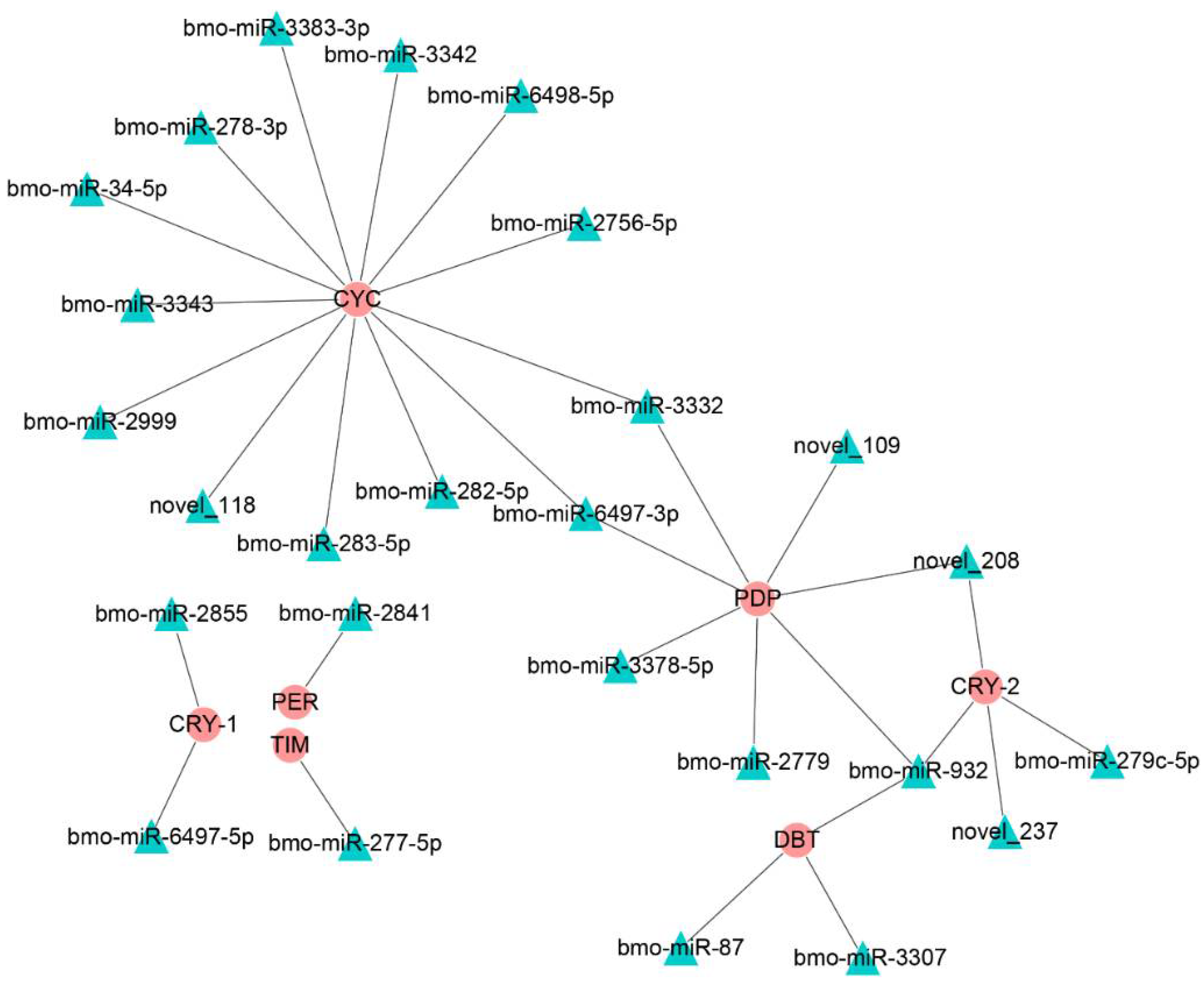
3.5. Interaction Analysis of bmo-miR-6497-3p and Circadian Clock Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Denlinger, D.L. Regulation of Diapause. Annu. Rev. Èntomol. 2002, 47, 93–122. [Google Scholar] [CrossRef] [PubMed]
- Macrae, T.H. Gene expression, metabolic regulation and stress tolerance during diapause. Cell. Mol. Life Sci. 2010, 67, 2405–2424. [Google Scholar] [CrossRef]
- Dickson, R.; Sanders, E. Factors Inducing Diapause in the Oriental Fruit Moth. J. Econ. Èntomol. 1945, 38, 605–606. [Google Scholar] [CrossRef]
- Emerson, K.J.; Bradshaw, W.E.; Holzapfel, C.M. Complications of complexity: Integrating environmental, genetic and hormonal control of insect diapause. Trends Genet. 2009, 25, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Valaitis, A.P.; Denlinger, D.L. Further evidence that diapause in the gypsy moth, Lymantria dispar, is regulated by ecdysteroids: A comparison of diapause and nondiapause strains. J. Insect Physiol. 1997, 43, 897–903. [Google Scholar] [CrossRef]
- Ohtaki, T.; Takahashi, M. Induction and termination of pupal diapause in relation to the change of ecdysone titer in the fleshfly, Sarcophaga peregrina. Jpn. J. Med. Sci. Biol. 1972, 25, 369–376. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Mohamed, A.A.M.; Takeda, M. Serotonin Receptor B May Lock the Gate of PTTH Release/Synthesis in the Chinese Silk Moth, Antheraea pernyi; A Diapause Initiation/Maintenance Mechanism? PLoS ONE 2013, 8, e79381. [Google Scholar] [CrossRef] [Green Version]
- Yin, C.M.; Chippendale, G.M. Juvenile hormone regulation of the larval diapause of the Southwestern corn borer, Diatraea grandiosella. J. Insect Physiol. 1973, 19, 2403–2420. [Google Scholar] [CrossRef]
- Readio, J.; Chen, M.-H.; Meola, R. Juvenile Hormone Biosynthesis in Diapausing and NondiapausingCulex pipiens (Diptera: Culicidae). J. Med. Èntomol. 1999, 36, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Singtripop, T.; Wanichacheewa, S.; Sakurai, S. Juvenile hormone-mediated termination of larval diapause in the bamboo borer, Omphisa fuscidentalis. Insect Biochem. Mol. Biol. 2000, 30, 847–854. [Google Scholar] [CrossRef]
- Bradshaw, W.E.; Holzapfel, C.M. Circadian clock genes, ovarian development and diapause. BMC Biol. 2010, 8, 115. [Google Scholar] [CrossRef] [Green Version]
- Edery, I. Circadian rhythms in a nutshell. Physiol. Genom. 2000, 3, 59–74. [Google Scholar] [CrossRef]
- Gegear, R.J.; Foley, L.E.; Casselman, A.; Reppert, S.M. Animal cryptochromes mediate magnetoreception by an unconventional photochemical mechanism. Nat. Cell Biol. 2010, 463, 804–807. [Google Scholar] [CrossRef]
- Yoshii, T.; Ahmad, M.; Helfrich-Förster, C. Cryptochrome mediates light-dependent magnetosensitivity of Drosophila’s circadian clock. PLoS Biol. 2009, 7, e1000086. [Google Scholar] [CrossRef]
