Historical Changes in Honey Bee Wing Venation in Romania
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
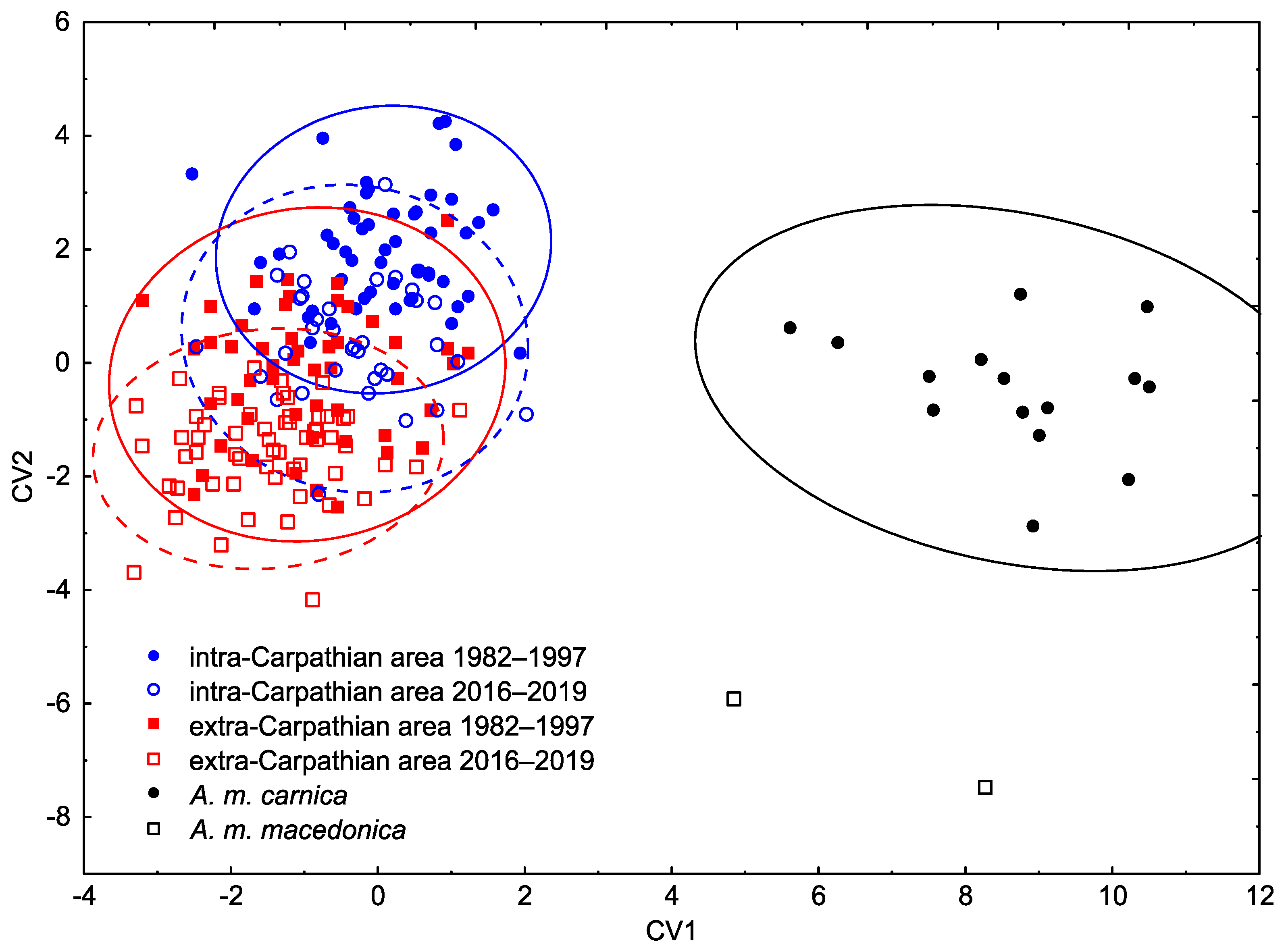
