Residual Tau-Fluvalinate in Honey Bee Colonies Is Coupled with Evidence for Selection for Varroa destructor Resistance to Pyrethroids
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Honey Bee Colonies Selection and Location
2.2. Wax and Beebread Samples
2.2.1. Sample Collection
2.2.2. Chemical Analysis
2.3. Varroa Mite Collection and Genotyping
2.3.1. Mite Collection
2.3.2. DNA Extraction and PCR-RLFP Assay
2.4. Allele Frequencies
3. Results
3.1. Tau-Fluvalinate Residues in Wax and Beebread
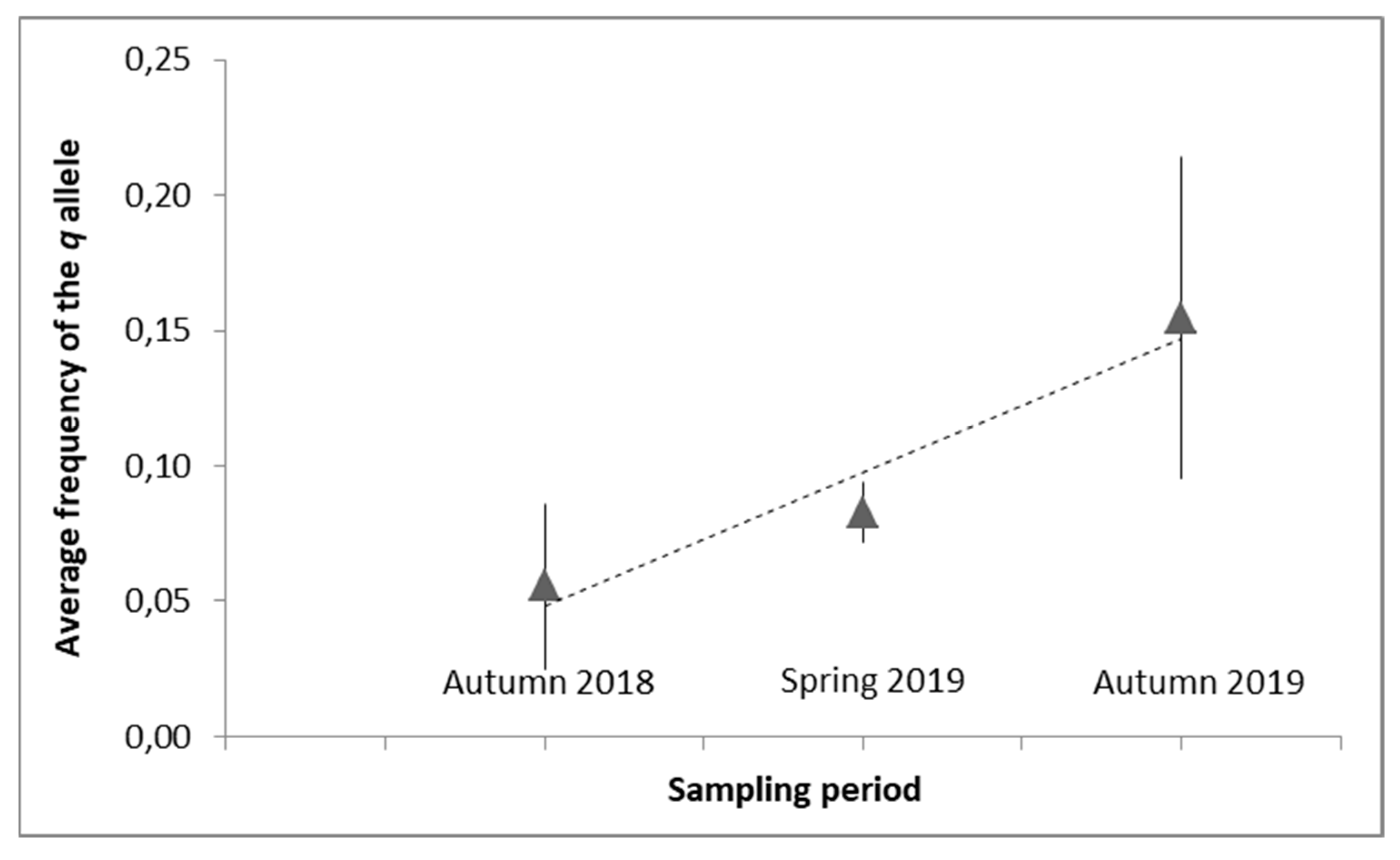
3.2. Pyrethroid-Resistance Allele Frequencies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosenkranz, P.; Aumeier, P.; Ziegelmann, B. Biology and Control of Varroa Destructor. J. Invertebr. Pathol. 2010, 103, S96–S119. [Google Scholar] [CrossRef]
- Evans, J.D.; Cook, S.C. Genetics and Physiology of Varroa Mites. Curr. Opin. Insect Sci. 2018, 26, 130–135. [Google Scholar] [CrossRef]
- Martin, S.J.; Brettell, L.E. Deformed Wing Virus in Honeybees and Other Insects. Annu. Rev. Virol. 2019, 6, 49–69. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, S.D.; Ochoa, R.; Bauchan, G.; Gulbronson, C.; Mowery, J.D.; Cohen, A.; Lim, D.; Joklik, J.; Cicero, J.M.; Ellis, J.D.; et al. Varroa Destructor Feeds Primarily on Honey Bee Fat Body Tissue and Not Hemolymph. Proc. Natl Acad. Sci. USA 2018. [Google Scholar] [CrossRef] [PubMed]
- Annoscia, D.; Brown, S.P.; Di Prisco, G.; De Paoli, E.; Del Fabbro, S.; Frizzera, D.; Zanni, V.; Galbraith, D.A.; Caprio, E.; Grozinger, C.M.; et al. Haemolymph Removal by Varroa Mite Destabilizes the Dynamical Interaction between Immune Effectors and Virus in Bees, as Predicted by Volterra’s Model. Proc. R. Soc. B Biol. Sci. 2019, 286, 2019033. [Google Scholar] [CrossRef] [PubMed]
- Buendía, M.; Martín-Hernández, R.; Ornosa, C.; Barrios, L.; Bartolomé, C.; Higes, M. Epidemiological Study of Honeybee Pathogens in Europe: The Results of Castilla-La Mancha (Spain). Spanish J. Agric. Res. 2018, 16, 1–10. [Google Scholar] [CrossRef]
- Tremolada, P.; Bernardinelli, I.; Colombo, M.; Spreafico, M.; Vighi, M. Coumaphos Distribution in the Hive Ecosystem: Case Study for Modeling Applications. Ecotoxicology 2004, 13, 589–601. [Google Scholar] [CrossRef]
- Sammataro, D.; De Guzman, L.; George, S.; Ochoa, R.; Otis, G. Standard Methods for Tracheal Mite Research. J. Apic. Res. 2013, 52, 1–20. [Google Scholar] [CrossRef]
