Entomological Survey Confirms Changes in Mosquito Composition and Abundance in Senegal and Reveals Discrepancies among Results by Different Host-Seeking Female Traps
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
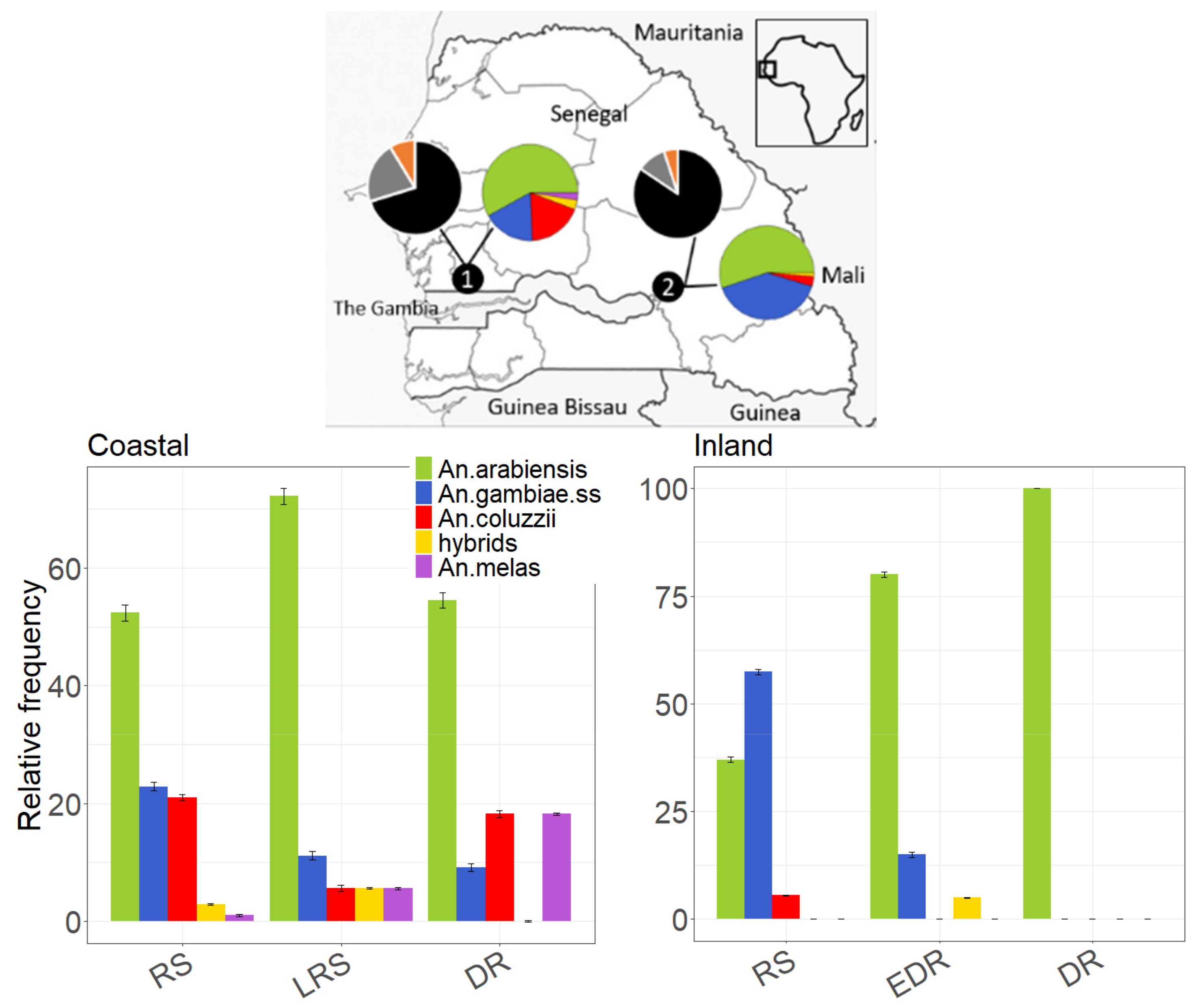
2.1. Study Areas
2.2. Mosquito Sampling and Processing
2.3. Statistical Methods
3. Results
3.1. Species Composition and Descriptive Statistics
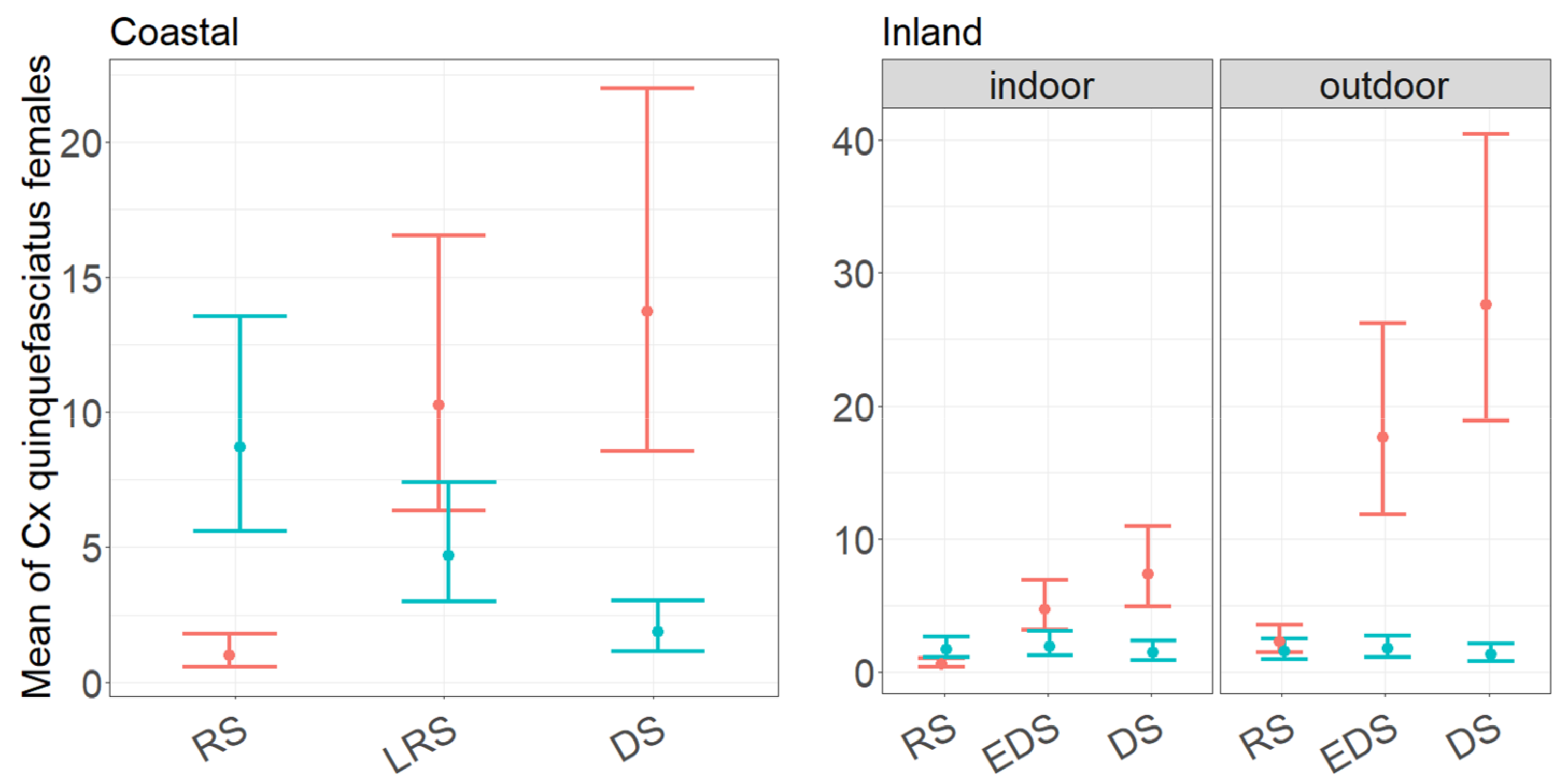
3.2. Performance of CDC and BG Traps in Collecting Culex quinquefasciatus Females as a Function of Month of Collection and Trapping Location
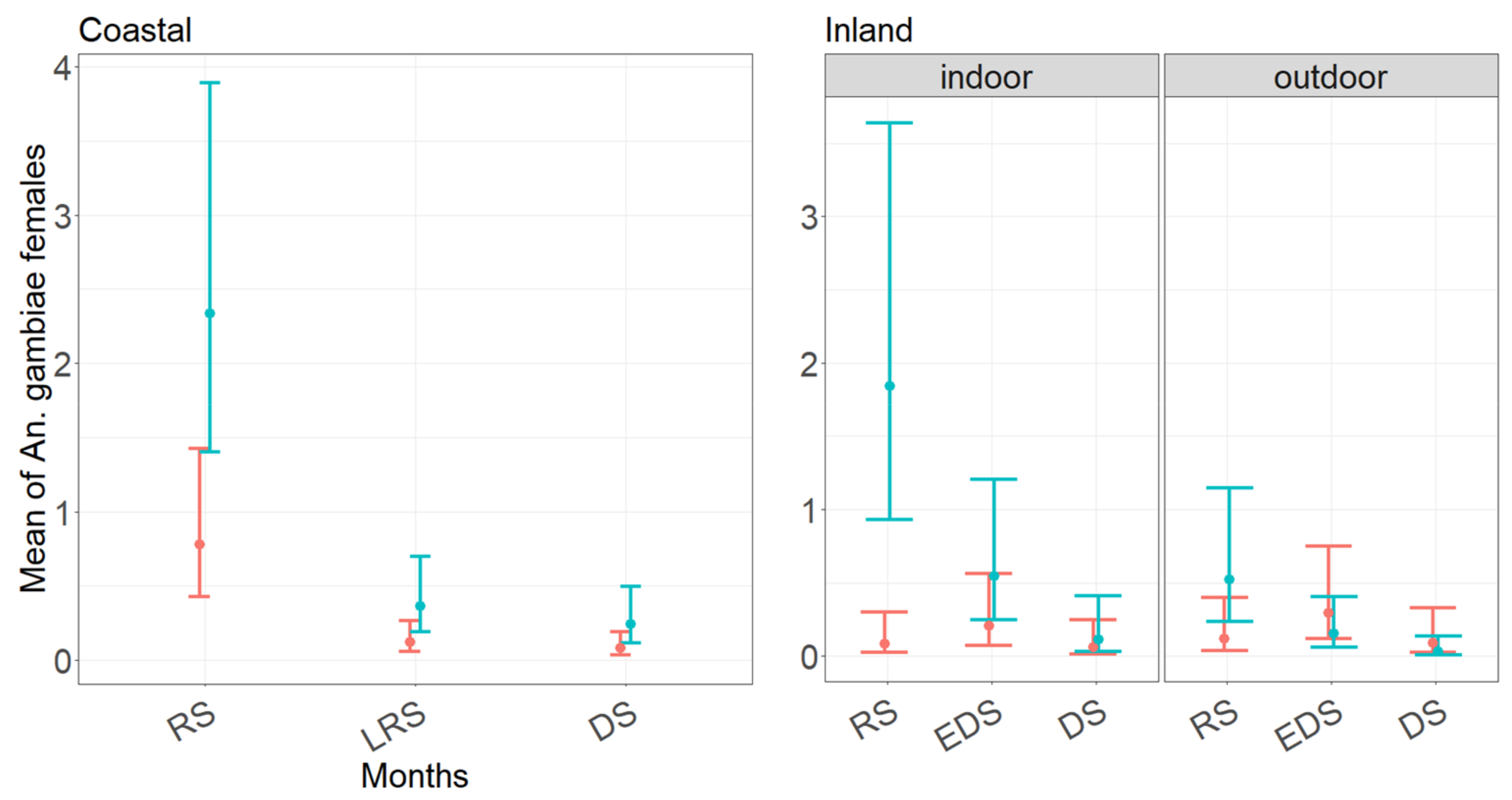
3.3. Performance of CDC and BG Traps in Collecting Anopheles gambiae s.l. Females as Function of Month of Collection and Trapping Location
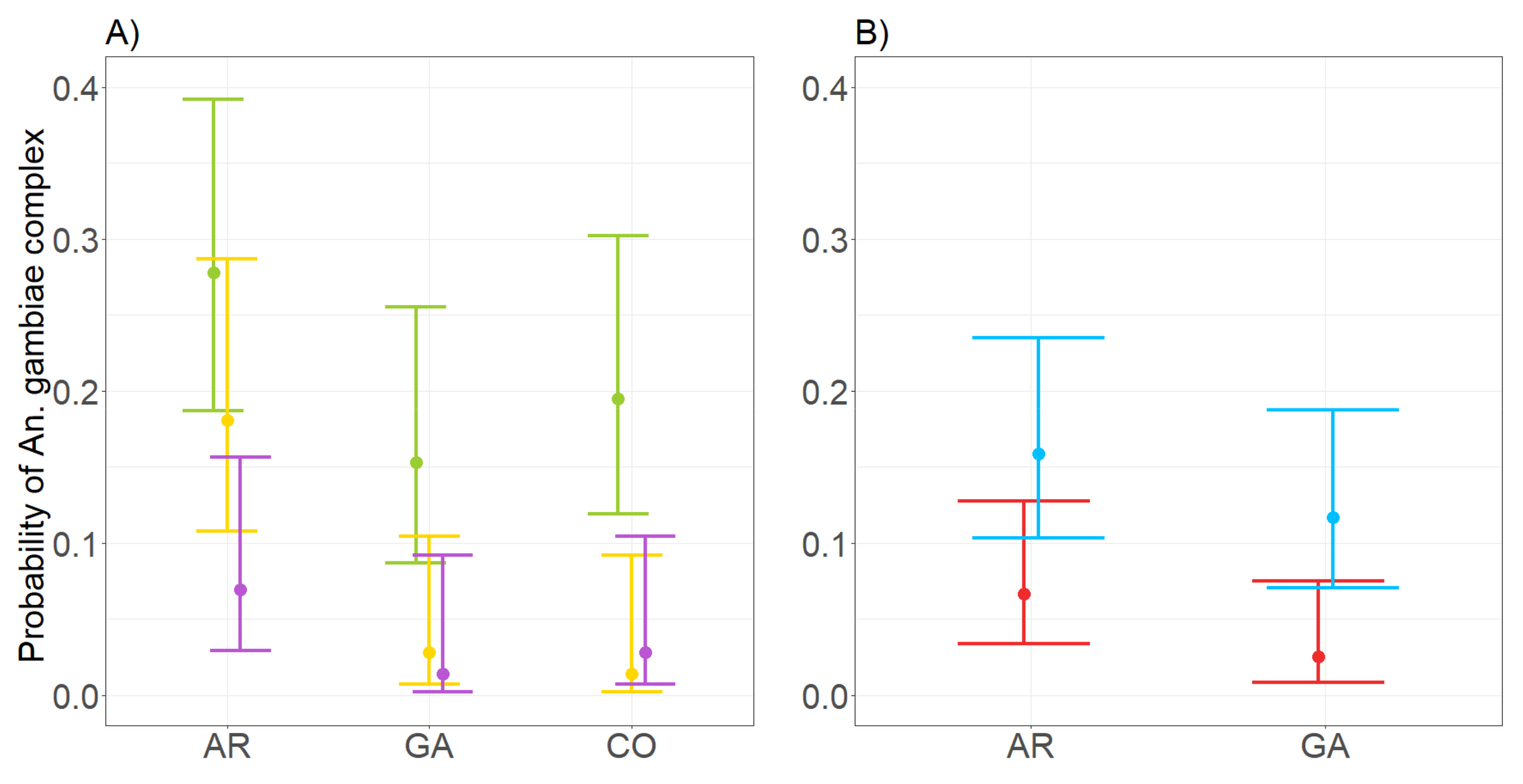
3.4. Performance of CDC and BG in Assessing the Probability to Detect Members of Anopheles gambiae Complex
4. Discussion
4.1. Performance of CDC and BG Traps in Collecting Culex quinquefasciatus Host-Seeking Females
4.2. Performance of CDC and BG Traps in Collecting Species of the Anopheles gambiae Complex
4.3. Culex quinquefasciatus Female Abundance, Seasonality, and Indoor/Outdoor Preferences in the Coastal and Inland Village
4.4. Anopheles gambiae Complex Species Abundance, Seasonality, and Indoor/Outdoor References in the Coastal and Inland Village
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campbell-Lendrum, D.; Manga, L.; Bagayoko, M.; Sommerfeld, J. Climate change and vector-borne diseases: What are the implications for public health research and policy? Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20130552. [Google Scholar] [CrossRef] [Green Version]
