Simple Summary
Predicting the distribution of endemic insects is vital to continual study and conservation efforts. Here we used ecological niche models and pH data to determine which environmental factors may be influencing the distribution of a group of damselflies in Vanuatu. We tested the utility of niche models in this context and found pH to be a strong predictor for this genus.
Abstract
Vanuatubasis Ober and Staniczek is a genus of damselfly endemic to Vanuatu. Little is known about the distribution and general natural history of the genus. We present the results of 14 weeks of fieldwork in Vanuatu to provide a better understanding of the biology of this genus. Specifically, we tested ecological niche models to predict the presence of Vanuatubasis throughout the region and explored how water pH may play a role in their distribution and ecology. The results of this fieldwork refined our model and further predicted the presence of this genus on additional islands. We also found stream pH as a strong predictor for the presence of Vanuatubasis, with their presence in alkaline streams significantly higher (p < 0.001). The mean pH for those streams where the genus was collected was 8.44 (n = 53).
1. Introduction
The ability to reliably predict and record the distribution of organisms is critical to both biodiversity research (e.g., taxonomy, systematics, phylogenetics) and conservation efforts [1,2]. Recording distributions is particularly important for organisms more vulnerable to extinction, such as highly endemic or rare species [3]. Freshwater ecosystems represent a fraction (~1%) of terrestrial area and are a major epicenter of animal diversity, with insects representing the majority of aquatic diversity (~80%; [4]). Island fresh-water habitats are among the most unique and threatened ecosystems on earth [5]. Island organisms are often highly unique and inhabit small areas, making them more prone to extinction [6,7]. Thus, endemic aquatic insects in freshwater island habitats are potentially the animals most at risk of disappearing before they can be properly identified by science. Documentation of the natural history, distribution, and diversity of such species, or even groups of closely related species with similar ecologies, should be an urgent focus of biodiversity research.
Vanuatu is a relatively young island chain (~14 Ma) composed of ~82 islands, mostly volcanic, located on the convergent boundary between the Australian and Pacific plates [8]. Many of these islands have little infrastructure and are largely covered with primary and secondary rainforest, streams, lakes, and active volcanoes [9]. The islands are relatively small, with the largest being ~3955 km2 and over fourteen islands being less than 100 km2 [9]. Although there is a relatively large amount of underdeveloped area [10] compared to other island nations in the South Pacific, the islands of Vanuatu are changing rapidly. Deforestation, overgrazing, mining, and globalization are all immediate factors that contribute to land degradation and destruction of natural land cover [11,12]. Rising sea levels also threaten the limited suitable habitat for geographically restricted island endemics [13].
Vanuatubasis is an endemic damselfly genus in the family Coenagrionidae. Little is known about their natural history, distribution, diversity, and ecological tolerances. Currently, this genus is composed of three species described from the islands of Aneityum, Espiritu Santo, and Malekula [14]. Additional work suggests there are several new species awaiting formal description [10]. All species described and awaiting formal description appear to be endemic to a single island [15]. All species appear to share similar ecologies, yet the genus lacks documentation of ecological factors that could predict their distribution.
Ecological factors influencing the distribution of Vanuatubasis throughout Vanuatu are unclear. Predictive models can help to investigate some of these potential factors. Species distribution modeling tools are common and widely used in ecology, and provide insight into species’ distributions, including odonates [16,17,18,19,20,21]. These models determine relationships between occurrences of species and environmental factors at given locations and predict suitable habitat distributions based on these factors [22]. Studies have shown that species distribution models are useful for guiding field surveys toward regions where there is an increased probability of finding new populations of a known species [23,24,25]. These models are especially useful to gain a basic understanding of rare and understudied groups [26], such as Vanuatubasis. These models have also been shown to be accurate across a genus when the species are closely related and tend to inhabit similar ecological niches. This shared niche space between closely related species is known as ‘phylogenetic niche conservatism’ and is seen in many different taxa [27,28,29].
By increasing the discovery rate of new populations, conservation planning in poorly known and highly threatened landscapes will become more efficient [30]. Ecological niche models are a tool that can be used to more efficiently direct efforts to discover these populations, particularly in groups with limited ranges in areas that are logistically problematic to survey [20,31]. Often these models are implemented in programs such as MaxEnt [32]. Several recent studies utilize this program in various animal groups around the world. For example, Patten et al. [33] discuss the predictive power of adult presence on odonate breeding across Oklahoma. These methods have also been used to predict the effects of climate change on high-elevation insects in Italy [34].
Ecological niche models, however, are limited by which environmental layers are provided by the user [35]. Thus, potentially important ecological factors that are not able to be included in these data layers will be ignored [30]. This may be impactful for species whose distribution is largely limited by abiotic characteristics of freshwater habitats whose data are not available in certain parts of the world. Factors that are known to influence odonate distributions include sunlight availability [36,37], water turbidity [38,39], substrate type [40], vegetation [41], and the water pH [42,43]. In regard to Vanuatubasis, prior observations seemed to show that streams where the genus was collected had large mineral deposits, likely a result of high amounts of calcium carbonate, which can lead to basic pH levels [44]. Thus, water pH may be an important factor influencing Vanuatubasis distribution that is not directly accounted for in the MaxEnt model.
