1. Introduction
Bemisia tabaci (Hemiptera: Aleyrodidae) is among the most devastating insect pests of agricultural crops worldwide [
1].
B. tabaci comprises a complex of 36 morphologically indistinguishable species that have a wide host plant range [
2,
3], including staple food crops such as cassava (
Manihot esculenta Crantz).
B. tabaci species complex causes three types of damage: direct feeding damage through ingestion of phloem sap, indirect feeding damage through the excretion of honeydew which results in the development of black sooty mold on leaf surfaces [
4], and transmission of more than 200 plant viruses [
5,
6]. Outbreaks of species in this pest complex are predominantly controlled by the use of insecticides [
7], which can cause further problems through disrupting natural enemies, such as predators and parasitoids that can then lead to secondary pest outbreaks. Researchers in some countries have released natural enemies to try to control outbreaks, e.g., the release of
Eretmocerus hayati Zolnerowich & Rose in Australia [
8], as well as understanding how host plant type and landscape resources support high population growth. Host plant resistance to another whitefly species,
Aleurotrachelus socialis Bondar was successfully bred into cassava varieties in South America [
9]. For non-high value crops such as cassava, especially in countries where pesticides are inaccessible to smallholder farmers, alternative control options such as host plant tolerance or resistance and natural enemies become critically important.
B. tabaci species complex in Sub-Saharan Africa transmit viruses that cause two economically important diseases of cassava: cassava mosaic disease (CMD) and cassava brown streak disease (CBSD) [
10,
11]. In Uganda, nearly 80% of the population, especially those in rural areas depend on cassava as a key food crop [
12]. Limited whitefly management options are available to smallholder farmers in Uganda, and pesticides are not readily accessible. However, there is a long history of breeding cassava genotypes with desirable traits for farmers, such as resistance to disease and other insect pests such as cassava mealybug (
Phenacoccus manihoti Matile–Ferrero) [
13,
14]. There is a large diversity of cassava genotypes, with very diverse form and growth habits, planted nearby each other in Uganda. This creates an ideal situation for understanding how the environment, host plant traits and cassava genotypes, and their interactions affect
B. tabaci field populations. Identification of cassava genotype traits that consistently support low
B. tabaci populations might be useful information for breeding programs around the world, particularly in developing countries.
As with many pests, we can say that the traits of the host plant itself interact with seasonal conditions to influence the population dynamics of
B. tabaci [
15]. However, when we compare studies from different crop types, there are conflicting results. A negative effect of temperature and a positive effect of relative humidity (RH) were observed on
B. tabaci population size on tomato (
Solanum esculentum Mill.) [
16] and brinjal (
Solanum melongena L.) [
17] in India. Conversely, a positive effect of temperature and a negative effect of RH was also observed on
B. tabaci population size on tomato in India [
18]. In Venezuela, rainfall was reported to negatively affect
B. tabaci population size on tomato [
19]. However, researchers concluded that rainfall had a positive effect population size of
B. tabaci on brinjal in India [
17]. Plant height and canopy closure indices had no effect on infestation levels of the whiteflies
B. tabaci (reported as
Bemisia argentifolii) and
Trialeurodes abutilonea on soybean in Tift county, Georgia, North America [
20]. However, plant height had a positive effect on
B. tabaci population size on chili (
Capsicum annuum L.) in Malaysia [
21], while it had a negative relationship with cotton (
Gossypium hirsutum L.) in Pakistan [
22].
In Uganda, the factors affecting
B. tabaci population dynamics on cassava are not fully understood. Previous outbreaks of CMD led to the development and deployment of several CMD-resistant genotypes with no emphasis on the vector. Unfortunately, these genotypes appear to support very high
B. tabaci populations [
23], and it is not known whether these genotypes have morphological traits that enhance
B. tabaci development. There is an increased status of
B. tabaci as both a direct pest of cassava and a vector of CMD and CBSD [
24].
B. tabaci was reported in most agro-ecological zones (AEZs) of Uganda in varying densities [
25,
26] and reported effects of climate factors on
B. tabaci population size are variable and so far inconclusive [
27,
28]. Indeed, previously high populations occurred in the Lake Victoria Crescent (LVC), which is a hot and humid environment. However, relatively high populations have recently been reported in other AEZs such as the North Eastern Savanna grassland, which is a hot and dry environment. These two AEZs are characterized by the widespread adoption of disease-resistant cassava genotypes. It is not known whether these genotypes are interacting with the environment to favor
B. tabaci population increases in certain AEZs.
It is important to determine how the environment, cassava genotypes, and their interactions affect B. tabaci field populations, as this knowledge will aid the design of effective and sustainable management strategies, as well as guide the development and deployment of future cassava genotypes. The objective of this study was to determine how morphological traits of cassava genotypes and changes in temperature, rainfall, and RH affect B. tabaci population dynamics in three contrasting regions.
4. Discussion
This study aimed to determine the effects of environmental conditions and cassava morphological traits on