- Dmitryjuk, M.; Zółtowska, K.; Fraczek, R.; Lipiński, Z. Esterases of Varroa Destructor (Acari: Varroidae), Parasitic Mite of the Honeybee. Exp. Appl. Acarol. 2014, 62, 499–510. [Google Scholar] [CrossRef] [PubMed]
- González-Cabrera, J.; Rodríguez-Vargas, S.; Davies, T.G.E.; Field, L.M.; Schmehl, D.; Ellis, J.D.; Krieger, K.; Williamson, M.S. Novel Mutations in the Voltage-Gated Sodium Channel of Pyrethroid-Resistant Varroa Destructor Populations from the Southeastern USA. PLoS ONE 2016, 11, e0155332. [Google Scholar] [CrossRef]
- Kamler, M.; Nesvorna, M.; Stara, J.; Erban, T.; Hubert, J. Comparison of Tau-Fluvalinate, Acrinathrin, and Amitraz Effects on Susceptible and Resistant Populations of Varroa Destructor in a Vial Test. Exp. Appl. Acarol. 2016, 69, 1–9. [Google Scholar] [CrossRef]
- Higes, M.; Martín-Hernández, R.; Hernández-Rodríguez, C.S.; González-Cabrera, J. Assessing the Resistance to Acaricides in Varroa Destructor from Several Spanish Locations. Parasitol. Res. 2020, 119, 3595–3601. [Google Scholar] [CrossRef]
- Rinkevich, F.D. Detection of Amitraz Resistance and Reduced Treatment Efficacy in the Varroa Mite, Varroa Destructor, within Commercial Beekeeping Operations. PLoS ONE 2020, 15, e0227264. [Google Scholar] [CrossRef]
- Trouiller, J. Monitoring Varroa Jacobsoni Resistance to Pyrethroids in Western Europe. Apidologie 1998, 29, 537–546. [Google Scholar] [CrossRef]
- Milani, N. The Resistance of Varroa Jacobsoni Oud to Pyrethroids: A Laboratory Assay. Apidologie 1995, 26, 415–429. [Google Scholar] [CrossRef]
- Martin, S.J. Acaricide (Pyrethroid) Resistance in Varroa Destructor. Bee World 2004, 85, 67–69. [Google Scholar] [CrossRef]
- Dong, K.; Du, Y.; Rinkevich, F.; Nomura, Y.; Xu, P.; Wang, L.; Silver, K.; Zhorov, B.S. Molecular Biology of Insect Sodium Channels and Pyrethroid Resistance. Insect Biochem. Mol. Biol. 2014, 50, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Huang, Z.Y.; Dong, K. Molecular Characterization of an Arachnid Sodium Channel Gene from the Varroa Mite (Varroa Destructor). Insect Biochem. Mol. Biol. 2003, 33, 733–739. [Google Scholar] [CrossRef]
- O’Reilly, A.O.; Khambay, B.P.S.; Williamson, M.S.; Field, L.M.; Wallace, B.A.; Davies, T.G.E. Modelling Insecticide-Binding Sites in the Voltage-Gated Sodium Channel. Biochem. J. 2006, 396, 255–263. [Google Scholar] [CrossRef] [PubMed]
- González-Cabrera, J.; Davies, T.G.E.; Field, L.M.; Kennedy, P.J.; Williamson, M.S. An Amino Acid Substitution (L925V) Associated with Resistance to Pyrethroids in Varroa Destructor. PLoS ONE 2013, 8, e82941. [Google Scholar] [CrossRef]