- Tauber, E.; Zordan, M.; Sandrelli, F.; Pegoraro, M.; Osterwalder, N.; Breda, C.; Daga, A.; Selmin, A.; Monger, K.; Benna, C.; et al. Natural Selection Favors a Newly Derived timeless Allele in Drosophila melanogaster. Science 2007, 316, 1895–1898. [Google Scholar] [CrossRef]
- Helfrich-Förster, C.; Stengl, M.; Homberg, U. Organization of the circadian system in insects. Chronobiol. Int. 1998, 15, 567–594. [Google Scholar] [CrossRef]
- Panda, S.; Antoch, M.P.; Miller, B.H.; Su, A.I.; Schook, A.B.; Straume, M.; Schultz, P.G.; Kay, S.A.; Takahashi, J.; Hogenesch, J.B. Coordinated Transcription of Key Pathways in the Mouse by the Circadian Clock. Cell 2002, 109, 307–320. [Google Scholar] [CrossRef] [Green Version]
- Schendzielorz, J.; Stengl, M. Candidates for the light entrainment pathway to the circadian clock of the Madeira cockroach Rhyparobia maderae. Cell Tissue Res. 2013, 355, 447–462. [Google Scholar] [CrossRef]
- Gentile, C.; Sehadova, H.; Simoni, A.; Chen, C.; Stanewsky, R. Cryptochrome Antagonizes Synchronization of Drosophila’s Circadian Clock to Temperature Cycles. Curr. Biol. 2013, 23, 185–195. [Google Scholar] [CrossRef] [Green Version]
- Meuti, M.E.; Stone, M.; Ikeno, T.; Denlinger, D.L. Functional circadian clock genes are essential for the overwintering diapause of the Northern house mosquito, Culex pipiens. J. Exp. Biol. 2015, 218, 412–422. [Google Scholar] [CrossRef] [Green Version]
- Ikeno, T.; I Tanaka, S.; Numata, H.; Goto, S.G. Photoperiodic diapause under the control of circadian clock genes in an insect. BMC Biol. 2010, 8, 116. [Google Scholar] [CrossRef] [Green Version]
- Ikeno, T.; Numata, H.; Goto, S. Circadian clock genes period and cycle regulate photoperiodic diapause in the bean bug Riptortus pedestris males. J. Insect Physiol. 2011, 57, 935–938. [Google Scholar] [CrossRef]
- Matsuda, N.; Numata, H.; Udaka, H. Transcriptomic changes in the pea aphid, Acyrthosiphon pisum: Effects of the seasonal timer and photoperiod. Comp. Biochem. Physiol. Part D Genom. Proteom. 2020, 36, 100740. [Google Scholar] [CrossRef]
- Kaniewska, M.; Vaněčková, H.; Doležel, D.; Kotwica-Rolinska, J. Light and Temperature Synchronizes Locomotor Activity in the Linden Bug, Pyrrhocoris apterus. Front. Physiol. 2020, 11, 242. [Google Scholar] [CrossRef]
- Jarwar, A.R.; Hao, K.; Bitume, E.V.; Ullah, H.; Cui, D.; Nong, X.; Wang, G.; Tu, X.; Zhang, Z. Comparative Transcriptomic Analysis Reveals Molecular Profiles of Central Nervous System in Maternal Diapause Induction of Locusta migratoria. G3 2019, 9, 3287–3296. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Tian, Z.; Guo, S.; Liu, W.; Zhu, F.; Wang, X.-P. Circadian clock genes link photoperiodic signals to lipid accumulation during diapause preparation in the diapause-destined female cabbage beetles Colaphellus bowringi. Insect Biochem. Mol. Biol. 2019, 104, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ambros, V.; Bartel, B.; Bartel, D.P.; Burge, C.B.; Carrington, J.; Chen, X.; Dreyfuss, G.; Eddy, S.; Griffiths-Jones, S.; Marshall, M.; et al. A uniform system for microRNA annotation. RNA 2003, 9, 277–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holley, C.L.; Topkara, V.K. An Introduction to Small Non-coding RNAs: miRNA and snoRNA. Cardiovasc. Drugs Ther. 2011, 25, 151–159. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target Recognition and Regulatory Functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabian, M.R.; Sonenberg, N.; Filipowicz, W. Regulation of mRNA Translation and Stability by microRNAs. Annu. Rev. Biochem. 2010, 79, 351–379. [Google Scholar] [CrossRef] [Green Version]
- Hadj-Moussa, H.; Storey, K.B. The OxymiR response to oxygen limitation: A comparative microRNA perspective. J. Exp. Biol. 2020, 223, jeb204594. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Sun, Y.-H.; Chiang, V.L. Stress-responsive microRNAs inPopulus. Plant J. 2008, 55, 131–151. [Google Scholar] [CrossRef]
- Qiu, C.-W.; Liu, L.; Feng, X.; Hao, P.-F.; He, X.; Cao, F.; Wu, F. Genome-Wide Identification and Characterization of Drought Stress Responsive microRNAs in Tibetan Wild Barley. Int. J. Mol. Sci. 2020, 21, 2795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyons, P.J.; Storey, K.B.; Morin, P.J. Expression of miRNAs in response to freezing and anoxia stresses in the freeze tolerant fly Eurosta solidaginis. Cryobiology 2015, 71, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Morin, M.D.; Frigault, J.J.; Lyons, P.J.; Crapoulet, N.; Boquel, S.; Storey, K.B.; Morin, P.J. Amplification and quantification of cold-associated microRNAs in the Colorado potato beetle (Leptinotarsa decemlineata) agricultural pest. Insect Mol. Biol. 2017, 26, 574–583. [Google Scholar] [CrossRef]
- Zhang, X.; Zabinsky, R.; Teng, Y.; Cui, M.; Han, M. microRNAs play critical roles in the survival and recovery of Caenorhabditis elegans from starvation-induced L1 diapause. Proc. Natl. Acad. Sci. USA 2011, 108, 17997–18002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teleman, A.A.; Cohen, S.M. Drosophila lacking microRNA miR-278 are defective in energy homeostasis. Genes Dev. 2006, 20, 417–422. [Google Scholar] [CrossRef] [Green Version]
- Ramírez, C.M.; Goedeke, L.; Rotllan, N.; Yoon, J.-H.; Cirera-Salinas, D.; Mattison, J.A.; Suárez, Y.; de Cabo, R.; Gorospe, M.; Fernández-Hernando, C. MicroRNA 33 Regulates Glucose Metabolism. Mol. Cell. Biol. 2013, 33, 2891–2902. [Google Scholar] [CrossRef] [Green Version]
- Brennecke, J.; Hipfner, D.R.; Stark, A.; Russell, R.B.; Cohen, S.M. bantam Encodes a Developmentally Regulated microRNA that Controls Cell Proliferation and Regulates the Proapoptotic Gene hid in Drosophila. Cell 2003, 113, 25–36. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.-L.; Jin, F.; Ye, X.; Zhu, L.; Yang, J.-S.; Yang, W.-J. Expression profiles of miRNAs and involvement of miR-100 and miR-34 in regulation of cell cycle arrest in Artemia. Biochem. J. 2015, 470, 223–231. [Google Scholar] [CrossRef]
