Diel and Circadian Patterns of Locomotor Activity in the Adults of Diamondback Moth (Plutella xylostella)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Sample
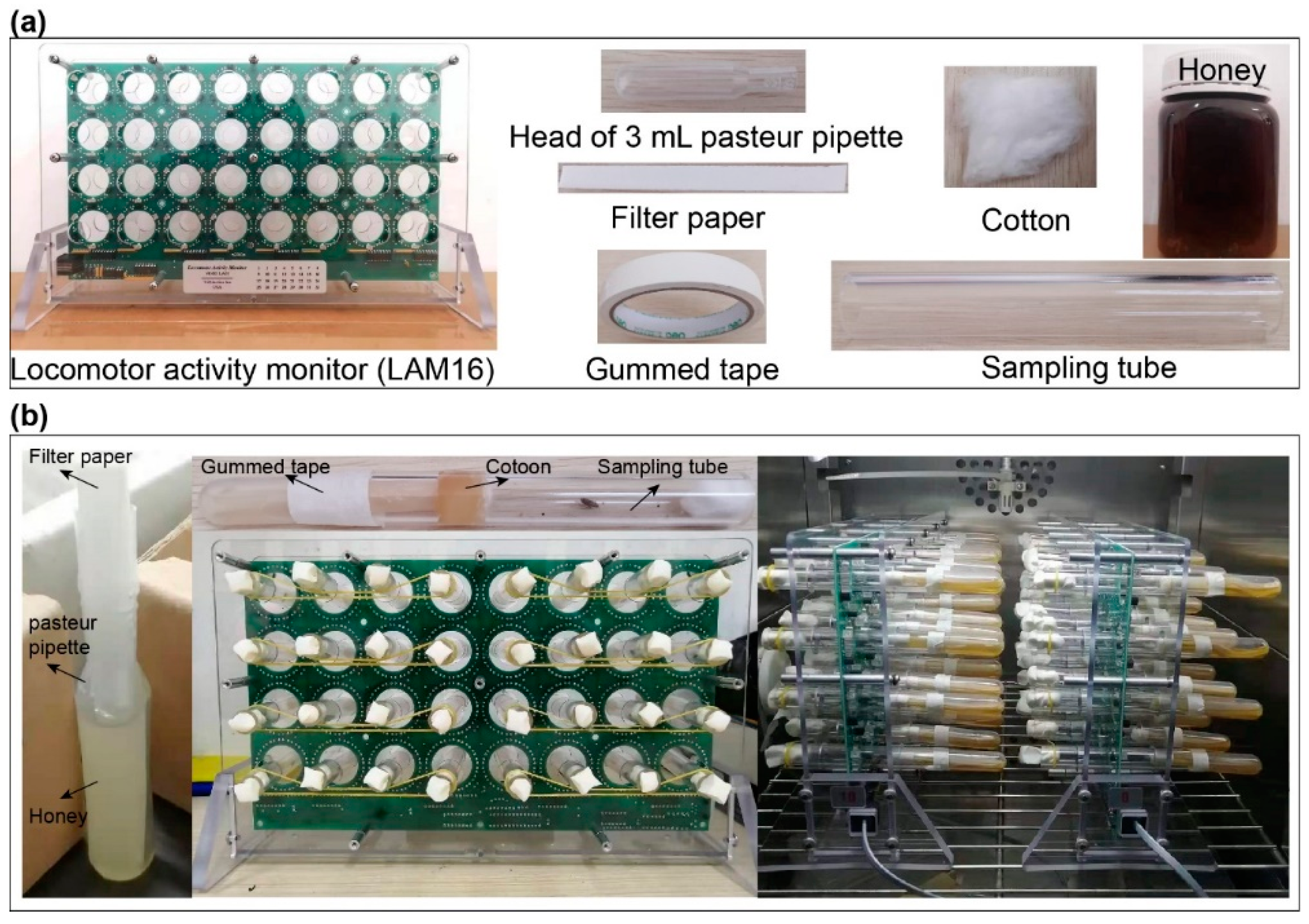
2.2. Activity Monitor System
2.3. Locomotor Activity Behaviors Analysis
3. Results
3.1. System for Measuring the Individual Locomotor Activities of P. xylostella
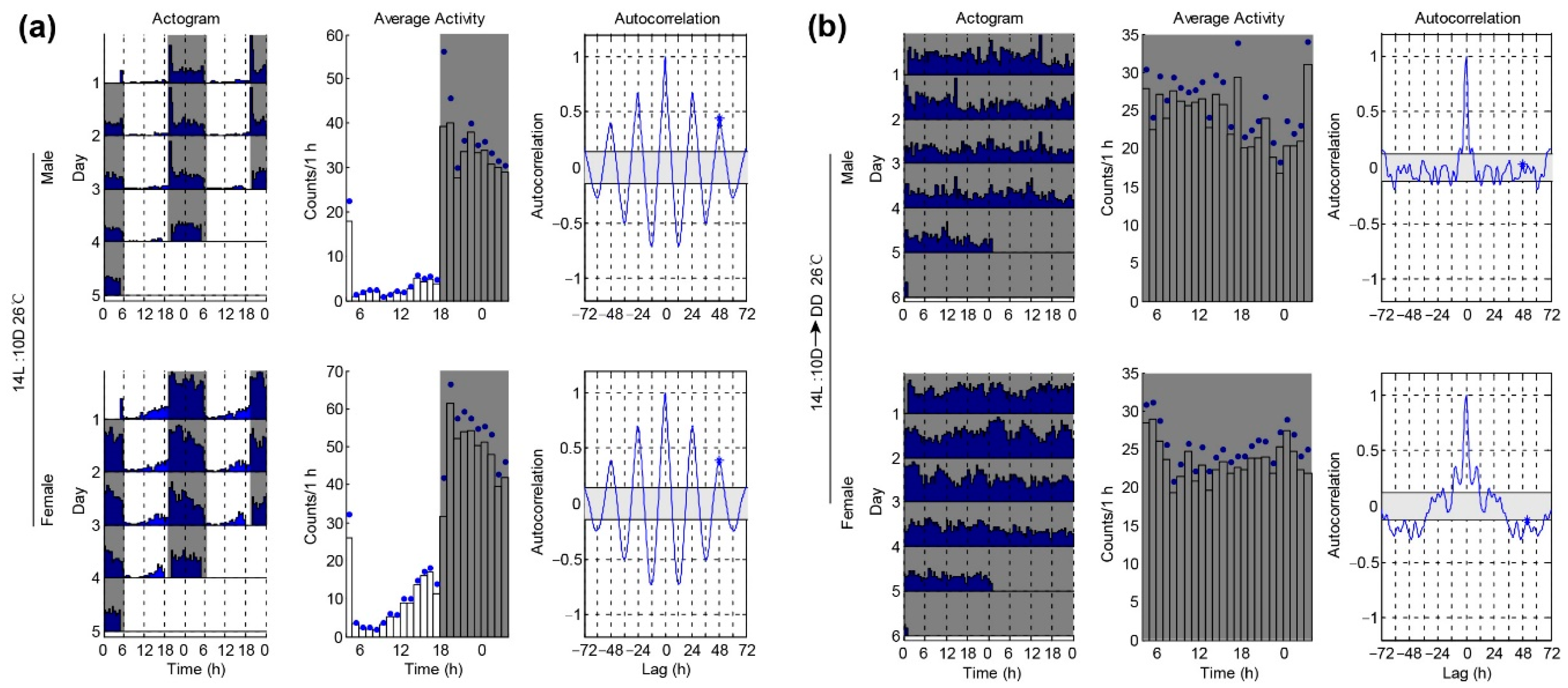
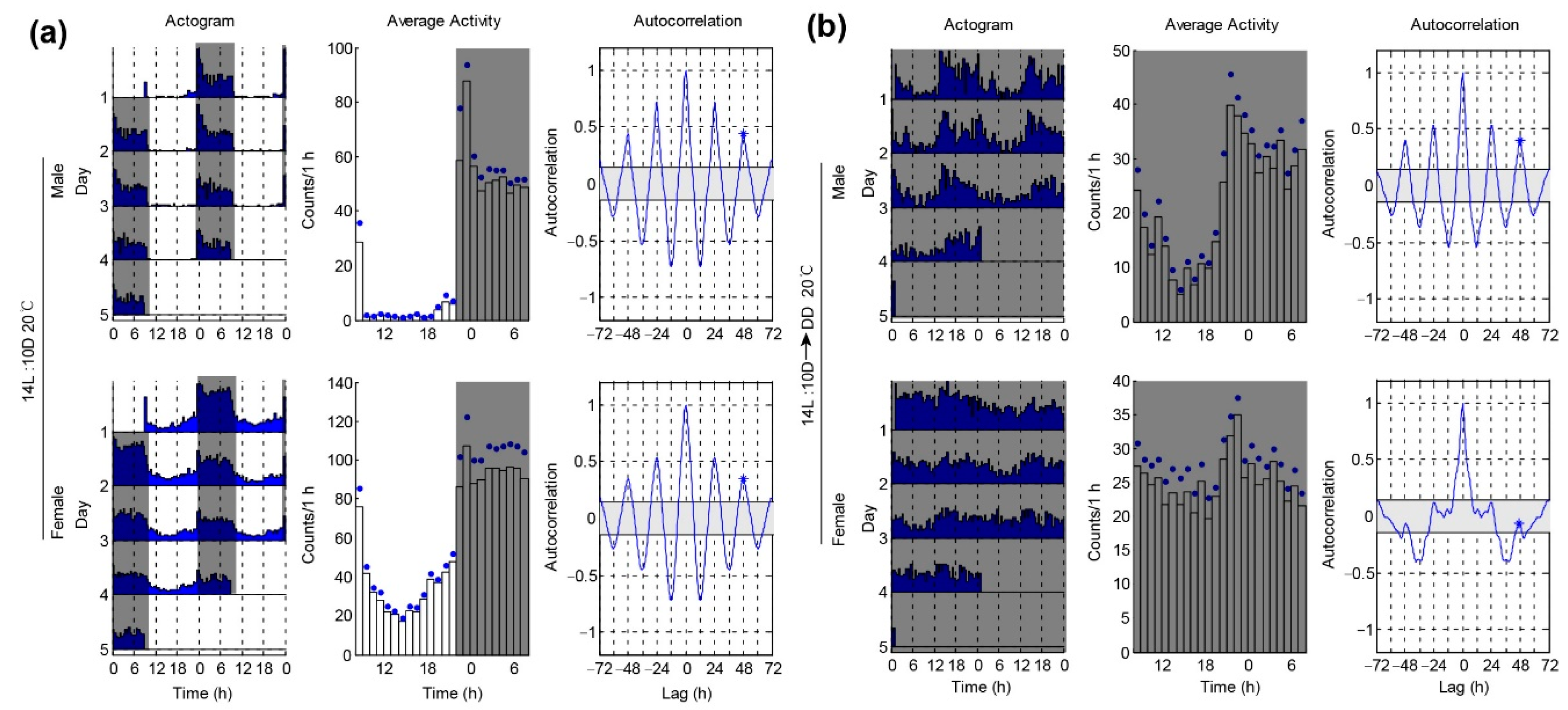
3.2. Locomotor Activities of P. xylostella under Different Temperatures
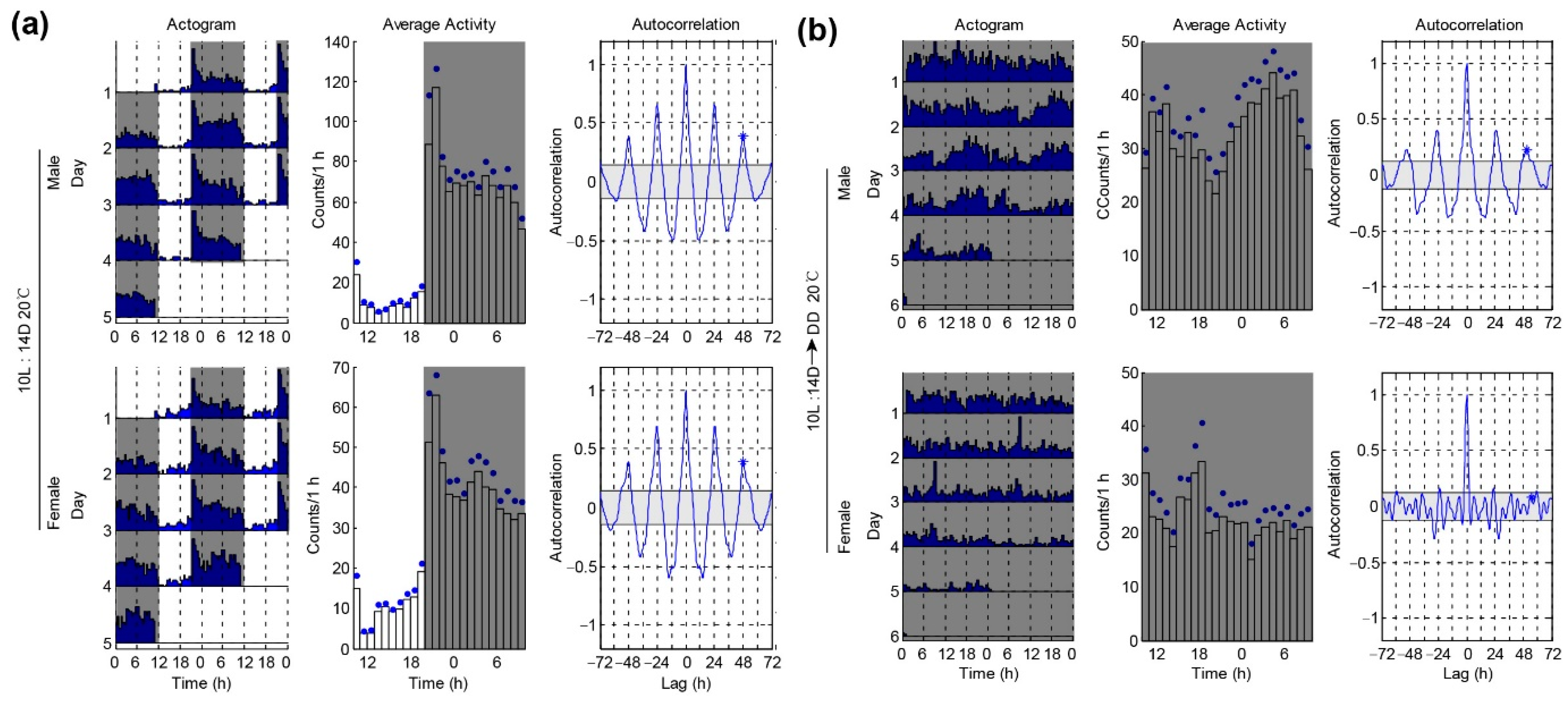
3.3. Locomotor Activities of P. xylostella under a Winter-like Short Day
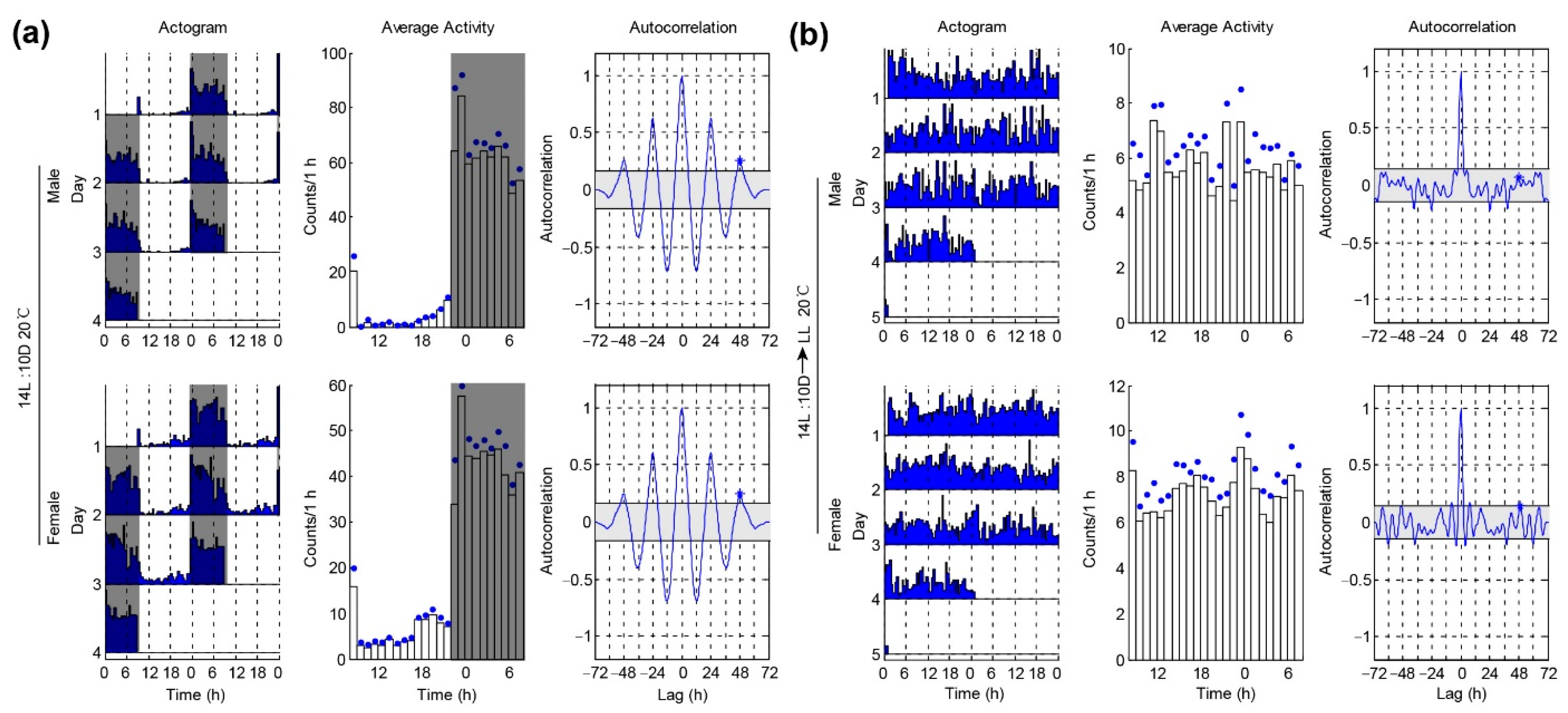
3.4. Locomotor Activities of P. xylostella under Constant Light Condition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Numata, H.; Miyazaki, Y.; Ikeno, T. Common features in diverse insect clocks. Zool. Lett. 2015, 1, 10. [Google Scholar] [CrossRef] [PubMed]
- Allada, R.; Chung, B.Y. Circadian organization of behavior and physiology in Drosophila. Annu. Rev. Physiol. 2010, 72, 605–624. [Google Scholar] [CrossRef]
- Malik, K.I.; Seth, R.K. Insect clocks: Implication in an effective pest management. Biol. Rhythm Res. 2017, 48, 777–788. [Google Scholar] [CrossRef]
- Niepoth, N.; Ke, G.; de Roode, J.C.; Groot, A.T. Comparing behavior and clock gene expression between caterpillars, butterflies and moths. J. Biol. Rhythm. 2018, 33, 52–64. [Google Scholar] [CrossRef]