- González-Cabrera, J.; Bumann, H.; Rodríguez-Vargas, S.; Kennedy, P.J.; Krieger, K.; Altreuther, G.; Hertel, A.; Hertlein, G.; Nauen, R.; Williamson, M.S. A Single Mutation Is Driving Resistance to Pyrethroids in European Populations of the Parasitic Mite, Varroa Destructor. J. Pest Sci. 2018, 91, 1137–1144. [Google Scholar] [CrossRef]
- Alissandrakis, E.; Ilias, A.; Tsagkarakou, A. Resistencia Diana Al Piretroide En Poblaciones Griegas Del Parásito de La Miel de Abeja Varroa Destructor (Acari: Varroidae). J. Apic. Res. 2017, 56, 625–630. [Google Scholar] [CrossRef]
- Panini, M.; Reguzzi, M.C.; Chiesa, O.; Cominelli, F.; Lupi, D.; Moores, G.; Mazzoni, E. Pyrethroid Resistance in Italian Populations of the Mite Varroa Destructor: A Focus on the Lombardy Region. Bull. Insectology 2019, 72, 227–232. [Google Scholar]
- Reeves, A.M.; O’Neal, S.T.; Fell, R.D.; Brewster, C.C.; Anderson, T.D. In-Hive Acaricides Alter Biochemical and Morphological Indicators of Honey Bee Nutrition, Immunity, and Development. J. Insect Sci. 2018, 18, 8. [Google Scholar] [CrossRef]
- Ogihara, S.; Inoue, O.; Yamagami, T.; Yanagimoto, K.; Uematsu, K.; Hisada, Y.; Uchida, T.; Ohta, M.; Suzuki-Inoue, K. Clinical Characteristics and Molecular Analysis of USA300 and ST 764 Methicillin-Resistant Staphylococcus Aureus Isolates from Outpatients in Japan by PCR-Based Open Reading Frame Typing. J. Infect. Chemother. 2021, 27, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Chauzat, M.-P.; Faucon, J.-P.; Martel, A.-C.; Lachaize, J.; Cougoule, N.; Aubert, M. A Survey of Pesticide Residues in Pollen Loads Collected by Honey Bees in France. J. Econ. Entomol. 2009, 99, 253–262. [Google Scholar] [CrossRef]
- Mullin, C.A.; Frazier, M.; Frazier, J.L.; Ashcraft, S.; Simonds, R.; vanEngelsdorp, D.; Pettis, J.S. High Levels of Miticides and Agrochemicals in North American Apiaries: Implications for Honey Bee Health. PLoS ONE 2010, 5, e9754. [Google Scholar] [CrossRef] [PubMed]
- Orantes-Bermejo, F.J.; Pajuelo, A.G.; Megías, M.M.; Fernández-Píñar, C.T. Pesticide Residues in Beeswax and Beebread Samples Collected from Honey Bee Colonies (Apis mellifera L.) in Spain. Possible Implications for Bee Losses. J. Apic. Res. 2010, 49, 243–250. [Google Scholar] [CrossRef]
- Simon-Delso, N.; Martin, G.S.; Bruneau, E.; Minsart, L.A.; Mouret, C.; Hautier, L. Honeybee Colony Disorder in Crop Areas: The Role of Pesticides and Viruses. PLoS ONE 2014, 9, e103073. [Google Scholar] [CrossRef] [PubMed]