B. tabaci adult and nymph populations in the field.
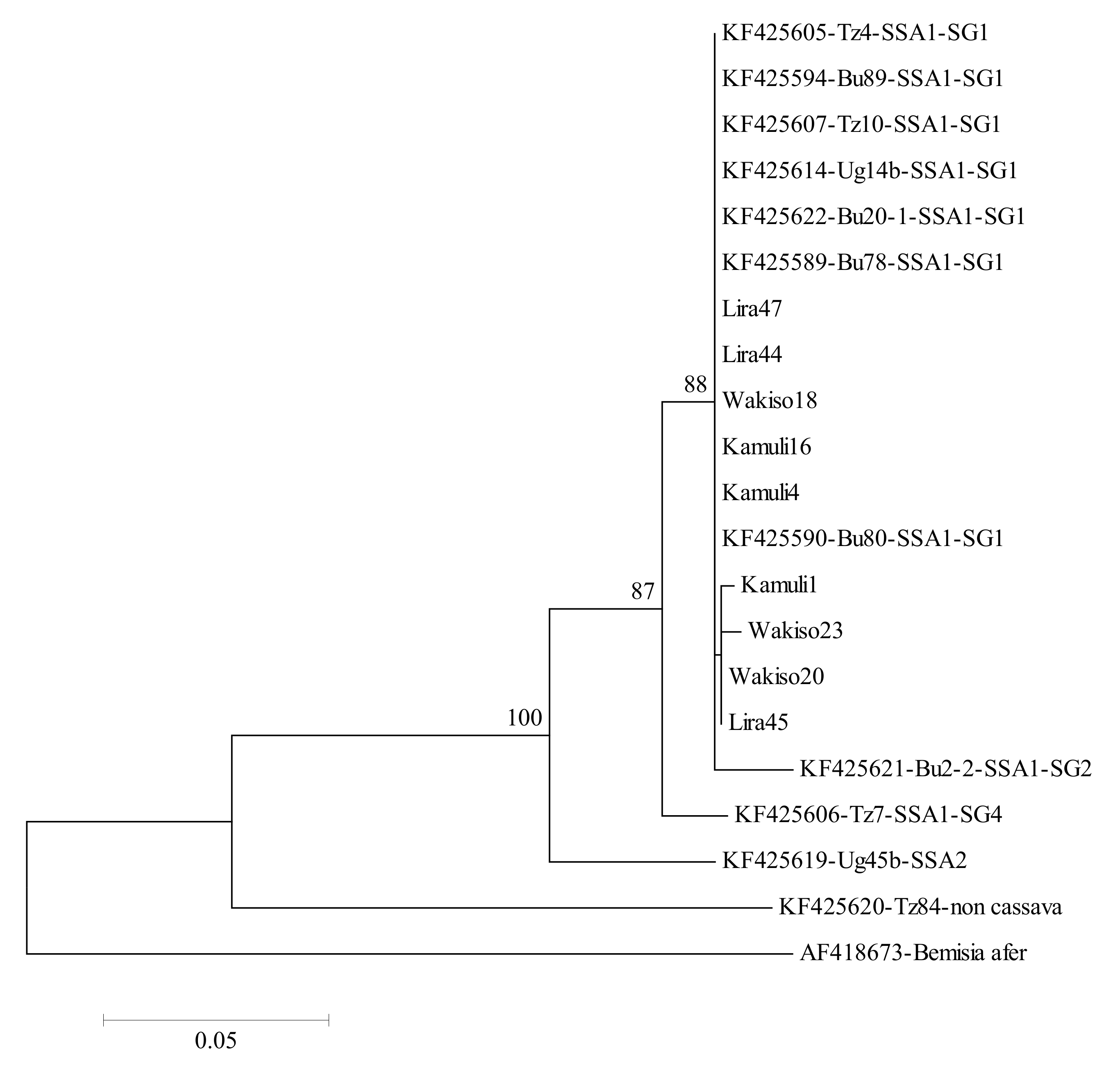
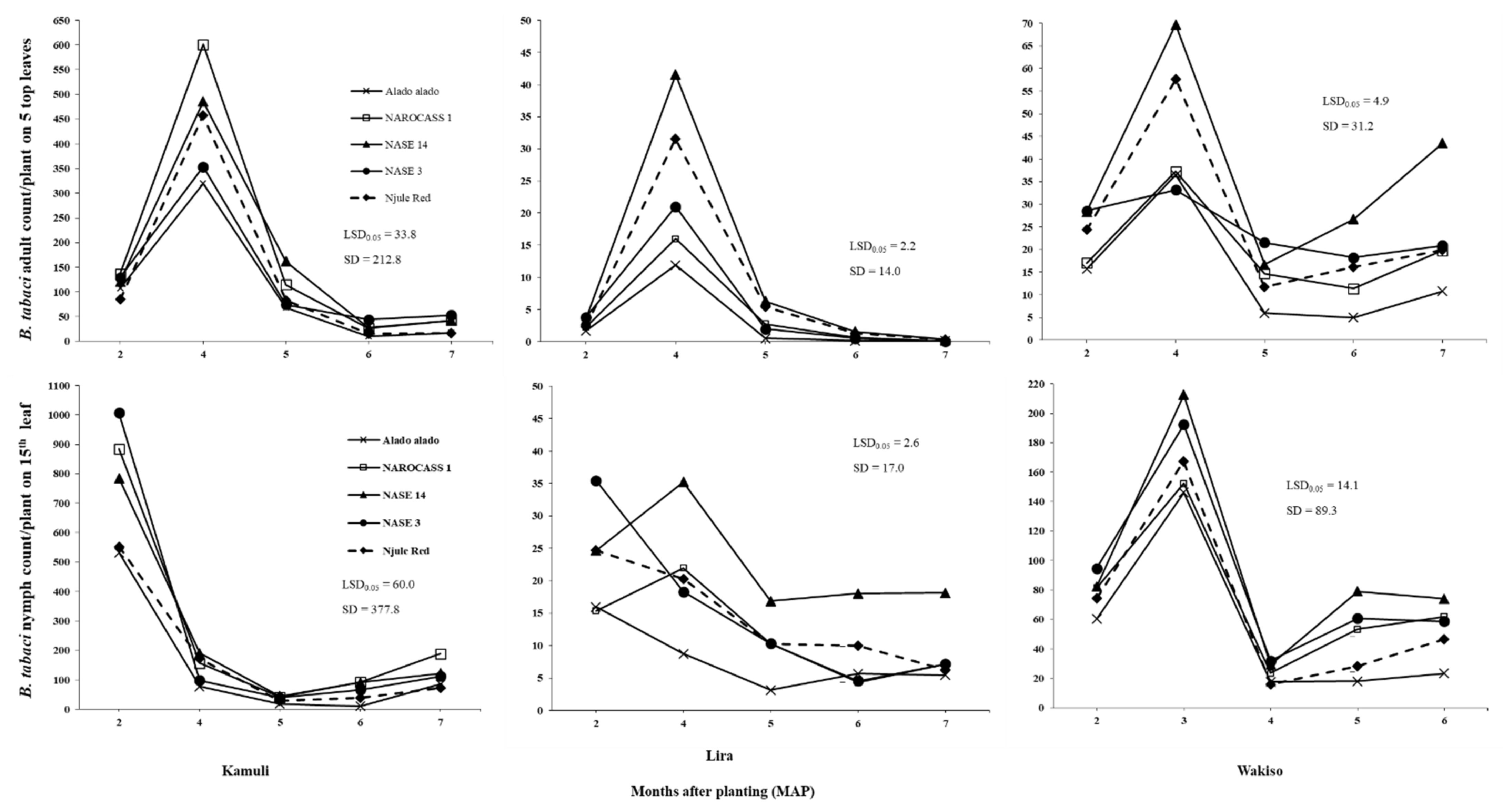
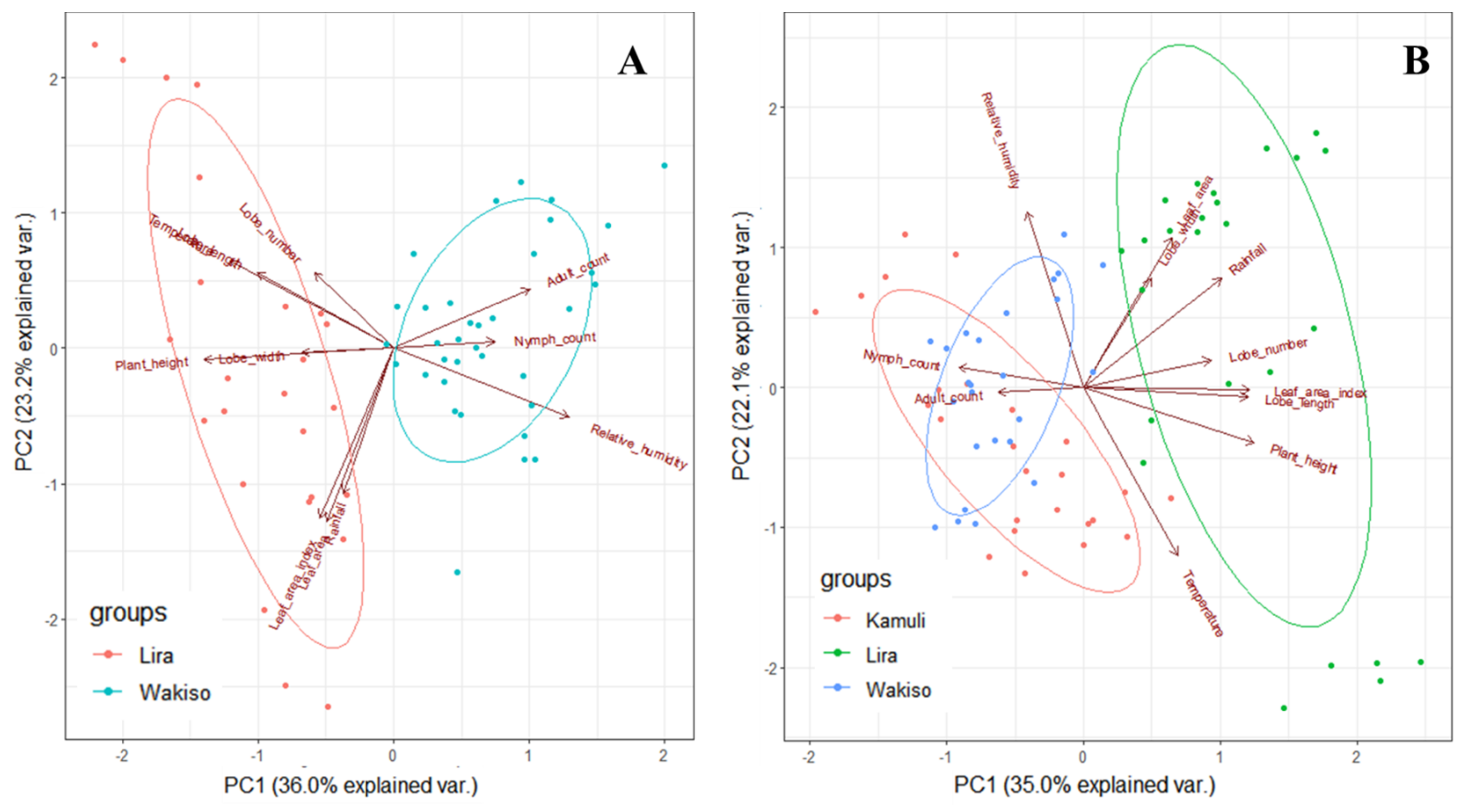
B. tabaci populations (identified as SSA1 species) were present on cassava in varying numbers in all locations in both years. The variation in
B. tabaci adult and nymph abundance in our trials demonstrated marked differences in the response of this pest to the different cassava genotypes as well as the environmental conditions in each AEZ. The mechanisms underlying these patterns may be through a combination of direct and indirect effects. Direct influences can be observed through limiting and stimulating the activity of adults and altering survival during adverse weather conditions; indirect influences can be through the effect on host plant growth and food quality [
44,
45].