- Linn, C.E.; Campbell, M.G.; Roelofs, W.L. Photoperiod cues and the modulatory action of octopamine and 5-hydroxytryptamine on locomotor and pheromone response in male gypsy moths, Lymantria dispar. Arch. Insect Biochem. Physiol. 1992, 20, 265–284. [Google Scholar] [CrossRef]
- Minis, D.H.; Pittendrigh, C.S. Circadian oscillation controlling hatching: Its ontogeny during embryogenesis of a moth. Science 1968, 159, 534–536. [Google Scholar] [CrossRef] [PubMed]
- Kobelkova, A.; Zavodska, R.; Sauman, I.; Bazalova, O.; Dolezel, D. Expression of clock genes period and timeless in the central nervous system of the Mediterranean flour moth, Ephestia kuehniella. J. Biol. Rhythm. 2015, 30, 104–116. [Google Scholar] [CrossRef]
- Groot, A.T. Circadian rhythms of sexual activities in moths: A review. Front. Ecol. Evol. 2014, 2, 43. [Google Scholar] [CrossRef][Green Version]
- Bhattarai, U.R.; Li, F.; Katuwal Bhattarai, M.; Masoudi, A.; Wang, D. Phototransduction and circadian entrainment are the key pathways in the signaling mechanism for the baculovirus induced tree-top disease in the lepidopteran larvae. Sci. Rep. 2018, 8, 17528. [Google Scholar] [CrossRef]
- Dubowy, C.; Sehgal, A. Circadian rhythms and sleep in Drosophila melanogaster. Genetics 2017, 205, 1373–1397. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffenberger, C.; Lear, B.C.; Keegan, K.P.; Allada, R. Locomotor activity level monitoring using the Drosophila Activity Monitoring (DAM) System. Cold Spring Harb. Protoc. 2010, 2010, pdb prot5518. [Google Scholar] [CrossRef]