- Wilmart, O.; Legrève, A.; Scippo, M.L.; Reybroeck, W.; Urbain, B.; De Graaf, D.C.; Steurbaut, W.; Delahaut, P.; Gustin, P.; Nguyen, B.K.; et al. Residues in Beeswax: A Health Risk for the Consumer of Honey and Beeswax? J. Agric. Food Chem. 2016, 64, 8425–8434. [Google Scholar] [CrossRef] [PubMed]
- Calatayud-Vernich, P.; Calatayud, F.; Simó, E.; Picó, Y. Pesticide Residues in Honey Bees, Pollen and Beeswax: Assessing Beehive Exposure. Environ. Pollut. 2018, 241, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Manning, R. Chemical Residues in Beebread, Honey, Pollen and Wax Samples Collected from Bee Hives Placed on Canola Crops in Western Australia. J. Apic. Res. 2018, 57, 696–708. [Google Scholar] [CrossRef]
- Alonso-Prados, E.; Muñoz, I.; De la Rúa, P.; Serrano, J.; Fernández-Alba, A.R.; García-Valcárcel, A.I.; Hernando, M.D.; Alonso, Á.; Alonso-Prados, J.L.; Bartolomé, C.; et al. The Toxic Unit Approach as a Risk Indicator in Honey Bees Surveillance Programmes: A Case of Study in Apis mellifera Iberiensis. Sci. Total Environ. 2020, 698, 134208. [Google Scholar] [CrossRef]
- Nozal, M.J.; Imaz, E.; Luis Bernal, J.; Nieto, J.L.; Higes, M.; Bernal, J.; Álvarez-Rivera, G.; Cifuentes, A. An Optimized Extraction Procedure for Determining Acaricide Residues in Foundation Sheets of Beeswax by Using Gas Chromatography-Mass Spectrometry. Agronomy 2021, 11, 804. [Google Scholar] [CrossRef]
- Bonzini, S.; Tremolada, P.; Bernardinelli, I.; Colombo, M.; Vighi, M. Predicting Pesticide Fate in the Hive (Part 1): Experimentally Determined τ-Fluvalinate Residues in Bees, Honey and Wax. Apidologie 2011, 42, 378–390. [Google Scholar] [CrossRef]
- Luna, A.; Alonso, R.; Cutillas, V.M.; Ferrer, C.M.; Gómez-Ramos, M.J.; Hernando, D.; Valverde, A.; Flores, J.M.; Fernández-Alba, A.R.; Fernández-Alba, A.R. Removal of Pesticide Residues from Beeswax Using a Methanol Extraction-Based Procedure: A Pilot-Scale Study. Environ. Technol. Innov. 2021, 23, 101606. [Google Scholar] [CrossRef]
- Li, Y.; Kelley, R.A.; Anderson, T.D.; Lydy, M.J. Development and Comparison of Two Multi-Residue Methods for the Analysis of Select Pesticides in Honey Bees, Pollen, and Wax by Gas Chromatography-Quadrupole Mass Spectrometry. Talanta 2015, 140, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Svečnjak, L.; Chesson, L.A.; Gallina, A.; Maia, M.; Martinello, M.; Mutinelli, F.; Muz, M.N.; Nunes, F.M.; Saucy, F.; Tipple, B.J.; et al. Standard Methods for Apis mellifera Beeswax Research. J. Apic. Res. 2019, 58, 1–108. [Google Scholar] [CrossRef]