Here, we provide a comprehensive survey of Vanuatubasis resulting from 14 weeks of fieldwork spanning three years. We provide an initial ecological niche model for the group using data from 2006, 2017, and 2018, which we were able to subsequently test, validate, and improve after additional fieldwork in 2019. We also explore the relationship between the distribution of this genus and the pH levels of streams in order to explore whether Vanuatubasis requires alkaline environments.
2. Materials and Methods
2.1. Fieldwork
In May and June of 2018, our research team traveled to Vanuatu, targeting the endemic genus, Vanuatubasis. We visited the islands of Efate, Erromango, Espiritu Santo, Gaua, Malekula, and Tanna (Figure 1b). Adult specimens were collected with aerial nets and preserved in 95% EtOH. Photographs were taken of these behaviors, and vouchers were collected. The GPS coordinates from any successful collecting event were combined with the coordinates taken during previous research expeditions in 2006 and 2017 [10,14] (Figure 1a) and used to generate a preliminary ecological niche model to guide future collecting efforts.
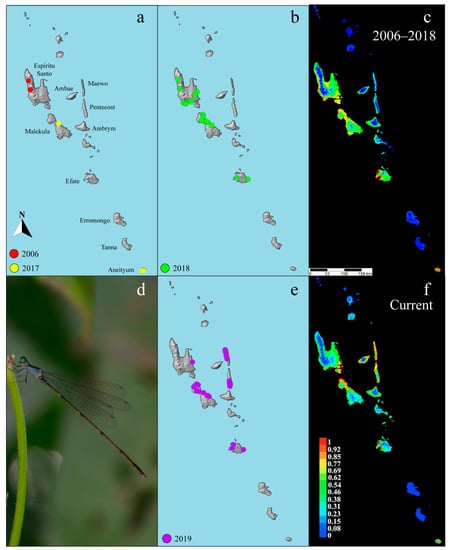
Figure 1.
(a) Collecting localities pre-2018 used in the first model including data from 2006 and 2017; (b) Collecting localities from 2018 used in the first model; (c) First ecological niche model produced in MaxEnt using data from 2006–2018; (d) undescribed male Vanuatubasis from Efate; (e) collecting localities added to the model from 2019 fieldwork; (f) updated ecological niche model produced in MaxEnt with all localities.
In order to test the accuracy of the 2018 ecological niche model, a follow-up expedition returned to Vanuatu in May and June of 2019 to collect on the islands of Ambrym, Efate, Espiritu Santo, Maewo, Malekula, Pentecost, and Tanna (Figure 1e). The 2018 model was used as a guide for choosing sampling areas based off of their predicted habitat suitability. Three previously unstudied islands, predicted to have habitat suitable for Vanuatubasis were included (i.e., Ambrym, Maewo, and Pentecost). Additionally, we expanded our research areas on the islands previously studied to further validate and refine the model.
2.2. Ecological Niche Models
The 2019 expedition was directed by the results of the 2018 model. Methods used to generate the first (2018) ecological niche model for Vanuatubasis included data points from expeditions prior to 2019 (Figure 1a,b). The updated ecological niche model generated after 2019 used all previous data and data newly collected in 2019 (Figure 1e) (See Table S1). Both ecological niche models were generated using the maximum entropy modeling software MaxEnt v3.4.1 [32] and followed basic methods discussed by Zaspel et al. [45]. MaxEnt uses species presence data and environmental variables to generate predictions and was chosen because it performs well for predictions of presence-only data [32,46].
We acquired environmental variables (i.e., climate data layers) from WorldClim v1.4 Current Conditions at the spatial resolution of 30 arcseconds [47]. Data layers included all 19 available bioclimatic variables and an additional four temperature and precipitation layers (e.g., monthly average temperatures (minimum, maximum, and median), monthly average precipitation, etc.). We did not reduce any of our layers since Elith et al. [19] states “MaxEnt has an inbuilt method for regularization (L1-regularization) that is reliable and known to perform well [48]. It implicitly deals with feature selection (relegating some coefficients to zero) and is unlikely to be improved—and more likely, degraded—by procedures that use other modelling methods to pre-select variables (e.g., [49]).” Each data layer was imported into DIVA-GIS v7.5 [50] and trimmed to only include Vanuatu (using bounding box coordinates: x = −13.07580, −20.34111 and y = 166.43738, 170.01268). Trimmed climate data files and all Vanuatubasis decimal degree localities were imported into MaxEnt [32]. We employed the jackknife method to measure variable importance. The model was generated with the default feature classes (linear, quadratic, product, and hinge) and a Cloglog output, as Cloglog is more robust with smaller data sets [51]. The model was also run with the experimental setting to write background predictions. An additional jackknife method was used to measure the importance of contributing variables. Each prediction point is given a value ranging from 0 to 1.0, with larger numbers representing a higher probability of species presence.