- Yuan, Q.; Metterville, D.; Briscoe, A.D.; Reppert, S.M. Insect cryptochromes: Gene duplication and loss define diverse ways to construct insect circadian clocks. Mol. Biol. Evol. 2007, 24, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Beer, K.; Helfrich-Forster, C. Model and non-model insects in chronobiology. Front Behav. Neurosci. 2020, 14, 601676. [Google Scholar] [CrossRef] [PubMed]
- You, M.; Ke, F.; You, S.; Wu, Z.; Liu, Q.; He, W.; Baxter, S.W.; Yuchi, Z.; Vasseur, L.; Gurr, G.M.; et al. Variation among 532 genomes unveils the origin and evolutionary history of a global insect herbivore. Nat. Commun. 2020, 11, 2321. [Google Scholar] [CrossRef]
- Furlong, M.J.; Wright, D.J.; Dosdall, L.M. Diamondback moth ecology and management: Problems, progress, and prospects. Annu. Rev. Entomol. 2013, 58, 517–541. [Google Scholar] [CrossRef] [PubMed]
- Rosato, E.; Kyriacou, C.P. Analysis of locomotor activity rhythms in Drosophila. Nat. Protoc. 2006, 1, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.D.; Funes, P.; Dowse, H.B.; Hall, J.C. Signal analysis of behavioral and molecular cycles. BMC Neurosci. 2002, 3, 1. [Google Scholar] [CrossRef]