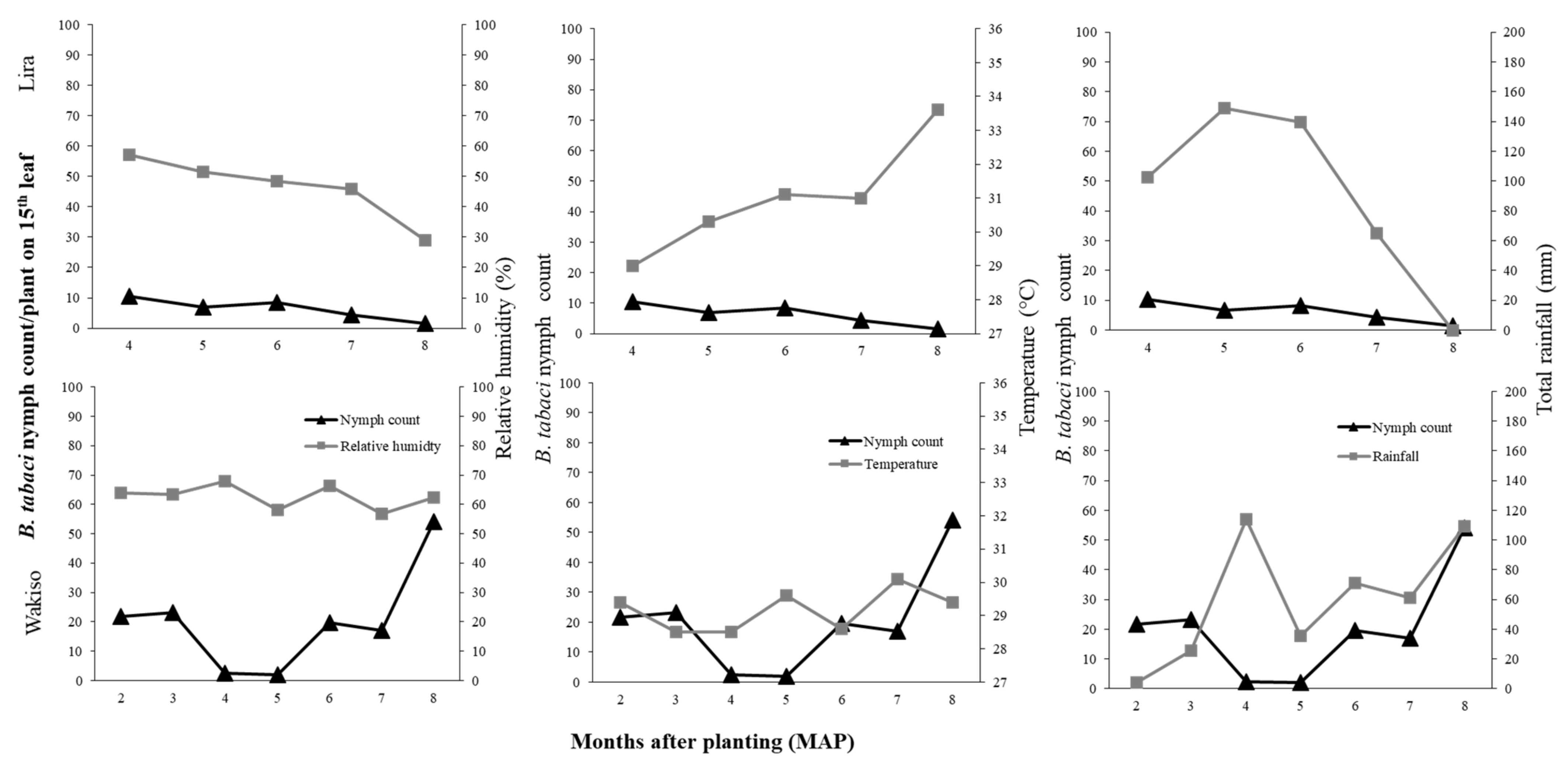
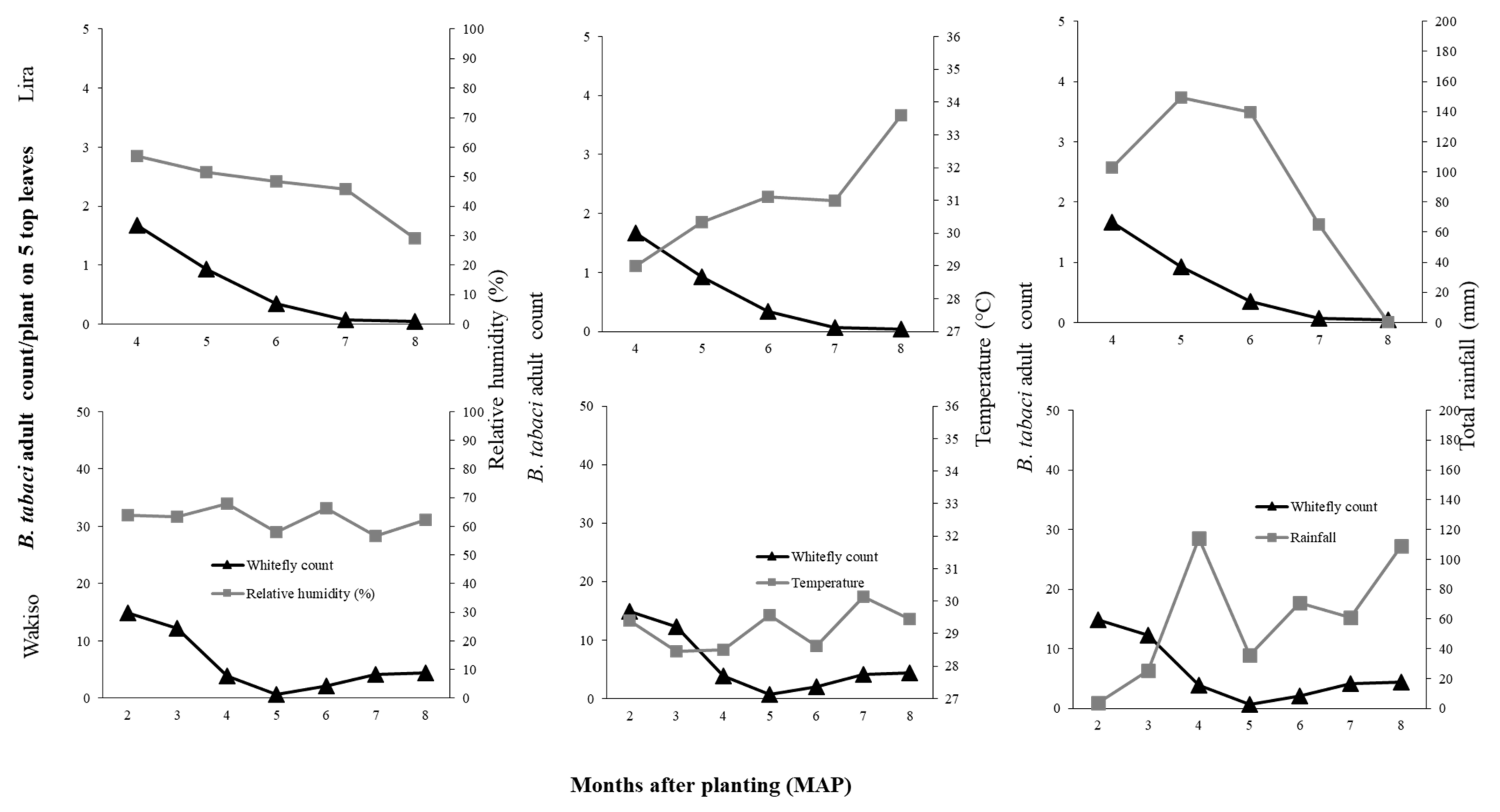
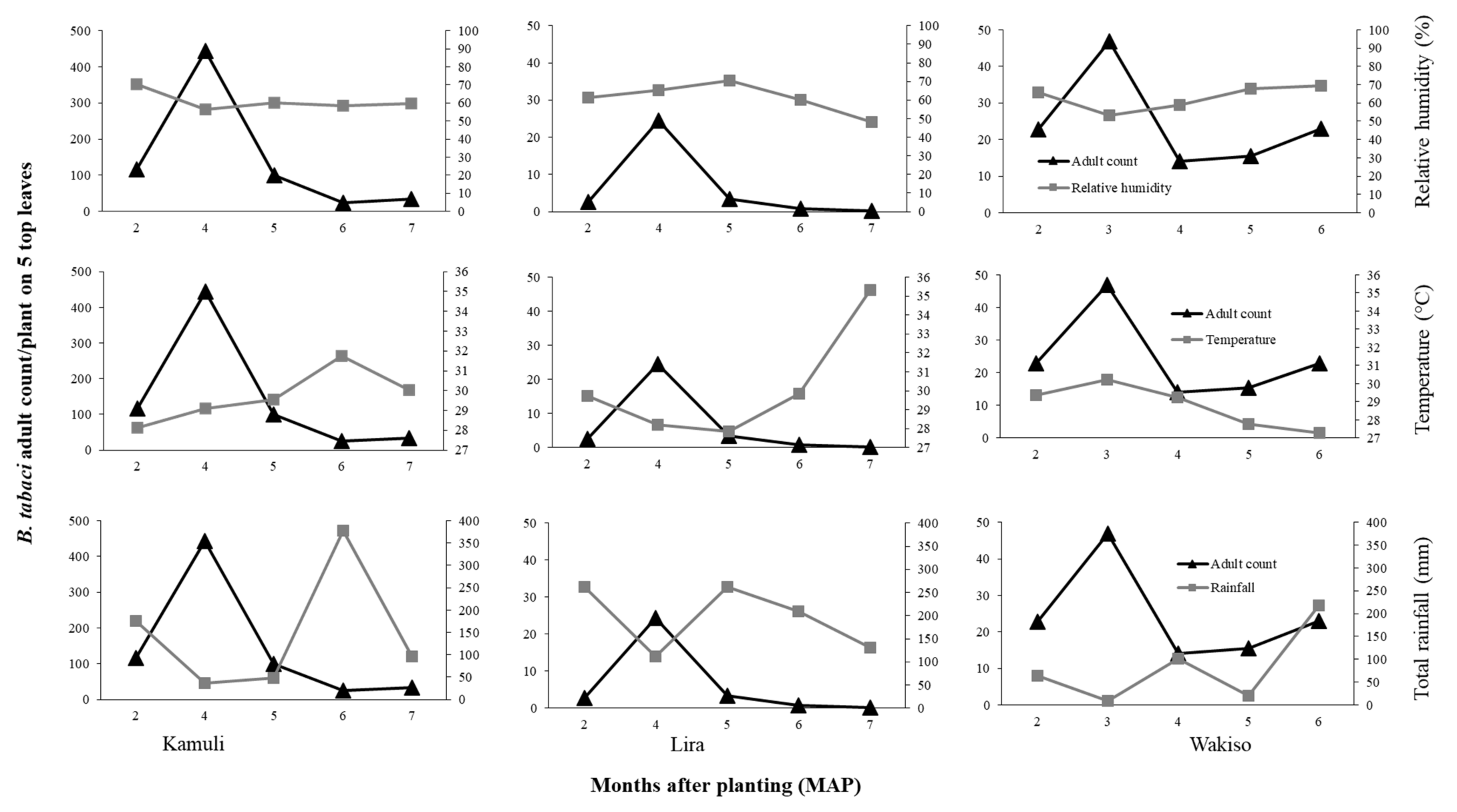
If we focus first on the direct effects of the environmental conditions, we observed that difference in
B. tabaci population dynamics in the different locations may be due to differences in temperature, rainfall, or relative humidity conditions. In Lira in 2017 where the highest temperature and rainfall were observed, we recorded low
B. tabaci numbers. High rainfall amounts is known to wash away whiteflies and other insect pests, thus causing significant mortality and population reduction [
46,
47,
48]. The negative relationship between
B. tabaci abundance and temperature possibly resulted from reduced adult longevity and reduced reproductive success at high temperature. [
49] reported mean
B. tabaci female adult longevity of 34.10 days at 20 °C and 16.88 days at 30 °C on cucumber. [
50] reported a decline in reproductive success of
B. tabaci with increasing temperature on collard; the highest reproductive success of over 80% was at 28 °C compared to 46% for that of 33 °C. Although the monthly average temperature was similar for Lira and Kamuli during 2–6 MAP (27.9–31.9 °C), in Lira there were many days of greater than 30 °C during 6–7 MAP. Conversely, high
B. tabaci populations were recorded in Kamuli in 2017, which experienced moderate rainfall, especially in the early crop growth stages (176.5 mm of total monthly rainfall compared to 262.9 mm at Lira at two MAP). Based on our results, the combination of high rainfall and high temperatures suppressed the population growth of
B. tabaci in Lira. The low
B. tabaci populations in 2016 may be attributed to differences in environmental conditions. In Lira, the low RH (29–57.1%), high temperatures (29–33.6 °C), and high rainfall (102.7–139.9 mm during 4–6 MAP followed by a drastic decline to 0 mm at 8 MAP) greatly suppressed
B. tabaci populations. These environmental factors all contribute to low reproductive success [
51] and high mortality, therefore leading to low population abundance. However, there is an urgent need for controlled studies on the effect of temperature and RH to validate our field results.