- de España, G. Disposición Final Quinta. In REAL DECRETO 608/2006, de 19 de Mayo, Por el Que se Establece y Regula un Programa Nacional de Lucha y Control de Las Enfermeda- des de Las Abejas de la Miel; de España, G., Ed.; Boletin Oficial del Estado: Madrid, Spain, 2006; pp. 1097–1102. [Google Scholar]
- Stara, J.; Pekar, S.; Nesvorna, M.; Kamler, M.; Doskocil, I.; Hubert, J. Spatio-Temporal Dynamics of Varroa Destructor Resistance to Tau-Fluvalinate in Czechia, Associated with L925V Sodium Channel Point Mutation. Pest Manag. Sci. 2019, 75, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Martín-Hernández, R.; Botías, C.; Bailón, E.G.; Martínez-Salvador, A.; Prieto, L.; Meana, A.; Higes, M. Microsporidia Infecting Apis mellifera: Coexistence or Competition. Is Nosema Ceranae Replacing Nosema Apis? Environ. Microbiol. 2012, 14, 2127–2138. [Google Scholar] [CrossRef] [PubMed]
- Millán-Leiva, A.; Hernández-Rodríguez, C.S.; González-Cabrera, J. New PCR–RFLP Diagnostics Methodology for Detecting Varroa Destructor Resistant to Synthetic Pyrethroids. J. Pest Sci. 2018, 91, 937–941. [Google Scholar] [CrossRef]
- Blenau, W.; Rademacher, E.; Baumann, A. Plant Essential Oils and Formamidines as Insecticides/Acaricides: What Are the Molecular Targets? Apidologie 2012, 43, 334–347. [Google Scholar] [CrossRef]
- Tremolada, P.; Bernardinelli, I.; Rossaro, B.; Colombo, M.; Vighi, M. Predicting Pesticide Fate in the Hive (Part 2): Development of a Dynamic Hive Model. Apidologie 2011, 42, 439–456. [Google Scholar] [CrossRef][Green Version]
- Sponsler, D.B.; Johnson, R.M. Mechanistic Modeling of Pesticide Exposure: The Missing Keystone of Honey Bee Toxicology. Environ. Toxicol. Chem. 2017, 36, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Ministerio de Agricultura, P.Y.A. Encuesta de Utilización de Productos Fitosanitarios 2013. In Plan de Acción Nacional Para el Uso Sostenible de Productos Fitosanitarios (PAN); Ministerio de Agricultura, P.Y.A., Ed.; Instituto Nacional de Estadistica: Madrid, Spain, 2013; p. 37. [Google Scholar]
- Dai, P.; Jack, C.J.; Mortensen, A.N.; Ellis, J.D. Acute Toxicity of Five Pesticides to Apis mellifera Larvae Reared in Vitro. Pest Manag. Sci. 2017, 73, 2282–2286. [Google Scholar] [CrossRef]
- Zhu, W.; Schmehl, D.R.; Mullin, C.A.; Frazier, J.L. Four Common Pesticides, Their Mixtures and a Formulation Solvent in the Hive Environment Have High Oral Toxicity to Honey Bee Larvae. PLoS ONE 2014, 9, e14720. [Google Scholar] [CrossRef] [PubMed]