- Sougoufara, S.; Harry, M.; Doucouré, S.; Sembène, P.M.; Sokhna, C. Shift in species composition in the Anopheles gambiae complex after implementation of long-lasting insecticidal nets in Dielmo, Senegal. Med. Vet. Entomol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Sougoufara, S.; Thiaw, O.; Cailleau, A.; Diagne, N.; Harry, M.; Bouganali, C.; Sembène, P.M.; Doucoure, S.; Sokhna, C. The impact of periodic distribution campaigns of long-lasting insecticidal-treated bed nets on malaria vector dynamics and human exposure in Dielmo, Senegal. Am. J. Trop. Med. Hyg. 2018, 98, 1343–1352. [Google Scholar] [CrossRef] [Green Version]
- Thiaw, O.; Doucouré, S.; Sougoufara, S.; Bouganali, C.; Konaté, L.; Diagne, N.; Faye, O.; Sokhna, C. Investigating insecticide resistance and knock-down resistance (kdr) mutation in Dielmo, Senegal, an area under long lasting insecticidal-treated nets universal coverage for 10 years. Malar. J. 2018, 17, 123. [Google Scholar] [CrossRef] [Green Version]
- Caputo, B.; Santolamazza, F.; Vicente, J.; Nwakanma, D.C.; Jawara, M.; Palsson, K.; Jaenson, T.; White, B.J.; Mancini, E.; Petrarca, V.; et al. The “far-west” of Anopheles gambiae molecular forms. PLoS ONE 2011, 6, e16415. [Google Scholar] [CrossRef] [Green Version]
- Vicente, J.L.; Clarkson, C.S.; Caputo, B.; Gomes, B.; Pombi, M.; Sousa, C.A.; Antao, T.; DInis, J.; Bottà, G.; Mancini, E.; et al. Massive introgression drives species radiation at the range limit of Anopheles gambiae. Sci. Rep. 2017, 7, 46451. [Google Scholar] [CrossRef] [Green Version]
- Herrmann, S.M.; Tappan, G.G. Vegetation impoverishment despite greening: A case study from central Senegal. J. Arid Environ. 2013, 90, 55–66. [Google Scholar] [CrossRef]