- Klarsfeld, A.; Leloup, J.C.; Rouyer, F. Circadian rhythms of locomotor activity in Drosophila. Behav. Process. 2003, 64, 161–175. [Google Scholar] [CrossRef]
- Chiu, J.C.; Low, K.H.; Pike, D.H.; Yildirim, E.; Edery, I. Assaying locomotor activity to study circadian rhythms and sleep parameters in Drosophila. J. Vis. Exp. 2010, e2157. [Google Scholar] [CrossRef]
- Giannoni-Guzman, M.A.; Avalos, A.; Marrero Perez, J.; Otero Loperena, E.J.; Kayim, M.; Medina, J.A.; Massey, S.E.; Kence, M.; Kence, A.; Giray, T.; et al. Measuring individual locomotor rhythms in honey bees, paper wasps and other similar-sized insects. J. Exp. Biol. 2014, 217, 1307–1315. [Google Scholar] [CrossRef]
- Gentile, C.; Rivas, G.B.; Meireles-Filho, A.C.; Lima, J.B.; Peixoto, A.A. Circadian expression of clock genes in two mosquito disease vectors: Cry2 is different. J. Biol. Rhythm. 2009, 24, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Rund, S.S.; Lee, S.J.; Bush, B.R.; Duffield, G.E. Strain- and sex-specific differences in daily flight activity and the circadian clock of Anopheles gambiae mosquitoes. J. Insect Physiol. 2012, 58, 1609–1619. [Google Scholar] [CrossRef]
- Pivnick, K.A.; Jarvis, B.J.; Gillott, C.; Slater, G.P.; Underhill, E.W. Daily patterns of reproductive activity and the influence of adult density and exposure to host plants on reproduction in the diamondback moth (Lepidoptera: Plutellidae). Environ. Entomol. 1990, 19, 587–593. [Google Scholar] [CrossRef]