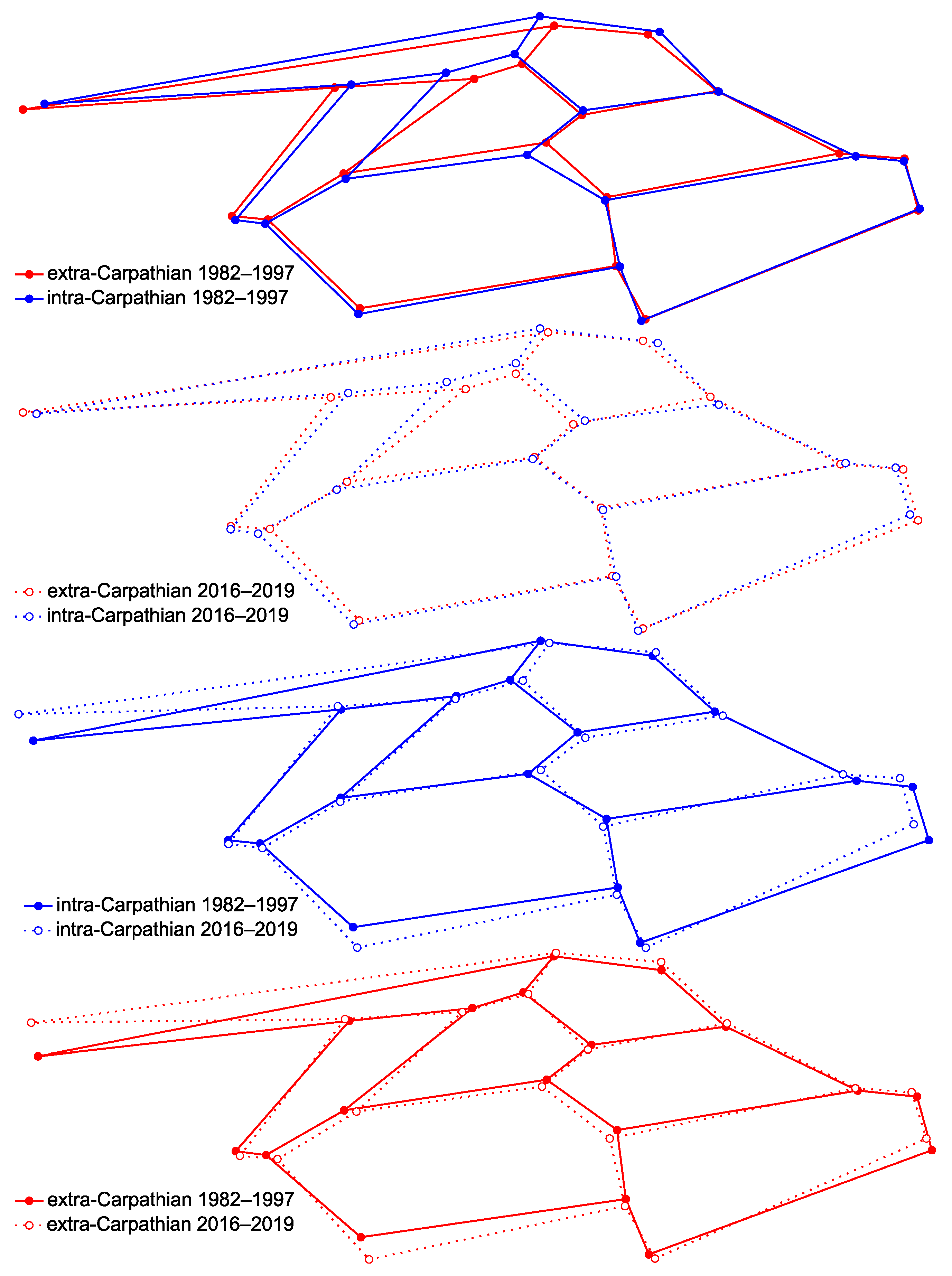
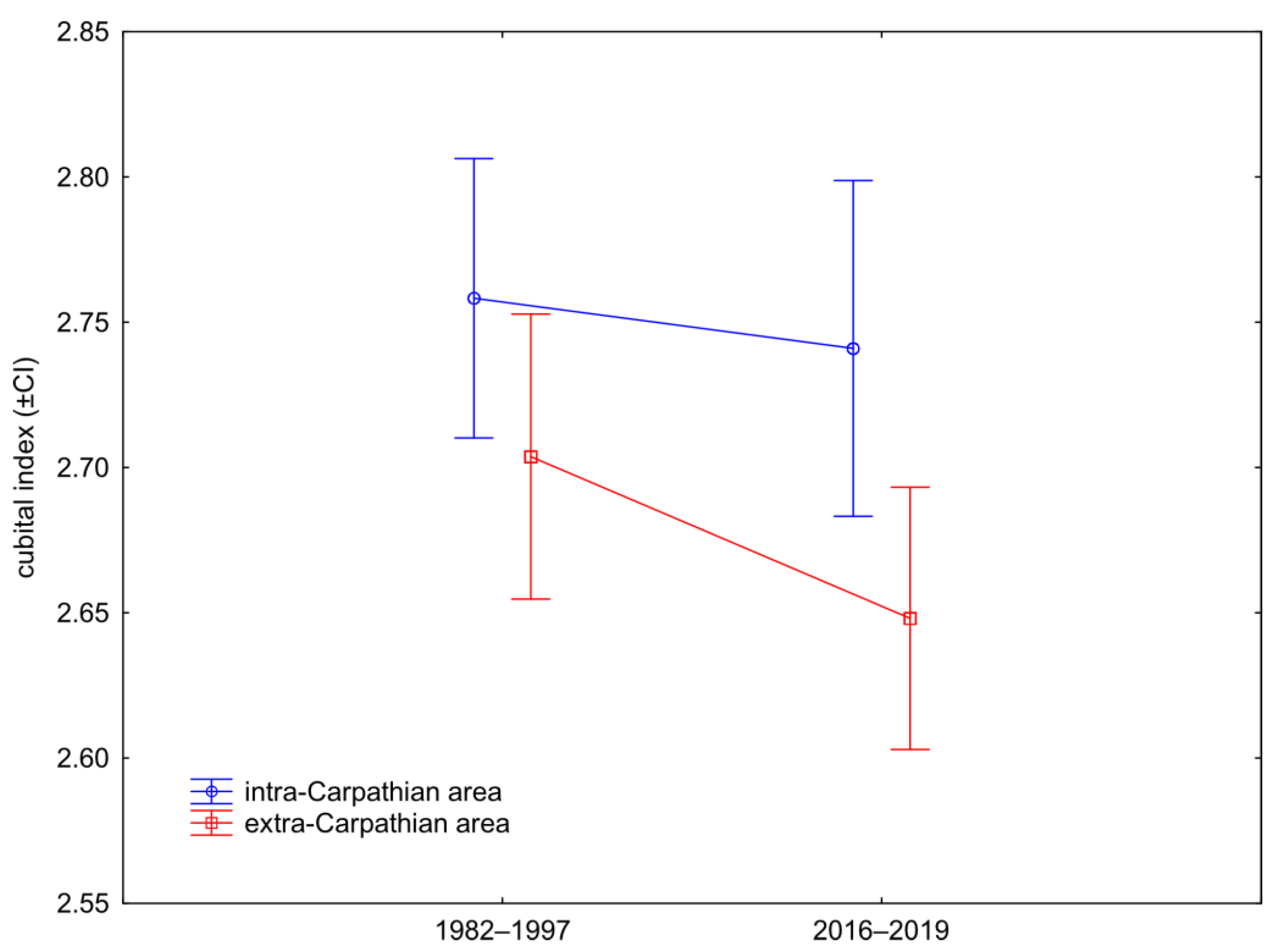