- Diallo, D.; Diagne, C.T.; Buenemann, M.; Ba, Y.; Dia, I.; Faye, O.; Sall, A.A.; Faye, O.; Watts, D.M.; Weaver, S.C.; et al. Biodiversity pattern of mosquitoes in southeastern Senegal, epidemiological implication in arbovirus and malaria transmission. J. Med. Entomol. 2019, 56, 453–463. [Google Scholar] [CrossRef]
- Jones, C.M.; Toé, H.K.; Sanou, A.; Namountougou, M.; Hughes, A.; Diabaté, A.; Dabiré, R.; Simard, F.; Ranson, H. Additional Selection for Insecticide Resistance in Urban Malaria Vectors: DDT Resistance in Anopheles arabiensis from Bobo-Dioulasso, Burkina Faso. PLoS ONE 2012, 7, e45995. [Google Scholar] [CrossRef]
- Niang, E.H.A.; Konaté, L.; Diallo, M.; Faye, O.; Dia, I. Reproductive isolation among sympatric molecular forms of An. gambiae from inland areas of south-eastern Senegal. PLoS ONE 2014, 9, e104622. [Google Scholar] [CrossRef] [Green Version]
- Sudia, W.; Chamberlain, R. Battery-operated light trap, an improved model. Mosq. News 1962, 22, 126–129. [Google Scholar]
- World Health Organisation. Division of Malaria and Other Parasitic Diseases; World Health Organisation: Geneva, Switzerland, 1975. [Google Scholar]
- Reisen, W.K.; Meyer, R.P.; Cummings, R.F.; Delgado, O. Effects of trap design and CO2 presentation on the measurement of adult mosquito abundance using Centers for Disease Control-style miniature light traps. J. Am. Mosq. Control Assoc. 2000, 166, 13–18. [Google Scholar]
- Costantini, C.; Sagnon, N.F.; Sanogo, E.; Merzagora, L.; Coluzzi, M. Relationship to human biting collections and influence of light and bednet in CDC light-trap catches of West African malaria vectors. Bull. Entomol. Res. 1998, 88, 503–511. [Google Scholar] [CrossRef] [Green Version]