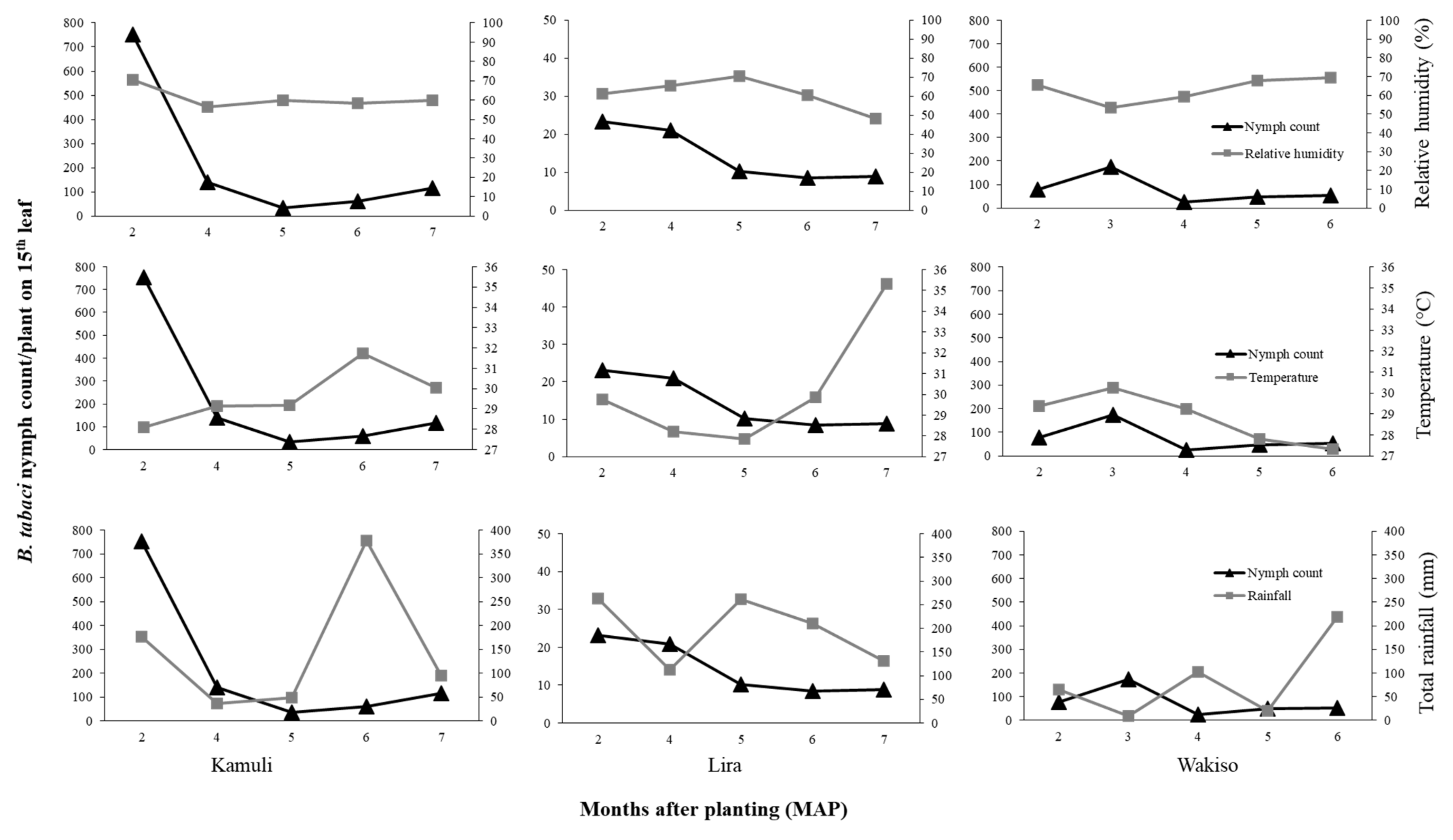
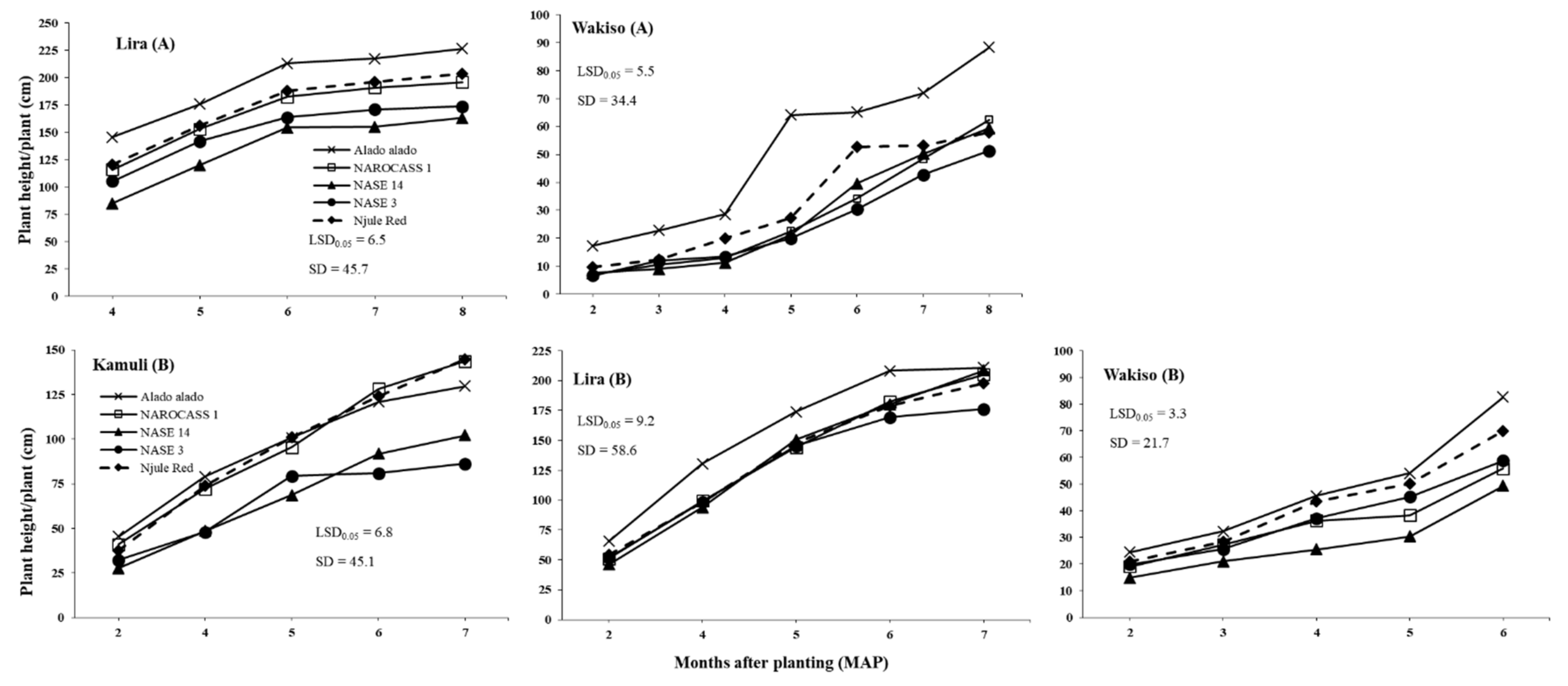
The environmental conditions can also lead to indirect effects through differences in cassava and morphological traits express themselves across locations. Differences in plant morphology influenced
B. tabaci population size in our trials. High growth and vigor of cassava occurred in Lira where the highest rainfall amount and temperature were recorded, supporting results of [
52] who reported that cassava growth is enhanced by high temperatures if adequate rainfall is received. Thus, the cassava crop in Lira escaped the vulnerable vegetative growth stage for
B. tabaci infestation. Therefore, there was low
B. tabaci populations in Lira, and this was even observed on the susceptible genotype NASE 14. However, Wakiso was largely dry in the first five months, and cassava grew poorly due to moisture stress as observed by the reduced plant height and restricted leaf area, a result consistent with reports of [
53] and [
54]. Thus, a stressed crop that may be nutritionally unfavorable for
B. tabaci development resulted in moderate
B. tabaci populations as observed in Wakiso. However, in Kamuli where moderate temperature, rainfall, and RH conditions occurred in the first five months, this resulted in moderate crop vigor and cassava plants with large succulent leaves that were preferred by
B. tabaci adults [
25,
55]. Hence, the highest
B. tabaci adult and nymph abundances were recorded in this trial.
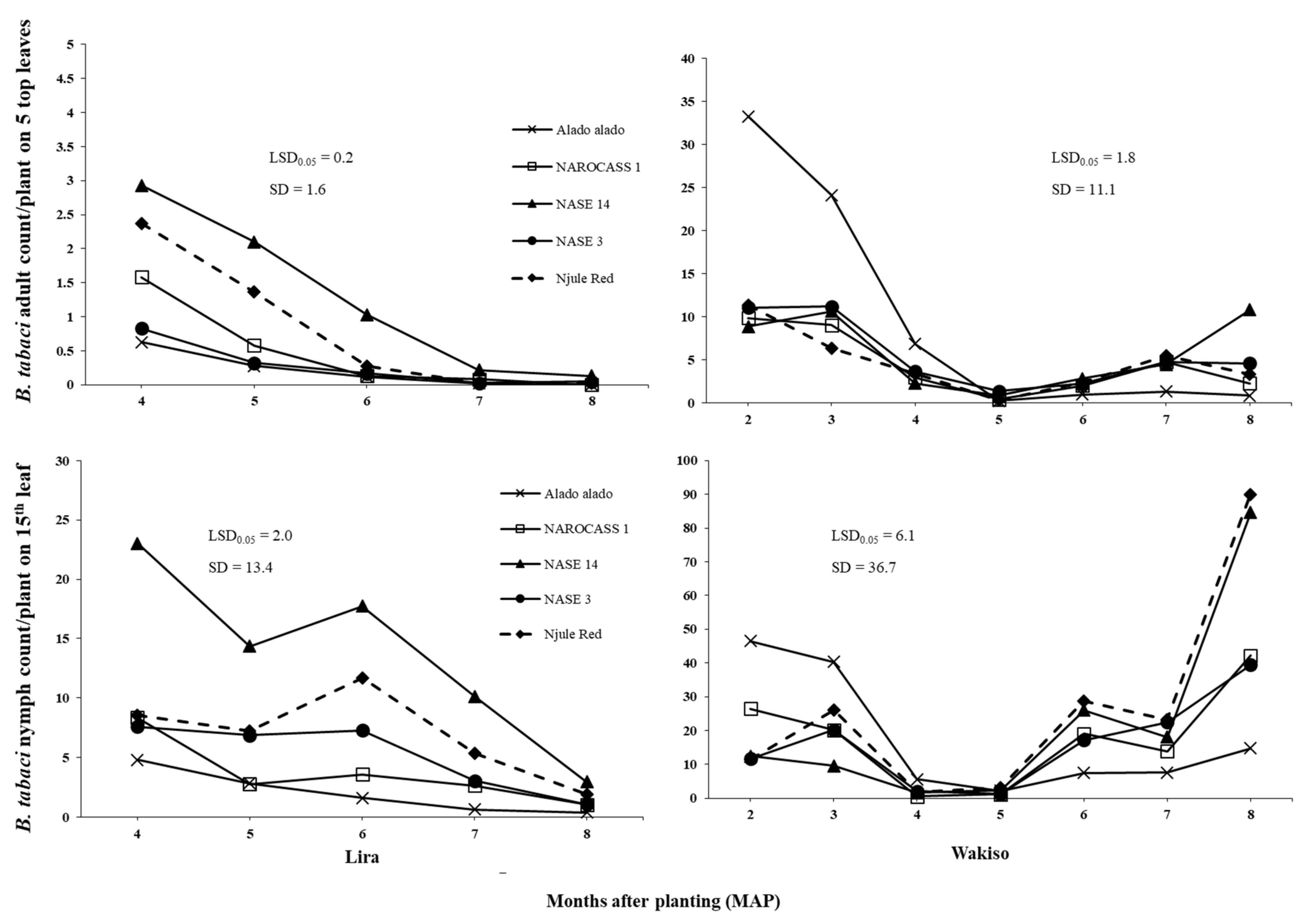
The fluctuations in
B. tabaci adult populations across all locations in 2017, involved low initial numbers at two MAP, followed by a rapid increase and peaks at three and four MAP, and eventual decline at five MAP (as illustrated in
Figure 2). Similar seasonal patterns were also observed in other studies [
21,
27]. This pattern may be associated with differences in host plant quality across time, with young crops with few leaves being less preferred by
B. tabaci adults, whereas large succulent leaves produced at later crop stages may be preferred by
