Comparative Morphology of the Stinger in Social Wasps (Hymenoptera: Vespidae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarmiento, C.E. Hymenopteran venom apparatus. In Encyclopedia of Social Insects; Starr, C.K., Ed.; Springer: Cham, Switzerland, 2021; pp. 504–512. [Google Scholar] [CrossRef]
- Macalintal, E.A.; Starr, C.K. Comparative morphology of the stinger in the social wasp genus Ropalidia (Hymenoptera: Vespidae). Mem. Entomol. Soc. Wash. 1996, 17, 108–115. [Google Scholar]
- Kumpanenko, A.S.; Gladun, D.V. Functional morphology of the sting apparatus of the spider wasp Cryptocheilus versicolor (Scopoli, 1763) (Hymenoptera: Pompilidae). Entomol. Sci. 2018, 21, 124–132. [Google Scholar] [CrossRef]
- Hermann, H.R.; Blum, M.S. Defensive mechanisms in the social Hymenoptera. In Social Insects; Hermann, H.R., Ed.; Academic: Cambridge, MA, USA, 1981; Volume 2, pp. 77–97. [Google Scholar]
- Hermann, H.R. Elaboration and reduction of the venom apparatus in the aculeate Hymenoptera. In Defensive Mechanisms in Social Insects; Hermann, H.R., Ed.; Praeger: Westport, CT, USA, 1984; pp. 201–259. [Google Scholar]
- Packer, L. Comparative morphology of the skeletal parts of the sting apparatus of bees (Hymenoptera: Apoidea). Zool. J. Linnaean Soc. 2003, 138, 1–38. [Google Scholar] [CrossRef] [Green Version]
- Quicke, D.L.J.; Leralec, A.; Vilhelmsen, L. Oviposition structure and function in the parasitic Hymenoptera with an exploration of new hypotheses. Atti Dell’accademia Naz. Ital. Di Entomol. Rend. 1999, 47, 197–239. [Google Scholar]
- Swammerdam, J. The Book of Nature; or, The History of Insects; Seyff, C.G., Ed.; Plus Tables and Indices: London, UK, 1758; p. 53. [Google Scholar]
- Hermann, H.R. Sting autotomy, a defensive mechanism in certain social Hymenoptera. Insectes Sociaux 1971, 18, 111–120. [Google Scholar] [CrossRef]
- Starr, C.K.; Sarmiento, C.E. Sting autotomy. In Encyclopedia of Social Insects; Starr, C.K., Ed.; Springer: Cham, Switzerland, 2021; pp. 881–883. [Google Scholar] [CrossRef]
- Snodgrass, R.E. Principles of Insect Morphology; McGraw-Hill: Ithaca, NY, USA, 1935; p. 687. [Google Scholar]