- Githeko, A.K.; Service, M.W.; Mbogo, C.M.; Atieli, F.A.; Juma, F.O. Sampling Anopheles arabiensis, A. gambiae sensu lato and A. funestus (Diptera: Culicidae) with CDC light-traps near a rice irrigation area and a sugarcane belt in western Kenya. Bull. Entomol. Res. 1994, 84, 319–324. [Google Scholar] [CrossRef]
- Kilama, M.; Smith, D.L.; Hutchinson, R.; Kigozi, R.; Yeka, A.; Lavoy, G.; Kamya, M.R.; Staedke, S.G.; Donnelly, M.J.; Drakeley, C.; et al. Estimating the annual entomological inoculation rate for Plasmodium falciparum transmitted by Anopheles gambiae sl using three sampling methods in three sites in Uganda. Malar. J. 2014, 13, 111. [Google Scholar] [CrossRef] [Green Version]
- Sougoufara, S.; Ottih, E.C.; Tripet, F. The need for new vector control approaches targeting outdoor biting Anopheline malaria vector communities. Parasites Vectors 2020, 13, 295. [Google Scholar] [CrossRef]
- Kröckel, U.; Rose, A.; Eiras, Á.E.; Geier, M. New tools for surveillance of adult yellow fever mosquitoes: Comparison of trap catches with human landing rates in an urban environment. J. Am. Mosq. Control Assoc. 2006, 22, 229–238. [Google Scholar] [CrossRef]
- Maciel-de-Freitas, R.; Eiras, Á.E.; Lourenço-de-Oliveira, R. Field evaluation of effectiveness of the BG-Sentinel, a new trap for capturing adult Aedes aegypti (Diptera: Culicidae). Mem. Inst. Oswaldo Cruz 2006, 101, 321–325. [Google Scholar] [CrossRef] [Green Version]