- Sokól, R. Wpływ Wielomiesięcznego Pozostawania. Med. Wet. 1996, 52, 718–720. [Google Scholar]
- Rinderer, T.E.; De Guzman, L.I.; Lancaster, V.A.; Delatte, G.T.; Stelzer, J.A. Varroa in the Mating Yard: I. The Effects of Varroa Jacobsoni and Apistan® on Drone Honey Bees. Am. Bee J. 1999, 139, 134–139. [Google Scholar]
- Haarmann, T.; Spivak, M.; Weaver, D.; Weaver, B.; Glenn, T. Effects of Fluvalinate and Coumaphos on Queen Honey Bees (Hymenoptera: Apidae) in Two Commercial Queen Rearing Operations. J. Econ. Entomol. 2002, 95, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Anelli, C.M.; Sheppard, W.S. Sub-Lethal Effects of Pesticide Residues in Brood Comb on Worker Honey Bee (Apis mellifera) Development and Longevity. PLoS ONE 2011, 6, e14720. [Google Scholar] [CrossRef]
- Beaurepaire, A.L.; Krieger, K.J.; Moritz, R.F.A. Seasonal Cycle of Inbreeding and Recombination of the Parasitic Mite Varroa Destructor in Honeybee Colonies and Its Implications for the Selection of Acaricide Resistance. Infect. Genet. Evol. 2017, 50, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Tadei, R.; Domingues, C.E.C.; Malaquias, J.B.; Camilo, E.V.; Malaspina, O.; Silva-Zacarin, E.C.M. Late Effect of Larval Co-Exposure to the Insecticide Clothianidin and Fungicide Pyraclostrobin in Africanized Apis mellifera. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Fulton, C.A.; Huff Hartz, K.E.; Fell, R.D.; Brewster, C.C.; Reeve, J.D.; Lydy, M.J. An Assessment of Pesticide Exposures and Land Use of Honey Bees in Virginia. Chemosphere 2019, 222, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Kochansky, J.; Wilzer, K.; Feldlaufer, M. Comparison of the Transfer of Coumaphos from Beeswax into Syrup and Honey. Apidologie 2001, 32, 119–125. [Google Scholar] [CrossRef]
- Kliot, A.; Ghanim, M. Fitness Costs Associated with Insecticide Resistance. Pest Manag. Sci. 2012, 68, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
| Colony | Autumn 18 | Spring 19 | Autumn 19 | |||
|---|---|---|---|---|---|---|
| Wax | Beebread | Wax | Beebread | Wax | Beebread | |
| A11 | - | - | 90.6 | 680.1 | 90.2 | 167.1 |
| A12 | 24.4 | 1863.3 | 58.4 | 713.7 | 57.5 | 172.3 |
| A13 | 92.1 | 1943.6 | 103.6 | 660.6 | - | - |
| A14 | 82.6 | 1960.7 | 58.0 | 704.3 | - | - |
| A15 | 68.2 | 1902.9 | 69.1 | 715.2 | 64.0 | 260.9 |
| A16 | 107.7 | 1940.1 | 72.4 | 723.1 | 70.1 | 340.2 |