- Jayasvasti, S.; Wongsiri, S. Stings of honey bees in Asia. Honeybee Sci. 1993, 14, 105–109. [Google Scholar]
- Tautz, J. The Buzz about Bees; Springer: Berlin, Germany, 2008; p. 284. [Google Scholar]
- Darwin, C. On the Origin of Species by Means of Natural Selection; Murray, J., Ed.; Online at Project Gutenberg: London, UK, 1859; p. 502. [Google Scholar]
- Laboulène, A. Sur la physiologie de l’aiguillon des insectes hyménoptères. Comptes Rendus Séances Mémoires Sociètè Biologie 1852, 4, 17–18. [Google Scholar]
- Rietschel, P. Bau und Funktion des Wehrstachels der staatenbildenden Bienen und Wespen. Z. Morphol. Okol. Tiere 1937, 33, 313–357. [Google Scholar] [CrossRef]
- Poore, D.M. Comparative study of the lancets and sheaths of some aculeate Hymenoptera. Bull. South. Calif. Acad. Sci. 1974, 73, 42–47. [Google Scholar]
- Gadallah, N.S.; Asserv, B.J. Comparative study of the skeletal parts of the sting apparatus in some sphecid species from Saudi Arabia (Hymenoptera: Sphecidae). Linz. Biologische. Beiträge 2004, 36, 1393–1412. [Google Scholar]
- Khadri, S.N.E.N. Comparative sting morphology of aculeate Hymenoptera. Master’s Thesis, University of Agricultural Sciences, Bangalore, Bengaluru, India, 2014; p. 79. [Google Scholar]
- Blum, M.S.; Hermann, H.R. Venoms and venom apparatuses of the Formicidae: Myrmeciinae, Ponerinae, Dorylinae, Pseudomyrmecinad, Myrmicinae, and Formicinae). In Handbook of Experimental Pharmacology; Bettini, S., Ed.; Springer: Berlin/Heidelberg, Germany, 1978; pp. 801–869. [Google Scholar]
- Hermann, H.R. The hymenopterous poison apparatus: Evolutionary trends in three closely related subfamilies of ants (Hymenoptera: Formicidae). J. Ga. Entomol. Soc. 1969, 4, 123–141. [Google Scholar]
- Hermann, H.R.; Blum, M.S.; Wheeler, J.W.; Overal, W.L.; Schmidt, J.O.; Chao, J.T. Comparative anatomy and chemistry of the venom apparatus and mandibular glands in Dinoponera grandis (Guérin) and Paraponera clavata (F.) (Formicidae). Ann. Ent. Soc. Am. 1984, 77, 272–279. [Google Scholar] [CrossRef]