- Gama, R.A.; da Silva, I.M.; Geier, M.; Eiras, Á.E. Development of the BG-Malaria trap as an alternative to human-landing catches for the capture of Anopheles darlingi. Mem. Inst. Oswaldo Cruz 2013, 108, 63–771. [Google Scholar] [CrossRef] [Green Version]
- Mukabana, W.R.; Mweresa, C.K.; Otieno, B.; Omusula, P.; Smallegange, R.C.; van Loon, J.J.A.; Takken, W. A Novel Synthetic Odorant Blend for Trapping of Malaria and Other African Mosquito Species. J. Chem. Ecol. 2012, 38, 235–244. [Google Scholar] [CrossRef] [Green Version]
- Pombi, M.; Guelbeogo, W.M.; Calzetta, M.; Sagnon, N.; Petrarca, V.; La Gioia, V.; Della Torre, A. Evaluation of a protocol for remote identification of mosquito vector species reveals BG-Sentinel trap as an efficient tool for Anopheles gambiae outdoor collection in Burkina Faso. Malar. J. 2015, 14. [Google Scholar] [CrossRef] [Green Version]
- Trape, J.F.; Rogier, C.; Konate, L.; Diagne, N.; Bouganali, H.; Canque, B.; Legros, F.; Badji, A.; Ndiaye, G.; Ndiaye, P.; et al. The Dielmo project: A longitudinal study of natural malaria infection and the mechanisms of protective immunity in a community living in a holoendemic area of Senegal. Am. J. Trop. Med. Hyg. 1994, 51, 123–137. [Google Scholar] [CrossRef] [Green Version]
- Dia, I.; Lochouarn, L.; Boccolini, D.; Costantini, C.; Fontenille, D. Spatial and temporal variations of the chromosomal inversion polymorphism of Anopheles funestus in Senegal. Parasite 2000, 7, 179–184. [Google Scholar] [CrossRef] [Green Version]
- Niang, E.H.A.; Konaté, L.; Faye, O.; Diallo, M.; Dia, I. Vector bionomics and malaria transmission in an area of sympatry of An. arabiensis, An. coluzzii and An. gambiae. Acta Trop. 2019, 189, 129–136. [Google Scholar] [CrossRef]