2.3. Stream PH
In 2019, we sampled 53 streams. Fieldwork was conducted during the sunniest part of the day, between 09:00 and 14:00 h. At each surveyed location we recorded the pH using a High Accuracy pH meter (Dr. Meter, Union City, CA, USA). We recorded several points throughout the stream and the average pH was recorded. Data were only used in the final analysis if it met three criteria: (1) GPS coordinates and pH values were collected; (2) a minimum threshold of collecting time was met (>4.5 working hours); (3) the weather was favorable for odonate collecting (i.e., sunny) and wind and/or rain were not a confounding factor. In total, 42 rivers met our criteria and were included in the analysis (See Table S2). Tableau software (Salesforce, Mountain View, CA, USA) was used to depict locations surveyed and the range of pH levels across the islands. We performed an independent samples Mann–Whitney U test using SPSS Statistics v.27 (IBM, Chicago, IL, USA) to test for a significant correlation between pH-level and the presence or absence of Vanuatubasis.
3. Results
3.1. 2018. Ecological Niche Model
Vanuatubasis were collected on the islands of Aneityum, Efate, Espiritu Santo, and Malekula at 23 different sites across these islands during 2018. Vanuatubasis was not found on Erromango, Gaua, or Tanna. Based on climate data associated with the confirmed collection sites, the 2018 ecological niche model predicts the presence of Vanuatubasis on the islands of Ambae, Ambrym, Aneityum, Efate, Espiritu Santo, Maewo, Malekula, and Pentecost with greater than 50% statistical support (Figure 1c, Table 1). The 2018 ecological niche model recovered five environmental factors that had >10% contribution to the overall model (Table 2). Here, temperature seasonality refers to the amount of temperature variation over a given year based on the standard deviation of monthly temperatures. The mean temperature of the wettest quarter is an index that approximated the mean temperature during the wettest three months of the year [52].

Table 1.
Prediction of Vanuatubasis presence (50–90%) at different cut-offs above 50% for the 2018 model, compared to the results of 2019 fieldwork (Presence).

Table 2.
The five variables that contributed the most to the MaxEnt ecological niche models for 2018 and 2019.
3.2. Field Validation of 2018 Model
Fieldwork in 2019 was used to validate and refine the 2018 model. In 2019, we surveyed Ambrym, Efate, Espiritu Santo, Maewo, Malekula, Pentecost, and Tanna for Vanuatubasis. Specimens were collected on the islands of Efate, Espiritu Santo, Malekula, Maewo and Pentecost at 31 localities (Figure 1e). The 2018 model predicted three previously unsampled islands (Ambrym, Maewo, and Pentecost) as suitable for Vanuatubasis. We collected Vanuatubasis on two of these islands (Pentecost and Maewo). No specimens were found on the islands of Ambrym or Tanna (Table 1). Ambrym was the only sampled island where Vanuatubasis was predicted but none were found. Ambae was also predicted to have Vanuatubasis present, but due to recent volcanic activity the island was not sampled.
3.3. 2019 Ecological Niche Model
The updated ecological niche model showed an overall refinement of the predicted distribution when compared with the 2018 model (Figure 1c,f). The probability of Vanuatubasis presence on the islands of Pentecost and Maewo increased, with a larger portion of the islands exhibiting probabilities close to 1.0. The probability of Vanuatubasis presence on the island of Malekula increased on the northern coast and shifted towards the eastern coast on Espirtu Santo. In addition, the five environmental factors that each had greater than 10% contribution to the overall model shifted so that only two of these factors were in common with the 2018 model (Table 2). Temperature seasonality and October precipitation, both in the top five for the 2018 model, increased in their contribution to the 2019 model.
3.4. Stream PH
The pH is shown to be highly correlated with the presence of Vanuatubasis (p <0.001). The range of pH for streams where Vanuatubasis was collected was 7.76 to 8.82 (N = 32, mean = 8.44, SD = 0.267; Figure 2). On two occasions specimens were collected in streams with a pH < 8.0; a small population was found in a river with a pH of 7.90, and two males were found on a seep with a pH of 7.76 (Figure 3). In streams where other factors (e.g., stream bottom composition, vegetation, size and overall appearance) seemed favorable but Vanuatubasis was absent, the mean pH was 7.76 and the median was 7.85. (N = 10, mean = 7.76, SD = 0.467). As pH is treated on a logarithmic scale the difference between the means of streams with Vanautubasis present and those without is approximately 7 times [53].