- Kugler, C. A comparative study of the myrmicine sting apparatus (Hymenoptera, Formicidae). Studia Entomol. 1978, 20, 413–548. [Google Scholar]
- Brothers, D.J.; Finnamore, A.T. Superfamily Vespoidea. In Hymenoptera of the World: An Identification Guide to Families; Goulet, H., Huber, J.T., Eds.; Agriculture: Ottawa, IL, Canada, 1993; pp. 161–270. [Google Scholar]
- Cowan, D.P. The solitary and presocial Vespidae. In The Social Biology of Wasps; Ross, K.G., Matthews, R.W., Eds.; Cornell University Press: Ithaca, NY, USA, 1991; pp. 33–73. [Google Scholar]
- Field, J.P. Hover wasps (Stenogastrinae). In Encyclopedia of Social Insects; Starr, C.K., Ed.; Springer: Cham, Switzerland, 2021; pp. 500–504. [Google Scholar] [CrossRef]
- Sumner, S.; Cini, A. Paper wasps (Polistes). In Encyclopedia of Social Insects; Starr, C.K., Ed.; Springer: Cham, Switzerland, 2021; pp. 697–709. [Google Scholar] [CrossRef]
- Jeanne, R.L. Swarm-founding wasps. In Encyclopedia of Social Insects; Starr, C.K., Ed.; Springer: Cham, Switzerland, 2021; pp. 918–934. [Google Scholar] [CrossRef]
- Yamane, S.; Yamane, S. Vespinae. In Encyclopedia of Social Insects; Starr, C.K., Ed.; Springer: Cham, Switzerland, 2021; pp. 1000–1008. [Google Scholar] [CrossRef]
- Da Silva, M.; Noll, F.B.; Carpenter, J.M. The usefulness of the sting apparatus in phylogenetic reconstructions in vespids, with emphasis on the Epiponini. Neotrop. Entomol. 2014, 43, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Piekarski, P.K.; Carpenter, J.M.; Lemmon, A.R.; Lemmon, E.M.; Sharanowski, B.J. Phylogenetic evidence overturns current conceptions of social evolution in wasps (Vespidae). Mol. Biol. Evol. 2019, 35, 2097–2109. [Google Scholar] [CrossRef] [PubMed]
- Hermann, H.R.; Chao, J.T. Morphology of the venom apparatus of Mischocyttarus mexicanus cubicola in the United States. Fla. Entomol. 1984, 67, 516–520. [Google Scholar] [CrossRef]
- Hermann, H.R.; Krispyn, J.W. The hymenopterous venom apparatus. XIV. Vespula maculata (Hymenoptera: Vespidae). J. Ga. Entomol. Soc. 1975, 10, 307–313. [Google Scholar]