B. tabaci adults. At later growth stages, the leaves senesce and the reduced food quality may prompt
B. tabaci adults to search for better host plants for both oviposition and feeding. Previous studies demonstrated that
B. tabaci population dynamics are strongly influenced by crop age [
21,
25,
56].
Results of this study revealed that
B. tabaci adult populations significantly varied with cassava genotype across all locations in both years (as illustrated in
Figure 1 and
Figure 2). This may be due to differences in plant morphological traits among the genotypes. Indeed, significant differences were observed in plant height, which was the only trait with a consistent negative effect on
B. tabaci adults across all locations. Hence, the consistently low and high
B. tabaci adult counts on Alado alado and NASE 14, respectively, the tallest and shortest genotypes across all locations in both years. This supports the findings of [
22,
57], who reported a negative relationship of
Leucinodes orbonalis Guenee and
B. tabaci adult populations with plant height in aubergine and cotton, respectively. In trials where the environmental conditions were suitable for growth and development of
B. tabaci populations, we observed that leaf area, leaf lobe width, and leaf lobe number positively influenced the
B. tabaci numbers that could be supported on cassava. Thus, high numbers were observed on NASE 14 and NAROCASS 1 in Kamuli in 2017. This is because large leaf area and lobe width provide
B. tabaci adults a large surface area for feeding and oviposition as well as offering suitable protection from wind and rain. These findings are in agreement with [
58], who reported a reduction in
B. tabaci adult numbers on cotton with lower leaf area. Large surface area, leaf lobe width and leaf lobe number ultimately result in dense canopy cover and so high LAI values, which provides protection from high temperatures and low RH [
59]. However, since this was a one study in Kamuli, further investigations in areas of high