- Emery, P.; So, W.V.; Kaneko, M.; Hall, J.C.; Rosbash, M. CRY, a Drosophila clock and light-regulated cryptochrome, is a major contributor to circadian rhythm resetting and photosensitivity. Cell 1998, 95, 669–679. [Google Scholar] [CrossRef]
- Lewis, T.; Taylor, L.R. Diurnal periodicity of flight by insects. Ecolo. Entomol. 1965, 116, 393–435. [Google Scholar] [CrossRef]
- Miranda-Anaya, M.; Guevara-Fefer, P.; García-Rivera, B.E. Circadian locomotor activity in the larva and adult fall armyworm, Spodoptera frugiperda (Noctuidae): Effect of feeding with the resistant variety of maize CML67. Biol. Rhythm Res. 2014, 33, 475–486. [Google Scholar] [CrossRef]
- Zavodska, R.; Fexova, S.; von Wowern, G.; Han, G.B.; Dolezel, D.; Sauman, I. Is the sex communication of two pyralid moths, Plodia interpunctella and Ephestia kuehniella, under circadian clock regulation? J. Biol. Rhythm. 2012, 27, 206–216. [Google Scholar] [CrossRef]
- Glaser, F.T.; Stanewsky, R. Temperature synchronization of the Drosophila circadian clock. Curr. Biol. 2005, 15, 1352–1363. [Google Scholar] [CrossRef]
- Bell-Pedersen, D.; Cassone, V.M.; Earnest, D.J.; Golden, S.S.; Hardin, P.E.; Thomas, T.L.; Zoran, M.J. Circadian rhythms from multiple oscillators: Lessons from diverse organisms. Nat. Rev. Genet. 2005, 6, 544–556. [Google Scholar] [CrossRef]