- Hunt, A.N.; Hermann, H.R. The hymenopterous poison apparatus. X. Polistes annularis (Hymenoptera: Vespidae). J. Ga. Entomol. Soc. 1970, 5, 201–216. [Google Scholar]
- De Fatima Manzolini-Palma, M.; Gobbi, N. Sting autotomy, sting morphology and sociality in neotropical vespids (Hymenoptera: Vespidae). J. Hymenopt. Res. 1997, 6, 152–162. [Google Scholar]
- Silveira, O.T.; Silveira, A.T. Comparative morphology of skeletal parts of the sting apparatus in neotropical polistine social wasps (Hymenoptera, Vespidae, Polistinae). Sociobiology 1994, 25, 295–327. [Google Scholar]
- Hermann, H.R.; Chao, J.T. Furcula, a major component of the hymenopterous poison apparatus. Int. J. Insects Morphol. Embryol. 1983, 12, 321–337. [Google Scholar] [CrossRef]
- Shorter, J.R.; Rueppell, O. A review of self-destructive behaviors in social insects. Insectes Sociaux 2012, 59, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Blackith, R.E. An analysis of of polymorphism in social wasps. Insectes Sociaux 1958, 5, 263–272. [Google Scholar] [CrossRef]
- Cockell, C.S. The Equations of Life: How Physics Shapes Evolution; Atlantic Books: London, UK, 2018; p. 352. [Google Scholar]
- Oster, G.F.; Wilson, E.O. Caste and Ecology in the Social Insects; Princeton University Press: Princeton, NJ, USA, 1978. [Google Scholar]
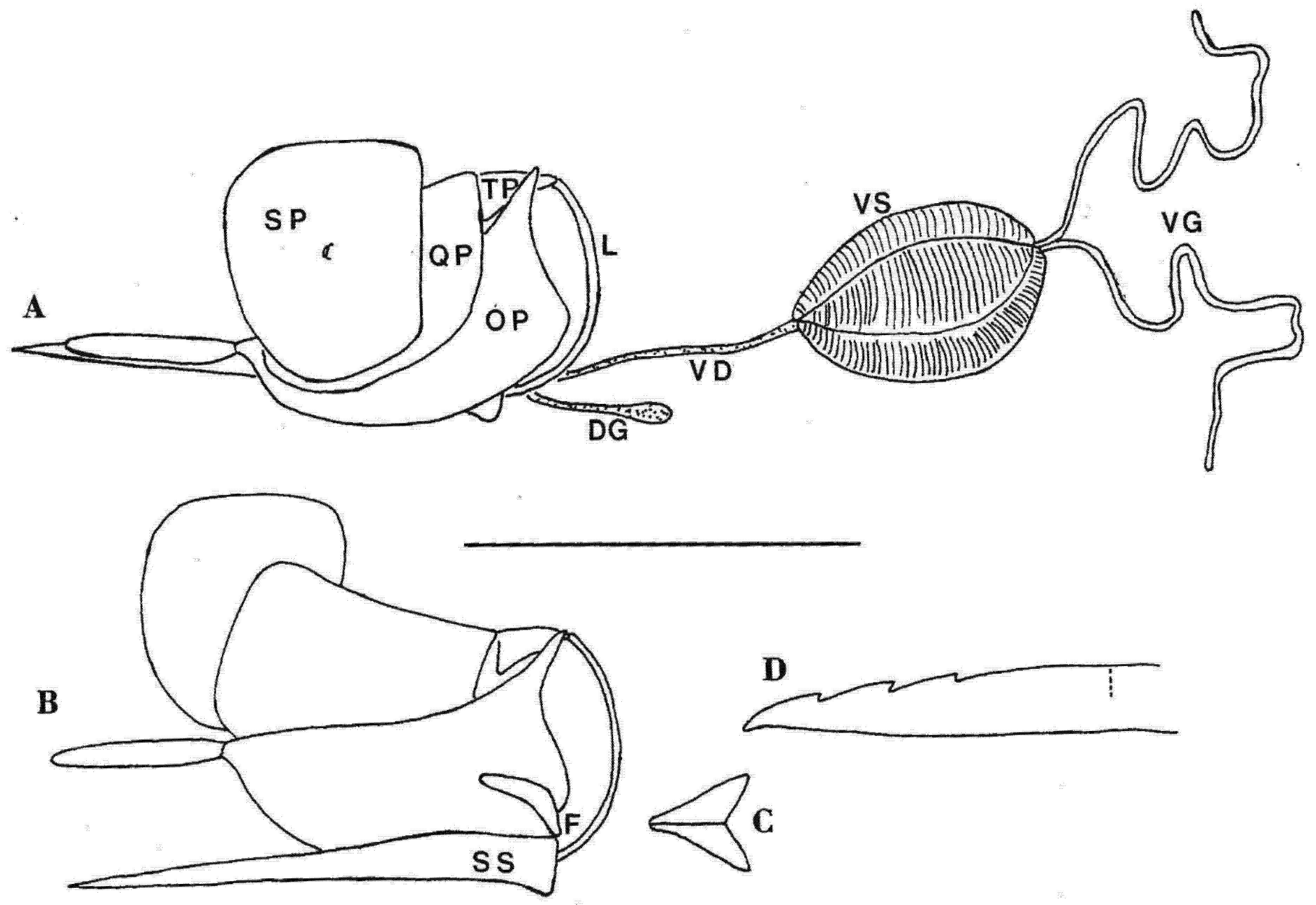

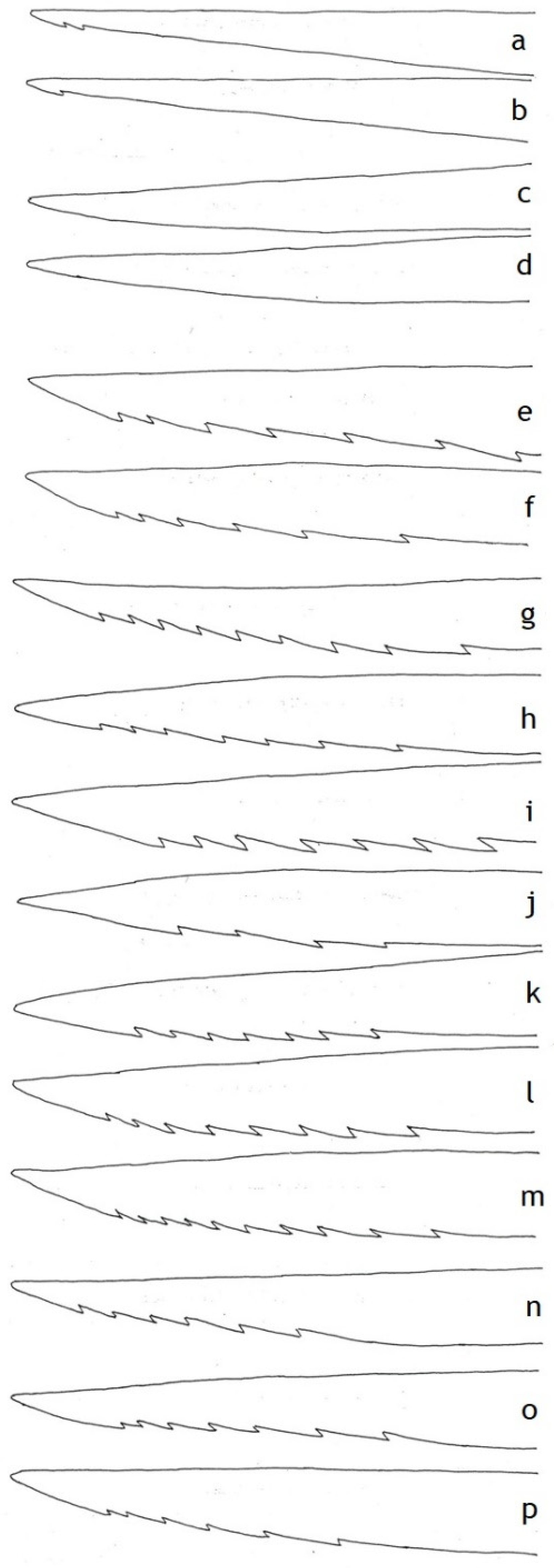
| Shaft Length (mm) | Wing Length (mm) | Shaft/Wing ± SD | Head Width (mm) | Shaft/Head | Lancet Barbs | Absolute Serration | Serration Index Sr | Sting Autotomy | |
|---|---|---|---|---|---|---|---|---|---|
| (μm) | |||||||||
| EUMENINAE and ZETHINAE | |||||||||
| Pachodynerus sp. A (3) | 2.99 | 14.5 | 0.21 ± 0.01 | 4.18 | 0.72 | 3 | 11 | 0.22 + 0.02 | |
| Pachodynerus sp. B (5) | 2.81 | 13.6 | 0.21 ± 0.01 | 3.86 | 0.73 | 2 | 5 | 0.10 + 0.02 | |
| Zethus sp. A (1) | 1.76 | 10.5 | 0.17 | 3.96 | 0.45 | 2 | 4 | 0.14 | |
| Zethus sp. B (3) | 1.67 | 9.2 | 0.18 ± 0.02 | 3.77 | 0.44 | 1 | 5 | 0.08 + 0.02 | |
| STENOGASTRINAE | |||||||||
| Eustenogaster calyptodoma (5) | 3.01 | 12.0 | 0.25 ± 0.01 | 3.71 | 0.81 | 0 | 0 | 0 | - |
| Parischnogaster striatula (4) | 1.57 | 8.8 | 0.18 ± 0.01 | 2.68 | 0.59 | 0 | 0 | 0 | |