- Gillies, M.T.; Coetzee, M. A Supplement to the Anophelinae of Africa South of the Sahara (Ethiopian zoogeographical region). Publ. S. Afr. Inst. Med. Res. 1987, 55, 1–146. [Google Scholar]
- Rider, M.A.; Byrd, B.D.; Keating, J.; Wesson, D.M.; Caillouet, K.A. PCR detection of malaria parasites in desiccated Anopheles mosquitoes is uninhibited by storage time and temperature. Malar. J. 2012, 11, 193. [Google Scholar] [CrossRef] [Green Version]
- Fanello, C.; Santolamazza, F.; Della Torre, A. Simultaneous identification of species and molecular forms of the Anopheles gambiae complex by PCR-RFLP. Med. Vet. Entomol. 2002, 16, 461–464. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2019; Available online: https://www.r-project.org/ (accessed on 1 April 2019).
- Lühken, R.; Pfitzner, W.P.; Börstler, J.; Garms, R.; Huber, K.; Schork, N.; Steinke, S.; Kiel, E.; Becker, N.; Tannich, E.; et al. Field evaluation of four widely used mosquito traps in Central Europe. Parasites Vectors 2014. [Google Scholar] [CrossRef] [Green Version]
- Hapairai, L.K.; Plichart, C.; Naseri, T.; Silva, U.; Tesimale, L.; Pemita, P.; Bossin, H.C.; Burkot, T.R.; Ritchie, S.A.; Graves, P.M.; et al. Evaluation of traps and lures for mosquito vectors and xenomonitoring of Wuchereria bancrofti infection in a high prevalence Samoan Village. Parasites Vectors 2015, 8, 287. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Su, X.; Zhou, G.; Zhang, H.; Puthiyakunnon, S.; Shuai, S.; Cai, S.; Gu, J.; Zhou, X.; Yan, G.; et al. Comparative evaluation of the efficiency of the BG-Sentinel trap, CDC light trap and Mosquito-oviposition trap for the surveillance of vector mosquitoes. Parasites Vectors 2016, 9, 446. [Google Scholar] [CrossRef] [Green Version]