B. tabaci population abundance are recommended to validate these findings.
In our trials, we used farmer preferred cassava genotypes with different levels of tolerance or resistance to major cassava diseases and
B. tabaci infestation (as illustrated in
Table 1). Generally, Alado alado, which is considered tolerant to
B. tabaci, performed as expected in terms of consistently low
B. tabaci adult and nymph abundance in our field trials. However, Njule Red often supported similar numbers of
B. tabaci to NAROCASS 1, NASE 3, and NASE 14 (the susceptible or moderately tolerant genotypes). Although Njule Red had very small leaf lobes as reflected in the low leaf lobe width values across all locations in both years, it had the highest leaf lobe number and leaf lobe length, which resulted in a large leaf area and high
B. tabaci numbers. Our findings suggest that Njule Red should not be considered tolerant to
B. tabaci in the field context.
Our trials showed that the risk of
B. tabaci outbreaks in cassava fields is heightened by the deployment of short, broad-leafed, cassava genotypes in areas that experience a moderately hot and humid environment, with moderate amounts of rainfall. However, we did not include all factors that may be important for
B. tabaci population dynamics in this field trial. Natural enemies such as predators and parasitoids can cause significant mortality to
B. tabaci populations, especially late in the season [
60,
61]. Additionally, our plot trials were small relative to the movement capabilities of
B. tabaci adults. Adults could easily move between plots in each trial. Although we assume that higher numbers of adults in one plot represent a biological preference for those plants over the others in the trial, there may also were a high amount of stochastic movement. Furthermore, in real smallholder production landscapes there is a diversity of cassava and non-cassava host plants that can support
B. tabaci populations (and their natural enemies) to some degree. This diversity of resources was not represented in our field trials but may have an impact.