| POLISTINAE (Independent-Founding) | |||||||||
| Belonogaster juncea (5) | 2.29 | 18.6 | 0.12 ± 0.01 | 3.82 | 0.60 | 6 | 47 | 0.62 + 0.06 | - |
| Mischocyttarus alfkeni (5) | 1.34 | 9.8 | 0.14 ± 0.01 | 2.58 | 0.52 | 4 | 33 | 0.53 + 0.04 | - |
| Mischocyttarus labiatus (5) | 1.43 | 14.7 | 0.10 + 0.01 | 3.31 | 0.44 | 5 | 31 | 0.66 + 0.06 | - |
| Parapolybia indica (3) | 2.70 | 16.0 | 0.17 + 0.07 | 3.86 | 0.70 | 6 | 53 | 0.77 + 0.22 | - |
| Parapolybia varia (5) | 1.90 | 10.9 | 0.17 + 0.07 | 3.09 | 0.62 | 6 | 42 | 0.87 + 0.25 | - |
| Polistes dominula (5) | 2.37 | 12.4 | 0.19 + 0.00 | 3.75 | 0.63 | 5 | 23 | 0.40 + 0.05 | - |
| Polistes dorsalis (5) | 2.01 | 11.3 | 0.18 + 0.01 | 3.19 | 0.63 | 5 | 30 | 0.55 + 0.08 | - |
| Polistes fuscatus (2) | 2.52 | 14.5 | 0.17 + 0.01 | 4.02 | 0.63 | 6 | 37 | 0.54 + 0.00 | - |
| Polistes gigas (4) | 5.42 | 26.0 | 0.21 + 0.00 | 6.50 | 0.84 | 7 | 84 | 0.64 + 0.13 | |
| Polistes lanio (5) | 3.26 | 19.1 | 0.17 + 0.01 | 4.41 | 0.74 | 6 | 49 | 0.65 + 0.05 | - |
| Polistes metricus (4) | 2.75 | 15.3 | 0.18 + 0.01 | 4.30 | 0.64 | 5 | 37 | 0.55 + 0.02 | - |
| Polistes olivaceus (5) | 3.05 | 15.4 | 0.20 + 0.01 | 4.48 | 0.68 | 6–7 | 50 | 0.64 + 0.18 | |
| Polistes stigma (8) | 2.19 | 9.4 | 0.23 + 0.01 | 3.22 | 0.68 | 4 | 26 | 0.44 + 0.14 | |
| Polistes strigosus (1) | 3.88 | 20.0 | 0.19 | 4.82 | 0.80 | 5 | 46 | 0.54 | |
| Polistes tenebricosus (2) | 3.77 | 20.5 | 0.19 + 0.01 | 4.90 | 0.77 | 6 | 59 | 0.64 + 0.09 | |
| Polistes versicolor (5) | 2.49 | 13.2 | 0.19 + 0.01 | 3.19 | 0.78 | 6 | 59 | 1.03 + 0.17 | - |
| POLISTINAE (Swarm-Founding) | |||||||||
| Agelaia centralis (4) | 1.23 | 9.5 | 0.13 + 0.01 | 2.73 | 0.45 | 9 | 100 | 1.66 + 0.23 | + |
| Angiopolybia pallens (5) | 1.12 | 7.5 | 0.15 + 0.01 | 2.15 | 0.52 | 6 | 25 | 0.68 + 0.08 | - |
| Apoica pallens (5) | 1.91 | 14.6 | 0.13 + 0.01 | 3.24 | 0.59 | 6 | 63 | 0.91 + 0.10 | - |
| Apoica pallida (5) | 2.21 | 16.1 | 0.14 + 0.01 | 3.40 | 0.65 | 8–9 | 75 | 1.14 + 0.03 | ± |
| Brachygastra bilineolata (5) | 1.60 | 5.3 | 0.31 + 0.04 | 2.54 | 0.63 | 6 | 47 | 0.93 + 0.09 | + |