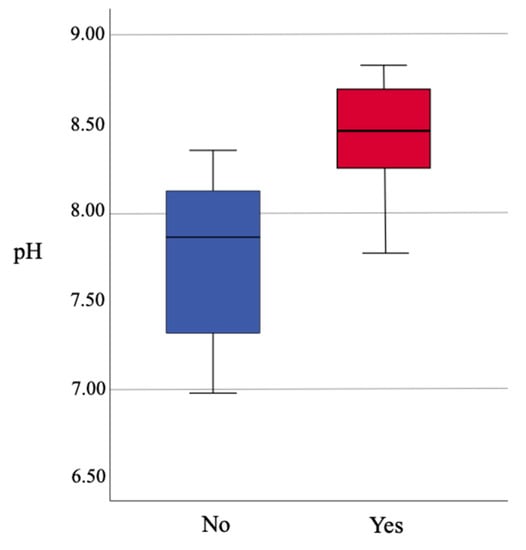
Figure 2.
Boxplot comparing water pH to presence/absence of Vanuatubasis.
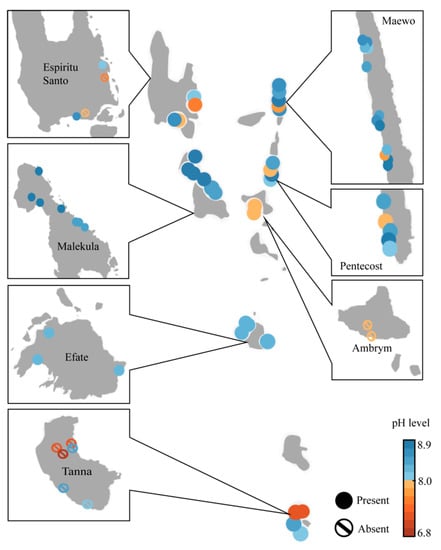
Figure 3.
Map of Vanuatu depicting the pH of each collecting locality. Solid circles represent localities where Vanuatubasis was collected, and the circle-backslash symbols represent locations where Vanuatubasis was not found.
4. Discussion
Vanuatubasis is only beginning to be understood in terms of its diversity, distribution, and natural history [10,14,15]. All species of Vanuatubasis are single island endemics, often found on a small portion of a single island. Therefore, most species within the genus are vulnerable to habitat and climate change. Ecologically, it appears that the genus Vanuatubasis displays a very narrow niche compared to other endemic genera of the South Pacific such as Nesobasis Selys and Megalagrion McLachlan [54,55]. Vanuatubasis inhabit nearly identical streams across the islands sampled here and appear to be highly endemic [15]. While some variation exists in the habitats Vanuatubasis occupies, they are generally found in lotic streams ranging from 7–10 m wide and 0.2–0.5 m deep. The banks of the streams tend to be sloped and densely covered with vegetation. Adult Vanuatubasis are frequently found in shady sites where sunspots peak through the canopy overhead, perched on vegetation, particularly ferns. The streams also appear to have high mineral content, often with visible dams formed from mineral deposits laid down over submerged mud, rocks, and branches [10]. This ecological specialization is not as pronounced in other island genera such as the Hawaiian Megalagrion that develop as nymphs in a wide range of habitats including seeps, pools, and leaf litter [55] allowing multiple species to be present on the same stream.
Future conservation efforts for these narrow endemics are reliant on studies focused on their distribution, as well as the environmental factors influencing this distribution. These studies will allow for better field surveys and management of their populations [35,56]. Improving our understanding of this genus, including more careful observation, sampling and taxonomy, will allow us to explore their origins, speciation, and general evolution.
4.1. Ecological Niche Models
The use of ecological niche models for Vanuatubasis across Vanuatu proved useful to guide fieldwork efforts. Of the eight main islands on which Vanuatubasis was predicted to be present, we were able to find specimens on six (Table 1). We performed fieldwork in 2017–2019. Ambae was inaccessible in 2017 due to cyclone Donna and in both 2018 and 2019 due to severe volcanic activity and subsequent evacuations of the entire island. Despite this, to date none of the other islands where we performed fieldwork that had active volcanoes had Vanuatubasis populations. Major volcanoes can be found across the central chain of islands in Vanua Lava, Gaua, Ambae, Ambrym, and Tanna [57]. For example, Ambrym was moderately predicted to have Vanuatubasis; however, despite being in what we considered suitable habitat, our field team did not find Vanuatubasis on this island. Ambrym had a limited number of accessible streams on the southwest portion of the island, the area most strongly predicted for Vanuatubasis for the 2006–2018 model, and also had relatively poor weather while fieldwork was conducted. Both factors could have impacted our ability to collect Vanuatubasis on Ambrym.
Fieldwork in 2019 also refined our model by increasing the probability of their presence on Ambae, Maewo, Pentecost, and Ambrym. This increase is likely due to additional presence points added, particularly in Maewo and Pentecost. Interestingly, the top five environmental factors contributing to the 2019 model only overlapped in two factors of the 2018 model (Table 2). This model was developed at a generic level due to both the lack of taxonomic work on the group as well as what appears to be very similar ecological niches among all species. Producing species-level ecological niche models in the future may provide more insight into whether certain factors are more significant to specific species. The variables that are used in niche modeling are based on the physical environment, but there may be other biotic and abiotic factors that are important to the distribution of these organisms, that are not represented by the model [30]. As mentioned previously, active volcanoes and the subsequent factors accompanying them (e.g., weather patterns that volcanoes can generate and/or the effect they can have on the water in surrounding rivers and streams) may not be accounted for in the model. Another factor that we observed and have statistical support for, but was not included in our ecological niche model, was water pH.