- Wilson, N.O.; Badara Ly, A.; Cama, V.A.; Cantey, P.T.; Cohn, D.; Diawara, L.; Direny, A.; Fall, M.; Feeser, K.R.; Fox, L.A.M.; et al. Evaluation of Lymphatic Filariasis and Onchocerciasis in Three Senegalese Districts Treated for Onchocerciasis with Ivermectin. PLoS Negl. Trop. Dis. 2016, 10, e0005198. [Google Scholar] [CrossRef]
- Subra, R. Biology and control of Culex pipiens quinquefasciatus Say, 1823 (Diptera, Culicidae) with special reference to Africa. Int. J. Trop. Insect Sci. 1981, 1, 319–338. [Google Scholar] [CrossRef]
- Doucoure, S.; Thiaw, O.; Wotodjo, A.N.; Bouganali, C.; Diagne, N.; Parola, P.; Sokhna, C. Anopheles arabiensis and Anopheles funestus biting patterns in Dielmo, an area of low level exposure to malaria vectors. Malar. J. 2020, 19, 230. [Google Scholar] [CrossRef]
- Ndiath, M.O.; Mazenot, C.; Gaye, A.; Konate, L.; Bouganali, C.; Faye, O.; Sokhna, C.; Trape, J.F. Methods to collect Anopheles mosquitoes and evaluate malaria transmission: A comparative study in two villages in Senegal. Malar. J. 2011, 10, 270. [Google Scholar] [CrossRef] [Green Version]
- Wotodjo, A.N.; Trape, J.F.; Richard, V.; Doucouré, S.; Diagne, N.; Tall, A.; Ndiath, O.; Faye, N.; Gaudart, J.; Rogier, C.; et al. No difference in the incidence of malaria in human-landing mosquito catch collectors and non-collectors in a Senegalese village with endemic malaria. PLoS ONE 2015, 10, e126187. [Google Scholar] [CrossRef] [Green Version]
- USAID. US President’s Malaria Initiative President’s Malaria Initiative—Senegal Abbreviated Malaria Operational Plan FY 2019; USAID: Washington, DC, USA, 2019; p. 15.