| Epipona tatua (5) | 1.46 | 10.2 | 0.14 + 0.00 | 3.73 | 0.39 | 9 | 124 | 2.27 + 0.42 | + |
| Metapolybia cingulata (5) | 1.05 | 5.3 | 0.20 + 0.03 | 5.25 | 0.47 | 7 | 48 | 0.82 + 0.04 | ± |
| Parachartergus colobopterus (5) | 1.26 | 6.7 | 0.19 + 0.01 | 2.36 | 0.53 | 4 | 20 | 0.43 + 0.12 | - |
| Parachartergus fraternus (5) | 2.11 | 11.2 | 0.19 + 0.01 | 3.22 | 0.66 | 4 | 34 | 0.54 + 0.11 | ± |
| Polybia occidentalis (5) | 0.42 | 6.0 | 0.07 + 0.07 | 2.00 | 0.21 | 8 | 54 | 1.20 + 0.10 | ± |
| Polybia rejecta (5) | 1.40 | 7.3 | 0.19 + 0.01 | 2.55 | 0.55 | 8 | 82 | 1.17 + 0.07 | ± |
| Protopolybia exigua (4) | 0.87 | 3.9 | 0.23 + 0.01 | 1.50 | 0.58 | 6 | 18 | 0.64 + 0.05 | + |
| Synoeca surinama (5) | 3.00 | 18.9 | 0.16 + 0.01 | 4.84 | 0.62 | 12 | 213 | 1.59 + 0.37 | + |
| VESPINAE | |||||||||
| Dolichovespula arenaria (5) | 1.95 | 9.4 | 0.21 + 0.00 | 3.50 | 0.56 | 6 | 48 | 0.61 + 0.04 | - |
| Dolichovespula maculata (5) | 2.61 | 13.9 | 0.19 + 0.01 | 4.72 | 0.56 | 7 | 68 | 0.76 + 0.03 | - |
| Dolichovespula norvegicoides (4) | 1.85 | 9.6 | 0.19 + 0.01 | 3.40 | 0.54 | 6–7 | 51 | 0.73 + 0.07 | - |
| Vespa affinis (4) | 3.16 | 17.6 | 0.18 + 0.00 | 5.72 | 0.55 | 7 | 68 | 0.72 + 0.06 | - |
| Vespa bicolor (5) | 2.91 | 13.9 | 0.21 + 0.01 | 5.17 | 0.57 | 7 | 82 | 0.84 + 0.08 | - |
| Vespa crabro (5) | 3.60 | 19.1 | 0.19 + 0.01 | 6.25 | 0.58 | 7 | 68 | 0.68 + 0.07 | - |
| Vespa ducalis (5) | 4.25 | 25.5 | 0.17 + 0.01 | 7.45 | 0.57 | 6 | 98 | 0.75 + 0.26 | - |
| Vespa luctuosa (5) | 2.97 | 13.8 | 0.20 + 0.01 | 7 | 0.75 + 0.07 | ||||
| Vespa tropica (5) | 3.71 | 21.3 | 0.17 + 0.00 | 6.16 | 0.59 | 6–7 | 82 | 0.81 + 0.10 | - |
| Vespa velutina (4) | 3.20 | 17.3 | 0.19 + 0.00 | 5.66 | 0.56 | 7 | 79 | 0.80 + 0.11 | - |
| Vespula atropilosa (4) | 2.24 | 10.8 | 0.21 + 0.01 | 3.92 | 0.57 | 9 | 70 | 1.09 + 0.19 | + |
| Vespula consobrina (5) | 1.94 | 9.8 | 0.20 + 0.01 | 3.54 | 0.55 | 8 | 57 | 0.83 + 0.07 | |
| Vespula germanica (6) | 1.92 | 9.5 | 0.20 + 0.01 | 3.75 | 0.51 | 8 | 69 | 0.99 + 0.06 | ± |
| Vespula maculifrons (7) | 1.97 | 10.1 | 0.19 + 0.00 | 3.40 | 0.58 | 8–9 | 70 | 1.15 + 0.18 | ± |