- Roessingh, S.; Rosing, M.; Marunova, M.; Ogueta, M.; George, R.; Lamaze, A.; Stanewsky, R. Temperature synchronization of the Drosophila circadian clock protein PERIOD is controlled by the TRPA channel PYREXIA. Commun. Biol. 2019, 2, 246. [Google Scholar] [CrossRef]
- Abhilash, L.; Kalliyil, A.; Sheeba, V. Responses of activity rhythms to temperature cues evolve in Drosophila populations selected for divergent timing of eclosion. J. Exp. Biol. 2020, 223. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Qin, Y. Effects of temperature and humidity on emergence dynamics of Plutella xylostella (Lepidoptera: Plutellidae). J. Econ. Entomol. 2010, 103, 2028–2033. [Google Scholar] [CrossRef]
- Syrova, Z.; Sauman, I.; Giebultowicz, J.M. Effects of light and temperature on the circadian system controlling sperm release in moth Spodoptera littoralis. Chronobiol. Int. 2003, 20, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, G.A.; Grossberg, S. A neural theory of circadian rhythms: Aschoff’s rule in diurnal and nocturnal mammals. Am. J. Physiol. 1984, 247, R1067–R1082. [Google Scholar] [CrossRef] [PubMed]
- Hooven, L.A.; Sherman, K.A.; Butcher, S.; Giebultowicz, J.M. Does the clock make the poison? Circadian variation in response to pesticides. PLoS ONE 2009, 4, e6469. [Google Scholar] [CrossRef]
- Khalid, M.F.; Lee, C.Y.; Doggett, S.L.; Veera Singham, G. Circadian rhythms in insecticide susceptibility, metabolic enzyme activity, and gene expression in Cimex lectularius (Hemiptera: Cimicidae). PLoS ONE 2019, 14, e0218343. [Google Scholar] [CrossRef]