4.2. Water PH
Water pH is notable as we were able to demonstrate a clear link between Vanuatubasis and alkaline streams. Studies looking at tolerance of water acidity among odonates have found that most groups can tolerate a wide range of pH levels [42,58]. Many studies attribute this wide pH tolerance to the broad distribution of odonates around the world [42]. Studies focusing on conservation of dragonfly groups have found that these species may even experience a population increase in response to acidification due to decreased competition and an increase in resource availability [58,59]. The general trend of tolerance to a wide range of pH has been explored across a limited set of odonates (i.e., common and easily identifiable species). The pH preferences and tolerance of more inconspicuous specialized and/or endemic odonate species are not as well known. It is likely that by studying such species, odonate distribution patterns, as they relate to water pH levels, will become clearer.
We used presence/absence data in conjunction with averaged pH measurements of streams to demonstrate that Vanuatubasis species are statistically more likely to be found in alkaline streams (p < 0.001). If pH is playing a role in the distribution of Vanuatubasis, it is probably most impactful on the immature, aquatic life-stage. Water pH has been recorded to affect nymphs in the past. For example, Correa et al. [60] found that in Somatochlora cingulata Selys, as they decreased the water pH, the respiration rate of the nymphs also decreased, particularly in the earlier stages of development. Odonate nymphs tend to live in much higher densities with both conspecifics and other congeners than adults and are often the primary source of competition for food resources [61]. This high rate of competition may force organisms to shift towards “extreme” habitats in order to limit competition [62]. Several instances of habitat shifts to avoid interspecific competition have been recorded in organisms including fish [63], lizards [64], and beetles [65].
Though the correlation between pH level and presence of Vanuatubasis is strong, it is difficult to determine if the pH level is a direct causal factor (e.g., egg survivorship, larval respiration) [42], or if a basic pH affects another biotic factor (e.g., predator and/or prey presence, plant communities, accumulation/decomposition of organic matter) that in turn impacts the presence of Vanuatubasis. Since pH can vary seasonally [66], studies would need to be done during months with higher precipitation and higher temperatures in Vanuatu to see how these results vary or persist. Our understanding of this phenomenon would improve with a broader sampling across additional islands at different elevations.
5. Conclusions
Vanuatubasis is an evolutionarily fascinating lineage. They exhibit extreme island endemism, selective habitat preference, and interesting behaviors. Our results provide compelling evidence supporting pH as a limiting factor impacting Vanautubasis distributions, as well as the utility of ecological niche models to guide fieldwork. We can use this predictive model as well as our observations of ideal stream pH conditions to guide our search for Vanuatubasis, as we work to fully understand the ecology and evolution of this group.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/insects12080670/s1, Table S1: Collecting localities, Table S2: pH data by locality.
Author Contributions
Conceptualization, G.S.P. and S.M.B.; Data curation, N.A.S. and G.S.P.; Formal analysis, N.A.S., E.M.P., A.M.D., C.R.J. and G.S.P.; Funding acquisition, S.M.B.; Investigation, E.M.P., A.M.D., C.R.J. and G.S.P.; Resources, S.M.B.; Validation, N.A.S., E.M.P., A.M.D., C.R.J., G.S.P. and S.M.B.; Visualization, N.A.S., E.M.P. and A.M.D.; Writing—original draft, N.A.S. and E.M.P.; Writing—review & editing, N.A.S., E.M.P., A.M.D., C.R.J., G.S.P. and S.M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by funding from the Department of Biology at Brigham Young University (Sant Grant) and the National Science Foundation (NSF DEB-1265714 and REU supplement to this award).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Supporting data can be found in the supplementary material accompanying this study.