- Barrio, L.; Dekanty, A.; Milán, M. MicroRNA-mediated regulation of Dp53 in the Drosophila fat body contributes to metabolic adaptation to nutrient deprivation. Cell Rep. 2014, 8, 528–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ezcurra, A.L.D.L.; Bertolin, A.P.; Kim, K.; Katz, M.J.; Gándara, L.; Misra, T.; Luschnig, S.; Perrimon, N.; Melani, M.; Wappner, P. miR-190 Enhances HIF-Dependent Responses to Hypoxia in Drosophila by Inhibiting the Prolyl-4-hydroxylase Fatiga. PLoS Genet. 2016, 12, e1006073. [Google Scholar] [CrossRef] [Green Version]
- Hammell, C.M.; Karp, X.; Ambros, V. A feedback circuit involving let-7-family miRNAs and DAF-12 integrates envi-ronmental signals and developmental timing in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2009, 106, 18668–18673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbott, A.L.; Alvarez-Saavedra, E.; Miska, E.A.; Lau, N.C.; Bartel, D.P.; Horvitz, H.R.; Ambros, V. The let-7 MicroRNA family members mir-48, mir-84, and mir-241 function together to regulate devel-opmental timing in Caenorhabditis elegans. Dev. Cell 2005, 9, 403–414. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, J.A.; Nachman, R.J.; Denlinger, D.L. Distinct microRNA and mRNA responses elicited by ecdysone, diapause hormone and a diapause hormone analog at diapause termination in pupae of the corn earworm, Helicoverpa zea. Gen. Comp. Endocrinol. 2019, 278, 68–78. [Google Scholar] [CrossRef]
- Batz, Z.A.; Goff, A.C.; Armbruster, P.A. MicroRNAs are differentially abundant during Aedes albopictus diapause maintenance but not diapause induction. Insect Mol. Biol. 2017, 26, 721–733. [Google Scholar] [CrossRef]
- Meuti, M.E.; Bautista-Jimenez, R.; Reynolds, J.A. Evidence that microRNAs are part of the molecular toolkit regulating adult reproductive diapause in the mosquito, Culex pipiens. PLoS ONE 2018, 13, e0203015. [Google Scholar] [CrossRef]
- Reynolds, J.A.; Peyton, J.T.; Denlinger, D.L. Changes in microRNA abundance may regulate diapause in the flesh fly, Sarcophaga bullata. Insect Biochem. Mol. Biol. 2017, 84, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.-H.; Sato, Y.; Ikeda, M.; Yamashita, O. Stage-dependent and Temperature-controlled Expression of the Gene Encoding the Precursor Protein of Diapause Hormone and Pheromone Biosynthesis Activating Neuropeptide in the Silkworm, Bombyx mori. J. Biol. Chem. 1995, 270, 3804–3808. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, K. Studies on the voltinism of the silkworm, Bombyx mori. Bull. Sericult. Exp. Stn. 1924, 6, 411–455. [Google Scholar]