| Vespula pensylvanica (6) | 1.99 | 10.1 | 0.20 + 0.01 | 3.59 | 0.53 | 8 | 74 | 1.04 + 0.18 | ± |
| Vespula squamosa (5) | 2.16 | 9.9 | 0.22 + 0.01 | 3.60 | 0.60 | 9 | 84 | 1.30 + 0.19 | |
| Vespula vidua (5) | 2.23 | 10.6 | 0.21 + 0.01 | 3.78 | 0.59 | 8–9 | 75 | 1.16 + 0.17 | + |
| Apis mellifera (4) | 1.81 | 8.6 | 0.21 + 0.01 | 3.48 | 0.52 | 8–9 | 102 | 2.43 + 0.48 | + |
| Stenogastrinae vs. Polistinae + Vespinae [30] | ||||
| shaft/forewing | z = 2.33, p = 0.02 | Sr | z = 5.07, p < 0.01 | |
| Stenogastinae vs. other vespids [31] | ||||
| shaft/forewing | z = 2.29, p = 0.02 | Sr | z = 5.08, p < 0.01 | |
| Polistinae vs. Vespinae | ||||
| shaft/forewing | z = 4.85, p < 0.01 | Sr | z = 3.19, p < 0.01 | |
| Polistini vs. Epiponini | ||||
| shaft/forewing | z = 3.53, p = 0.02 | Sr | z = 5.80, p < 0.01 | |
| Ropalidiini vs. other polistines | ||||
| shaft/forewing | z = 2.34, p = 0.02 | Sr | z = 0.10, p = 0.92 | |
| solitary wasps * vs. primitively social wasps * | ||||
| shaft/forewing | z = 2.23, p = 0.03 | Sr | z = 4.12, p < 0.01 | |
| primitively social wasps * vs. Epiponini | ||||
| shaft/forewing | z = 1.82, p = 0.07 | Sr | z = 6.83, p < 0.01 | |
| primitively social wasps * vs. Vespinae | ||||
| shaft/forewing | z = 3.09, p < 0.01 | Sr | z = 7.75, p < 0.01 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bissessarsingh, M.; Starr, C.K. Comparative Morphology of the Stinger in Social Wasps (Hymenoptera: Vespidae). Insects 2021, 12, 729. https://doi.org/10.3390/insects12080729
Bissessarsingh M, Starr CK. Comparative Morphology of the Stinger in Social Wasps (Hymenoptera: Vespidae). Insects. 2021; 12(8):729. https://doi.org/10.3390/insects12080729
Chicago/Turabian StyleBissessarsingh, Mario, and Christopher K. Starr. 2021. "Comparative Morphology of the Stinger in Social Wasps (Hymenoptera: Vespidae)" Insects 12, no. 8: 729. https://doi.org/10.3390/insects12080729
APA StyleBissessarsingh, M., & Starr, C. K. (2021). Comparative Morphology of the Stinger in Social Wasps (Hymenoptera: Vespidae). Insects, 12(8), 729. https://doi.org/10.3390/insects12080729