Acknowledgments
We thank the Department of Biology at Brigham Young University, the BYU Kennedy Center, Global Opportunity Scholarship, the Gilman Scholarship, the National Science Foundation, the BYU undergraduates who helped with data collection, the landowners in Vanuatu who allowed us to collect, and all government officials that assisted with permits and travel. Additional thanks to Milen Marinov for organismal knowledge, Makani Fisher for help using MaxEnt and DIVAGIS and Alexandra Duffy for advice on statistical analyses.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vaughan, I.P.; Ormerod, S.J. Improving the quality of distribution models for conservation by addressing shortcomings in the field collection of training data. Conserv. Biol. 2003, 17, 1601–1611. [Google Scholar] [CrossRef]
- Kaky, E.; Nolan, V.; Alatawi, A.; Gilbert, F. A comparison between ensemble and MaxEnt species distribution modelling approaches for conservation: A case study with Egyptian medicinal plants. Ecol. Inform. 2020, 60, 1–12. [Google Scholar] [CrossRef]
- Engler, R.; Guisan, A.; Rechsteiner, L. An improved approach for predicting the distribution of rare and endangered species from occurrence and pseudo-absence data. J. Appl Ecol. 2004, 41, 263–274. [Google Scholar] [CrossRef]
- Dijkstra, K.D.B.; Monaghan, M.T.; Pauls, S.U. Freshwater biodiversity and aquatic insect diversification. Annu. Rev. Entomol. 2014, 59, 143–163. [Google Scholar] [CrossRef] [Green Version]
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.; Soto, D.; Stiassny, M.L.J.; et al. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. 2006, 81, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Vitousek, P.M. Diversity and biological invasions of oceanic islands. Biodiversity 1988, 20, 181–189. [Google Scholar]
- Frankham, R. Inbreeding and extinction: Island populations. Conserv. Biol. 1998, 12, 665–675. [Google Scholar] [CrossRef]
- Hamilton, A.M.; Klein, E.R.; Austin, C.C. Biogeographic breaks in Vanuatu, a nascent oceanic archipelago. Pac. Sci. 2010, 64, 149–159. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, A.M.; Hartman, J.H.; Austin, C.C. Island area and species diversity in the southwest Pacific Ocean: Is the lizard fauna of Vanuatu depauperate? Ecography 2009, 32, 247–258. [Google Scholar] [CrossRef]
- Marinov, M.; Bybee, S.; Doscher, C.; Kalfatakmolis, D. Faunistic studies on Odonata of the Republic of Vanuatu (Insecta: Odonata). J. Int. Dragonfly Fund 2019, 26, 1–046. [Google Scholar]
- Granderson, A.A. Value conflicts and the politics of risk: Challenges in assessing climate change impacts and risk priorities in rural Vanuatu. Clim. Dev. 2018, 10, 481–494. [Google Scholar] [CrossRef]
- Komugabe-Dixson, A.F.; de Ville, N.S.; Trundle, A.; McEvoy, D. Environmental change, urbanisation, and socio-ecological resilience in the Pacific: Community narratives from Port Vila, Vanuatu. Ecosyst. Serv. 2019, 39, 1–13. [Google Scholar] [CrossRef]
- Wairiu, M. Land degradation and sustainable land management practices in Pacific Island Countries. Reg. Environ. Chang. 2017, 17, 1053–1064. [Google Scholar] [CrossRef]
- Ober, S.V.; Staniczek, A.H. A new genus and species of coenagrionid damselflies (Insecta, Odonata, Zygoptera, Coenagrionidae) from Vanuatu. Zoosystema 2009, 31, 485–498. [Google Scholar] [CrossRef]
- Saxton, N.A.; Powell, G.S.; Bybee, S.M. Prevalence of leg regeneration in damselflies reevaluated: A case study in Coenagrionidae. Arthropod Struct. Dev. 2020, 59, 1–6. [Google Scholar] [CrossRef]
- Bush, A.A.; Nipperess, D.A.; Duursma, D.E.; Theischinger, G.; Turak, E.; Hughes, L. Continental-scale assessment of risk to the Australian Odonata from climate change. PLoS ONE 2014, 9, e88958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, S.D.; McIntyre, N.E. Modeling the distribution of odonates: A review. Freshw. Sci. 2015, 34, 1144–1158. [Google Scholar] [CrossRef]
- De Almeida, M.C.; Cortes, L.G.; De Marco Junior, P. New records and a niche model for the distribution of two Neotropical damselflies: Schistolobos boliviensis and Tuberculobasis inversa (Odonata: Coenagrionidae). Insect Conserv. Divers. 2010, 3, 252–256. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef] [Green Version]
- Hassall, C. Predicting the distributions of under-recorded Odonata using species distribution models. Insect Conserv. Divers. 2012, 5, 192–201. [Google Scholar] [CrossRef]
- Peterson, A.T. Uses and requirements of ecological niche models and related distributional models. Biodivers. Inform. 2006, 3, 59–72. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stohlgren, T.J. MaxEnt modeling for predicting suitable habitat for threatened and endangered tree Canacomyrica monticola in New Caledonia. J. Ecol. Nat. 2009, 1, 094–098. [Google Scholar]
- Bourg, N.A.; McShea, W.J.; Gill, D.E. Putting a CART before the search: Successful habitat prediction for a rare forest herb. Ecology 2005, 86, 2793–2804. [Google Scholar] [CrossRef]
- Fleishman, E.; Nally, R.M.; Fay, J.P. Validation tests of predictive models of butterfly occurrence based on environmental variables. Conserv. Biol. 2003, 17, 806–817. [Google Scholar] [CrossRef]