- Morita, A.; Niimi, T.; Yamashita, O. Physiological differentiation of DH-PBAN-producing neurosecretory cells in the silkworm embryo. J. Insect Physiol. 2003, 49, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Trapnell, C.; Pop, M.; Salzberg, S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009, 10, R25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedlander, M.; Mackowiak, S.; Li, N.; Chen, W.; Rajewsky, N. miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Res. 2011, 40, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Wen, M.; Shen, Y.; Shi, S.; Tang, T. miREvo: An integrative microRNA evolutionary analysis platform for next-generation sequencing experi-ments. BMC Bioinform. 2012, 13, 140. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Chen, J.; Li, Z.; Li, X.; Hu, X.; Huang, Y.; Zhao, X.; Liang, C.; Wang, Y.; Sun, L.; et al. Integrated Profiling of MicroRNAs and mRNAs: MicroRNAs Located on Xq27.3 Associate with Clear Cell Renal Cell Carcinoma. PLoS ONE 2010, 5, e15224. [Google Scholar] [CrossRef]
- Storey, J. The positive false discovery rate: A Bayesian interpretation and the q-value. Ann. Stat. 2003, 31, 2013–2035. [Google Scholar] [CrossRef]
- Guo, H.; Jiang, L.; Xia, Q. Selection of reference genes for analysis of stress-responsive genes after challenge with viruses and temperature changes in the silkworm Bombyx mori. Mol. Genet Genomics 2016, 291, 999–1004. [Google Scholar] [CrossRef]
- Liak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar]
- Rehmsmeier, M.; Steffen, P.; Höchsmann, M.; Giegerich, R. Fast and effective prediction of microRNA/target duplexes. RNA 2004, 10, 1507–1517. [Google Scholar] [CrossRef] [Green Version]
- Andersen, J.P.; Schwartz, A.; Gramsbergen, J.B.; Loeschcke, V. Dopamine levels in the mosquito Aedes aegypti during adult development, following blood feeding and in response to heat stress. J. Insect Physiol. 2006, 52, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Bang, S.; Hyun, S.; Hong, S.-T.; Kang, J.; Jeong, K.; Park, J.-J.; Choe, J.; Chung, J. Dopamine Signalling in Mushroom Bodies Regulates Temperature-Preference Behaviour in Drosophila. PLoS Genet. 2011, 7, e1001346. [Google Scholar] [CrossRef]
- Ueno, T.; Tomita, J.; Tanimoto, H.; Endo, K.; Ito, K.; Kume, S.; Kume, K. Identification of a dopamine pathway that regulates sleep and arousal in Drosophila. Nat. Neurosci. 2012, 15, 1516–1523. [Google Scholar] [CrossRef] [PubMed]
- Chu, F.; Qiu, J.F.; Tao, H.; Li, X.; Shu, M.Y.; Liu, H.J.; SiMa, Y.; Xu, S. Impact of cyclical changes in temperature on circadian clock genes expression in bombyx Bmn cells. Arch. Insect Biochem. Physiol. 2016, 91, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Lee, J.-E.; Padgett, R.W.; Edery, I. Circadian regulation of a limited set of conserved microRNAs in Drosophila. BMC Genom. 2008, 9, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadener, S.; Menet, J.S.; Sugino, K.; Horwich, M.D.; Weissbein, U.; Nawathean, P.; Vagin, V.V.; Zamore, P.D.; Nelson, S.B.; Rosbash, M. A role for microRNAs in the Drosophila circadian clock. Genes Dev. 2009, 23, 2179–2191. [Google Scholar] [CrossRef] [Green Version]