- Mwesigwa, J.; Okebe, J.; Affara, M.; Di Tanna, G.L.; Nwakanma, D.; Janha, O.; Opondo, K.; Grietens, K.P.; Achan, J.; D’Alessandro, U. On-going malaria transmission in The Gambia despite high coverage of control interventions: A nationwide cross-sectional survey. Malar. J. 2015, 14, 314. [Google Scholar] [CrossRef] [Green Version]
- Nwakanma, D.C.; Neafsey, D.E.; Jawara, M.; Adiamoh, M.; Lund, E.; Rodrigues, A.; Loua, K.M.; Konate, L.; Sy, N.; Dia, I.; et al. Breakdown in the process of incipient speciation in Anopheles gambiae. Genetics 2013, 193, 1221–1231. [Google Scholar] [CrossRef] [Green Version]
- Caputo, B.; Nwakanma, D.; Jawara, M.; Adiamoh, M.; Dia, I.; Konate, L.; Petrarca, V.; Conway, D.J.; Torre, A. Anopheles gambiae complex along The Gambia river, with particular reference to the molecular forms of An. gambiae ss. Malar. J. 2008, 17, 182. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngom, E.H.M.; Virgillito, C.; Manica, M.; Rosà, R.; Pichler, V.; Sarleti, N.; Kassé, I.; Diallo, M.; della Torre, A.; Dia, I.; et al. Entomological Survey Confirms Changes in Mosquito Composition and Abundance in Senegal and Reveals Discrepancies among Results by Different Host-Seeking Female Traps. Insects 2021, 12, 692. https://doi.org/10.3390/insects12080692
Ngom EHM, Virgillito C, Manica M, Rosà R, Pichler V, Sarleti N, Kassé I, Diallo M, della Torre A, Dia I, et al. Entomological Survey Confirms Changes in Mosquito Composition and Abundance in Senegal and Reveals Discrepancies among Results by Different Host-Seeking Female Traps. Insects. 2021; 12(8):692. https://doi.org/10.3390/insects12080692
Chicago/Turabian StyleNgom, El Hadji Malick, Chiara Virgillito, Mattia Manica, Roberto Rosà, Verena Pichler, Noemi Sarleti, Isseu Kassé, Mawlouth Diallo, Alessandra della Torre, Ibrahima Dia, and et al. 2021. "Entomological Survey Confirms Changes in Mosquito Composition and Abundance in Senegal and Reveals Discrepancies among Results by Different Host-Seeking Female Traps" Insects 12, no. 8: 692. https://doi.org/10.3390/insects12080692
APA StyleNgom, E. H. M., Virgillito, C., Manica, M., Rosà, R., Pichler, V., Sarleti, N., Kassé, I., Diallo, M., della Torre, A., Dia, I., & Caputo, B. (2021). Entomological Survey Confirms Changes in Mosquito Composition and Abundance in Senegal and Reveals Discrepancies among Results by Different Host-Seeking Female Traps. Insects, 12(8), 692. https://doi.org/10.3390/insects12080692