- Guisan, A.; Broennimann, O.; Engler, R.; Vust, M.; Yoccoz, N.G.; Lehmann, A.; Zimmermann, N.E. Using niche-based models to improve the sampling of rare species. Conserv. Biol. 2006, 20, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Fois, M.; Cuena-Lombraña, A.; Fenu, G.; Bacchetta, G. Using species distribution models at local scale to guide the search of poorly known species: Review, methodological issues and future directions. Ecol. Model. 2018, 385, 124–132. [Google Scholar] [CrossRef] [Green Version]
- Losos, J.B. Phylogenetic niche conservatism, phylogenetic signal and the relationship between phylogenetic relatedness and ecological similarity among species. Ecol. Lett. 2008, 11, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Murienne, J.; Guilbert, E.; Grandcolas, P. Species’ diversity in the New Caledonian endemic genera Cephalidiosus and Nobarnus (Insecta: Heteroptera: Tingidae), an approach using phylogeny and species’ distribution modelling. Biol. J. Linn. Soc. 2009, 97, 177–184. [Google Scholar] [CrossRef] [Green Version]
- Wiens, J.J. Speciation and ecology revisited: Phylogenetic niche conservatism and the origin of species. Evolution 2004, 58, 193–197. [Google Scholar] [CrossRef]
- Pearson, R.G. Species’ distribution modeling for conservation educators and practitioners. Lessons Conserv. 2007, 3, 54–89. [Google Scholar]
- Finch, J.M.; Samways, M.J.; Hill, T.R.; Piper, S.E.; Taylor, S. Application of predictive distribution modelling to invertebrates: Odonata in South Africa. Biodivers. Conserv. 2006, 15, 4239–4251. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef] [Green Version]
- Patten, M.A.; Bried, J.T.; Smith-Patten, B.D. Survey data matter: Predicted niche of adult vs breeding Odonata. Freshw. Sci. 2015, 34, 1114–1122. [Google Scholar] [CrossRef] [Green Version]
- Urbani, F.; D’Alessandro, P.; Biondi, M. Using Maximum Entropy Modeling (MaxEnt) to predict future trends in the distribution of high altitude endemic insects in response to climate change. Bull. Insectology 2017, 70, 189–200. [Google Scholar]
- Bradie, J.; Leung, B. A quantitative synthesis of the importance of variables used in MaxEnt species distribution models. J. Biogeogr. 2017, 44, 1344–1361. [Google Scholar] [CrossRef]
- Watanabe, M. Thermoregulation and habitat preference in two wing color forms of Mnais damselflies (Odonata: Calopterygidae). Zool. Sci. 1991, 8, 983–989. [Google Scholar]
- Remsburg, A.J.; Olson, A.C.; Samways, M.J. Shade alone reduces adult dragonfly (Odonata: Libellulidae) abundance. J. Insect Behav. 2008, 21, 460–468. [Google Scholar] [CrossRef]
- d’Amico, F.; Darblade, S.; Avignon, S.; Blanc-Manel, S.; Ormerod, S.J. Odonates as indicators of shallow lake restoration by liming: Comparing adult and larval responses. Restor. Ecol. 2004, 12, 439–446. [Google Scholar] [CrossRef]
- Allen, K.A.; Le Duc, M.G.; Thompson, D.J. Habitat and conservation of the enigmatic damselfly. Ischnura Pumilio. J. Insect Conserv. 2010, 14, 689–700. [Google Scholar] [CrossRef]
- Juen, L.; Cabette, H.S.R.; De Marco, P. Odonate assemblage structure in relation to basin and aquatic habitat structure in Pantanal wetlands. Hydrobiologia 2007, 579, 125–134. [Google Scholar] [CrossRef]
- Lee Foote, A.; Rice Hornung, C.L. Odonates as biological indicators of grazing effects on Canadian prairie wetlands. Ecol. Entomol. 2005, 30, 273–283. [Google Scholar] [CrossRef]
- Hudson, J.; Berrill, M. Tolerance of low pH exposure by the eggs of Odonata (dragonflies and damselflies). Hydrobiologia 1986, 140, 21–25. [Google Scholar] [CrossRef]
- Gorham, C.T.; Vodopich, D.S. Effects of acidic pH on predation rates and survivorship of damselfly nymphs. Hydrobiologia 1992, 242, 51–62. [Google Scholar] [CrossRef]
- Larsen, T.H.; Lopera, A.; Forsyth, A. Extreme trophic and habitat specialization by Peruvian dung beetles (Coleoptera: Scarabaeidae: Scarabaeinae). Coleopt. Bull. 2006, 60, 315–324. [Google Scholar] [CrossRef]
- Zaspel, J.M.; Weller, S.J.; Wardwell, C.T.; Zahiri, R.; Wahlberg, N. Phylogeny and evolution of pharmacophagy in tiger moths (Lepidoptera: Erebidae: Arctiinae). PLoS ONE 2014, 9, e101975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yackulic, C.B.; Chandler, R.; Zipkin, E.F.; Royle, J.A.; Nichols, J.D.; Campbell Grant, E.H.; Veran, S. Presence-only modelling using MAXENT: When can we trust the inferences? Methods Ecol. Evol. 2013, 4, 236–243. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.; Parra, J.; Jones, P.; Jarvis, A.; Richardson, K. WorldClim, Version 1.3; University of California: Berkeley, CA, USA, 2005. [Google Scholar]
- Hastie, T.; Tibshirani, R.; Friedman, J.H. The Elements of Statistical Learning: Data Mining, Inference, and Prediction, 2nd ed.; Springer: New York, NY, USA, 2009; p. 745. [Google Scholar]
- Wollan, A.K.; Bakkestuen, V.; Kauserud, H.; Gulden, G.; Halvorsen, R. Modelling and predicting fungal distribution patterns using herbarium data. J. Biogeogr. 2008, 35, 2298–2310. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Guarino, L.; Bussink, C. DIVA-GIS. 7.5. A Geographic Information System for the Analysis of Species Distribution Data. 2012. Available online: http://www.diva-gis.org (accessed on 19 August 2018).