- Meuti, M.E.; Denlinger, D.L. Evolutionary Links between Circadian Clocks and Photoperiodic Diapause in Insects. Integr. Comp. Biol. 2013, 53, 131–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ragland, G.J.; Armbruster, P.; Meuti, M. Evolutionary and functional genetics of insect diapause: A call for greater integration. Curr. Opin. Insect Sci. 2019, 36, 74–81. [Google Scholar] [CrossRef]
- Saunders, D.S. Dormancy, Diapause, and the Role of the Circadian System in Insect Photoperiodism. Annu. Rev. Èntomol. 2020, 65, 373–389. [Google Scholar] [CrossRef] [Green Version]
- Ikeno, T.; Ishikawa, K.; Numata, H.; Goto, S.G. Circadian clock geneClockis involved in the photoperiodic response of the bean bugRiptortus pedestris. Physiol. Èntomol. 2013, 38, 157–162. [Google Scholar] [CrossRef]
- Kotwica-Rolinska, J.; Pivarciova, L.; Vaneckova, H.; Dolezel, D. The role of circadian clock genes in the photoperiodic timer of the linden bug Pyrrhocoris apterus during the nymphal stage. Physiol. Entomol. 2017, 42, 266–273. [Google Scholar] [CrossRef]
- Mukai, A.; Goto, S.G. The clock gene period is essential for the photoperiodic response in the jewel wasp Nasonia vitripennis (Hymenoptera: Pteromalidae). Appl. Èntomol. Zool. 2016, 51, 185–194. [Google Scholar] [CrossRef] [Green Version]
- Kawakami, A.; Kataoka, H.; Oka, T.; Mizoguchi, A.; Kimura-Kawakami, M.; Adachi, T.; Iwami, M.; Nagasawa, H.; Suzuki, A.; Ishizaki, H. Molecular cloning of the Bombyx mori prothoracicotropic hormone. Science 1990, 247, 1333–1335. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.M. Physiology of insect diapause: The role of the brain in the production and termination of pupal dormancy in the giant silkworm, Platysamia cecropia. Anat. Rec. 1946, 94, 425. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.-J.; Zhang, Q.; Kang, L.; Xu, W.-H.; Denlinger, D.L. Molecular characterization and expression of prothoracicotropic hormone during development and pupal diapause in the cotton bollworm, Helicoverpa armigera. J. Insect Physiol. 2005, 51, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, A.; Ohsumi, S.; Kobayashi, K.; Okamoto, N.; Yamada, N.; Tateishi, K.; Fujimoto, Y.; Kataoka, H. Prothoracicotropic hormone acts as a neuroendocrine switch between pupal diapause and adult de-velopment. PLoS ONE 2013, 8, e60824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akitomo, S.; Egi, Y.; Nakamura, Y.; Suetsugu, Y.; Oishi, K.; Sakamoto, K. Genome-wide microarray screening for Bombyx mori genes related to transmitting the determination outcome of whether to produce diapause or nondiapause eggs. Insect Sci. 2017, 24, 187–193. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Ge, X.; Ling, L.; Zeng, B.; Xu, J.; Aslam, A.F.; You, L.; Palli, S.R.; Huang, Y.; Tan, A. CYP18A1 regulates tissue-specific steroid hormone inactivation in Bombyx mori. Insect Biochem. Mol. Biol. 2014, 54, 33–41. [Google Scholar] [CrossRef] [Green Version]