| Condition | Phase (95% Confidence Limit) | Power | Average Rhythm Index (RI) | Phase (95% Confidence Limit) | Power | Average Rhythm Index (RI) | ||
|---|---|---|---|---|---|---|---|---|
| Male | Female | |||||||
| LD (4 days) | ||||||||
| 26 °C 14L:10D | 1.4 ± 1.1 | 54.2 ± 3.07 n.s. | 0.28 ± 0.02 n.s. | 2.8 ± 0.8 | 50.4 ± 3.07 | 0.26 ± 0.02 | ||
| 20 °C 14L:10D | 2.0 ± 0.6 | 70.9 ± 3.98 ** | 0.31 ± 0.02 ** | 2.4 ± 1.1 | 54.5 ± 3.36 | 0.23 ± 0.02 | ||
| 20 °C 10L:14D | 22.5 ± 0.9 | 55.9 ± 2.87 * | 0.29 ± 0.02 ** | 22.0 ± 0.3 | 50.4 ± 3.81 | 0.16 ± 0.04 | ||
| Condition | Arrhythmic Ratio | Period | Power | Average Rhythm Index (RI) | Arrhythmic Ratio | Period | Power | Average Rhythm Index (RI) |
| LD-DD or LD-LL (5–6 days) | ||||||||
| 26 °C 14L:10D-DD | 21/29 * | 24.3 ± 2.85 | 41.3 ± 6.07 * | 0.15 ± 0.01 n.s. | 33/35 | 25.8 ± 1.25 | 25.7 ± 2.58 | 0.12 ± 0.02 |
| 20 °C 14L:10D-DD | 20/38 *** | 24.0 ± 0.31 | 46.4 ± 3.80 ** | 0.19 ± 0.01 ** | 36/41 | 27.0 ± 1.81 | 37.6 ± 3.47 | 0.14 ± 0.02 |
| 20 °C 10L:14D-DD | 10/21 *** | 23.8 ± 0.28 | 36.6 ± 6.35 | 0.16 ± 0.02 ** | 20/20 | - | - | 0.10 ± 0.03 |
| 20 °C 14L:10D-LL | 20/20 | - | - | - | 16/16 | - | - | - |
| Condition | Number | Activity: Average/30 min | Number | Activity: Average/30 min |
|---|---|---|---|---|
| Male | Female | |||
| LD (4 days) | ||||