| A17 | 61.3 | 1929.6 | - | - | - | - |
| A18 | 70.6 | 2129.3 | 80.3 | 450.1 | - | - |
| A19 | 44.9 | 1954.7 | 54.1 | 520.4 | 60.1 | 340.3 |
| A20 | 82.0 | 2020.1 | 72.5 | 901.8 | 69.6 | 541.2 |
| Mean | 70.4 | 1960.5 | 73.2 | 674.4 | 68.6 | 303.7 |
| SD | 25.0 | 76.2 | 16.2 | 128.7 | 11.7 | 139.2 |
| Sampling Period | SS | SR | RR | p | |
|---|---|---|---|---|---|
| Autumn 2018 | Obs | 747 | 50 | 21 | |
| Exp | 728.6 | 86.8 | 2.6 | *** | |
| Spring 2019 | Obs | 75 | 8 | 3 | |
| Exp | 72.6 | 12.9 | 0.6 | *** | |
| Autumn 2019 | Obs | 459 | 117 | 36 | |
| Exp | 437.6 | 159.8 | 14.6 | *** |
| Colony | Frequency of q (Mean ± SE) | Tau-Fluvalinate in Wax | ||
|---|---|---|---|---|
| Autumn 2018 | Autumn 2019 | Autumn 2018 | Autumn 2019 | |
| A12 | 0.01 ± 0.01 | 0.08 ± 0.04 | 24.4 | 57.5 |
| A15 | 0.04 ± 0.01 | 0.16 ± 0.06 | 68.2 | 64.0 |
| A16 | 0.15 ± 0.04 | 0.18 ± 0.05 | 107.7 | 70.1 |
| A19 | 0.05 ± 0.05 | 0.19 ± 0.05 | 44.9 | 60.1 |
| A20 | 0.06 ± 0.05 | 0.00 ± 0.00 | 82.0 | 69.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benito-Murcia, M.; Bartolomé, C.; Maside, X.; Bernal, J.; Bernal, J.L.; del Nozal, M.J.; Meana, A.; Botías, C.; Martín-Hernández, R.; Higes, M. Residual Tau-Fluvalinate in Honey Bee Colonies Is Coupled with Evidence for Selection for Varroa destructor Resistance to Pyrethroids. Insects 2021, 12, 731. https://doi.org/10.3390/insects12080731
Benito-Murcia M, Bartolomé C, Maside X, Bernal J, Bernal JL, del Nozal MJ, Meana A, Botías C, Martín-Hernández R, Higes M. Residual Tau-Fluvalinate in Honey Bee Colonies Is Coupled with Evidence for Selection for Varroa destructor Resistance to Pyrethroids. Insects. 2021; 12(8):731. https://doi.org/10.3390/insects12080731
Chicago/Turabian StyleBenito-Murcia, María, Carolina Bartolomé, Xulio Maside, José Bernal, José Luis Bernal, María Jesús del Nozal, Aránzazu Meana, Cristina Botías, Raquel Martín-Hernández, and Mariano Higes. 2021. "Residual Tau-Fluvalinate in Honey Bee Colonies Is Coupled with Evidence for Selection for Varroa destructor Resistance to Pyrethroids" Insects 12, no. 8: 731. https://doi.org/10.3390/insects12080731
APA StyleBenito-Murcia, M., Bartolomé, C., Maside, X., Bernal, J., Bernal, J. L., del Nozal, M. J., Meana, A., Botías, C., Martín-Hernández, R., & Higes, M. (2021). Residual Tau-Fluvalinate in Honey Bee Colonies Is Coupled with Evidence for Selection for Varroa destructor Resistance to Pyrethroids. Insects, 12(8), 731. https://doi.org/10.3390/insects12080731