- Smykal, V.; Daimon, T.; Kayukawa, T.; Takaki, K.; Shinoda, T.; Jindra, M. Importance of juvenile hormone signaling arises with competence of insect larvae to metamorphose. Dev. Biol. 2014, 390, 221–230. [Google Scholar] [CrossRef] [Green Version]
- Chengcang, W.; Koichi, S.; Eiichi, K. Induction of Non-diapause Eggs by Imidazole Derivative KK-42 in the Diapause Type ofBombyx moriSilkworm. Biosci. Biotechnol. Biochem. 1996, 60, 1201–1203. [Google Scholar] [CrossRef]
- Fukuda, S. Hormonal control of diapause in the silkworm. Gen. Comp. Endocrinol. 1962, 1, 337–340. [Google Scholar] [CrossRef]
- Lin, X.; Yu, N.; Smagghe, G. FoxO mediates the timing of pupation through regulating ecdysteroid biosynthesis in the red flour beetle, Tribolium castaneum. Gen. Comp. Endocrinol. 2018, 258, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Sim, C.; Denlinger, D.L. Insulin signaling and FOXO regulate the overwintering diapause of the mosquito Culex pipiens. Proc. Natl. Acad. Sci. USA 2008, 105, 6777–6781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klappa, P.; Zimmermann, M.; Zimmermann, R. The membrane proteins TRAMp and sec61 alpha p may be involved in post-translational transport of presecretory proteins into mammalian microsomes. FEBS Lett. 1994, 341, 281–287. [Google Scholar] [CrossRef] [Green Version]
- Talley, E.M.; Solórzano, G.; Lei, Q.; Kim, D.; Bayliss, D.A. CNS Distribution of Members of the Two-Pore-Domain (KCNK) Potassium Channel Family. J. Neurosci. 2001, 21, 7491–7505. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Zhang, P.; Gao, Q.; Feng, X.; Han, L.; Zhang, F.; Bai, Y.; Han, M.; Hu, H.; Dai, F.; et al. Comparative Transcriptome Analysis Reveals bmo-miR-6497-3p Regulate Circadian Clock Genes during the Embryonic Diapause Induction Process in Bivoltine Silkworm. Insects 2021, 12, 739. https://doi.org/10.3390/insects12080739
Liu L, Zhang P, Gao Q, Feng X, Han L, Zhang F, Bai Y, Han M, Hu H, Dai F, et al. Comparative Transcriptome Analysis Reveals bmo-miR-6497-3p Regulate Circadian Clock Genes during the Embryonic Diapause Induction Process in Bivoltine Silkworm. Insects. 2021; 12(8):739. https://doi.org/10.3390/insects12080739
Chicago/Turabian StyleLiu, Lulu, Pan Zhang, Qiang Gao, Xiaoge Feng, Lan Han, Fengbin Zhang, Yanmin Bai, Minjin Han, Hai Hu, Fangyin Dai, and et al. 2021. "Comparative Transcriptome Analysis Reveals bmo-miR-6497-3p Regulate Circadian Clock Genes during the Embryonic Diapause Induction Process in Bivoltine Silkworm" Insects 12, no. 8: 739. https://doi.org/10.3390/insects12080739
APA StyleLiu, L., Zhang, P., Gao, Q., Feng, X., Han, L., Zhang, F., Bai, Y., Han, M., Hu, H., Dai, F., Zhang, G., & Tong, X. (2021). Comparative Transcriptome Analysis Reveals bmo-miR-6497-3p Regulate Circadian Clock Genes during the Embryonic Diapause Induction Process in Bivoltine Silkworm. Insects, 12(8), 739. https://doi.org/10.3390/insects12080739