- Wisz, M.S.; Hijman, R.J.; Li, J.; Peterson, A.T.; Graham, C.H.; Guisan, A. NCEAS Predicting Species Distributions Working Group. Effects of sample size on the performance of species distribution models. Divers. Distrib. 2008, 14, 763–773. [Google Scholar] [CrossRef]
- O’Donnell, M.S.; Ignizio, D.A. Bioclimatic predictors for supporting ecological applications in the conterminous United States. U.S. Geol. Surv. Data Ser. 2012, 691, 4–9. [Google Scholar]
- U.S. Geological Survey. pH and Water. 2020. Available online: https://www.usgs.gov/ (accessed on 9 February 2020).
- Beatty, C.D.; Van Gossum, H.; Sherratt, T.N. Nesobasis species diversity and abundance: Notes on an endemic genus of the island group of Fiji (Zygoptera: Coenagrionidae). Odonatologica 2007, 36, 13–26. [Google Scholar]
- Polhemus, D.A. Phylogenetic analysis of the Hawaiian damselfly genus Megalagrion (Odonata: Coenagrionidae): Implications for biogeography, ecology, and conservation biology. Pac. Sci. 1997, 51, 395–412. [Google Scholar]
- Groff, L.A.; Marks, S.B.; Hayes, M.P. Using ecological niche models to direct rare amphibian surveys: A case study using the Oregon Spotted Frog (Rana pretiosa). Herpeto.L Conserv. Biol. 2014, 9, 354–368. [Google Scholar]
- Gassner, P.; Molisa, V.; Westerveld, L.; Macmillan-Lawler, M.; Davey, K.; Baker, E.; Clark, M.; Kaitu’u, J.; Wendt, H.; Fernandes, L. Marine Atlas: Maximizing Benefits for Vanuatu; MACBIO (GIZ/IUCN/SPREP): Suva, Fiji, 2019; p. 83. [Google Scholar]
- Rychła, A.; Benndorf, J.; Buczyński, P. Impact of pH and conductivity on species richness and community structure of dragonflies (Odonata) in small mining lakes. Fundam. Appl. Limnol. 2011, 179, 41–50. [Google Scholar] [CrossRef]
- Kinvig, R.G.; Samways, M. Conserving dragonflies (Odonata) along streams running through commercial forestry. Odonatologica 2000, 29, 195–208. [Google Scholar]
- Correa, M.; Coler, R.A.; Yin, C.M. Changes in oxygen consumption and nitrogen metabolism in the dragonfly Somatochlora cingulata exposed to aluminum in acid waters. Hydrobiologia 1985, 121, 151–156. [Google Scholar] [CrossRef]
- Moore, N.W. Intra-and interspecific competition among dragonflies (Odonata). J. Anim. Ecol. 1964, 33, 49–71. [Google Scholar] [CrossRef]
- den Boer, P.J. The present status of the competitive exclusion principle. Trends Ecol. Evol. 1986, 1, 25–28. [Google Scholar] [CrossRef]
- Pörtner, H.O.; Schulte, P.M.; Wood, C.M.; Schiemer, F. Niche dimensions in fishes: An integrative view. Physiol. Biochem. Zool. 2010, 83, 808–826. [Google Scholar] [CrossRef] [Green Version]
- Capula, M.; Aloise, G. Extreme feeding behaviors in the Italian wall lizard. Podarcis Siculus. Acta Herpetol. 2011, 6, 11–14. [Google Scholar]
- Larson, T.E.; Buswell, A.M.; Ludwig, H.F.; Langelier, W.F. Calcium carbonate saturation index and alkalinity interpretations (with discussion). J. Am. Water Work. Ass. 1942, 1, 1667–1684. [Google Scholar] [CrossRef]
- Hornbeck, J.W.; Likens, G.E.; Eaton, J.S. Seasonal patterns in acidity of precipitation and their implications for forest stream ecosystems. Water Air Soil Pollut. 1977, 7, 355–365. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).