| 26 °C 14L:10D | 42 | 31.5 ± 4.05 | 46 | 30.4 ± 2.94 |
| 20 °C 14L:10D | 40 | 35.8 ± 3.22 | 42 | 31.7 ± 3.62 |
| 20 °C 10L:14D | 30 | 46.5 ± 4.55 | 28 | 26.7 ± 2.81 |
| Condition | Number | Activity: Average/30 min | Number | Activity: Average/30 min |
| DD (4 days) or LL (4 days) | ||||
| 26 °C 14L:10D-DD | 29 | 23.9 ± 3.11 | 35 | 26.0 ± 3.00 |
| 20 °C 14L:10D-DD | 38 | 20.3 ± 3.07 | 41 | 15.6 ± 2.31 |
| 20 °C 10L:14D-DD | 21 | 24.7 ± 2.85 | 20 | 19.3 ± 2.53 |
| 20 °C 14L:10D-LL | 20 | 6.1 ± 1.44 | 16 | 8.2 ± 1.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Yang, G.; Chen, W. Diel and Circadian Patterns of Locomotor Activity in the Adults of Diamondback Moth (Plutella xylostella). Insects 2021, 12, 727. https://doi.org/10.3390/insects12080727
Wang D, Yang G, Chen W. Diel and Circadian Patterns of Locomotor Activity in the Adults of Diamondback Moth (Plutella xylostella). Insects. 2021; 12(8):727. https://doi.org/10.3390/insects12080727
Chicago/Turabian StyleWang, Danfeng, Guang Yang, and Wenfeng Chen. 2021. "Diel and Circadian Patterns of Locomotor Activity in the Adults of Diamondback Moth (Plutella xylostella)" Insects 12, no. 8: 727. https://doi.org/10.3390/insects12080727
APA StyleWang, D., Yang, G., & Chen, W. (2021). Diel and Circadian Patterns of Locomotor Activity in the Adults of Diamondback Moth (Plutella xylostella). Insects, 12(8), 727. https://doi.org/10.3390/insects12080727