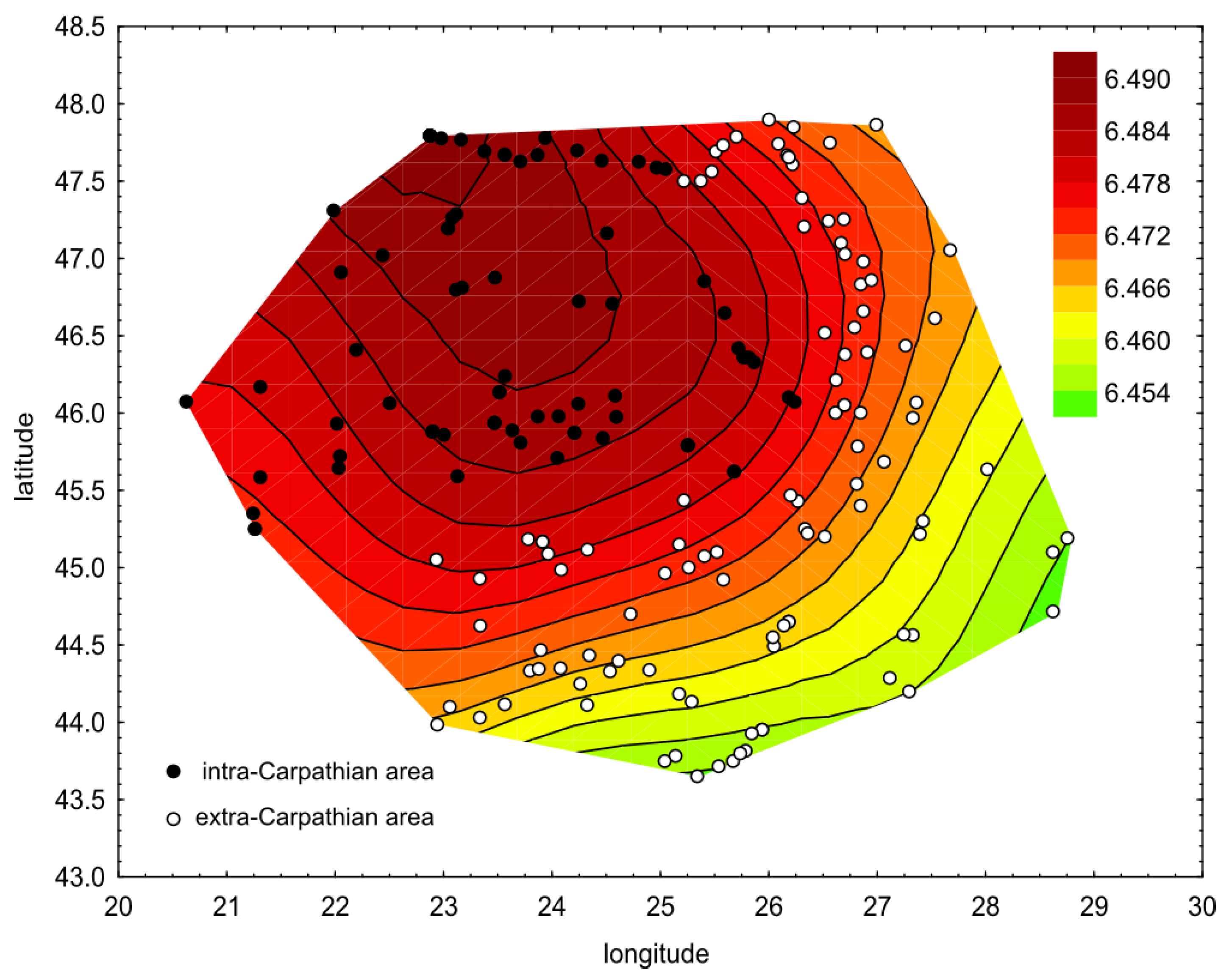
3.1. Wing Shape
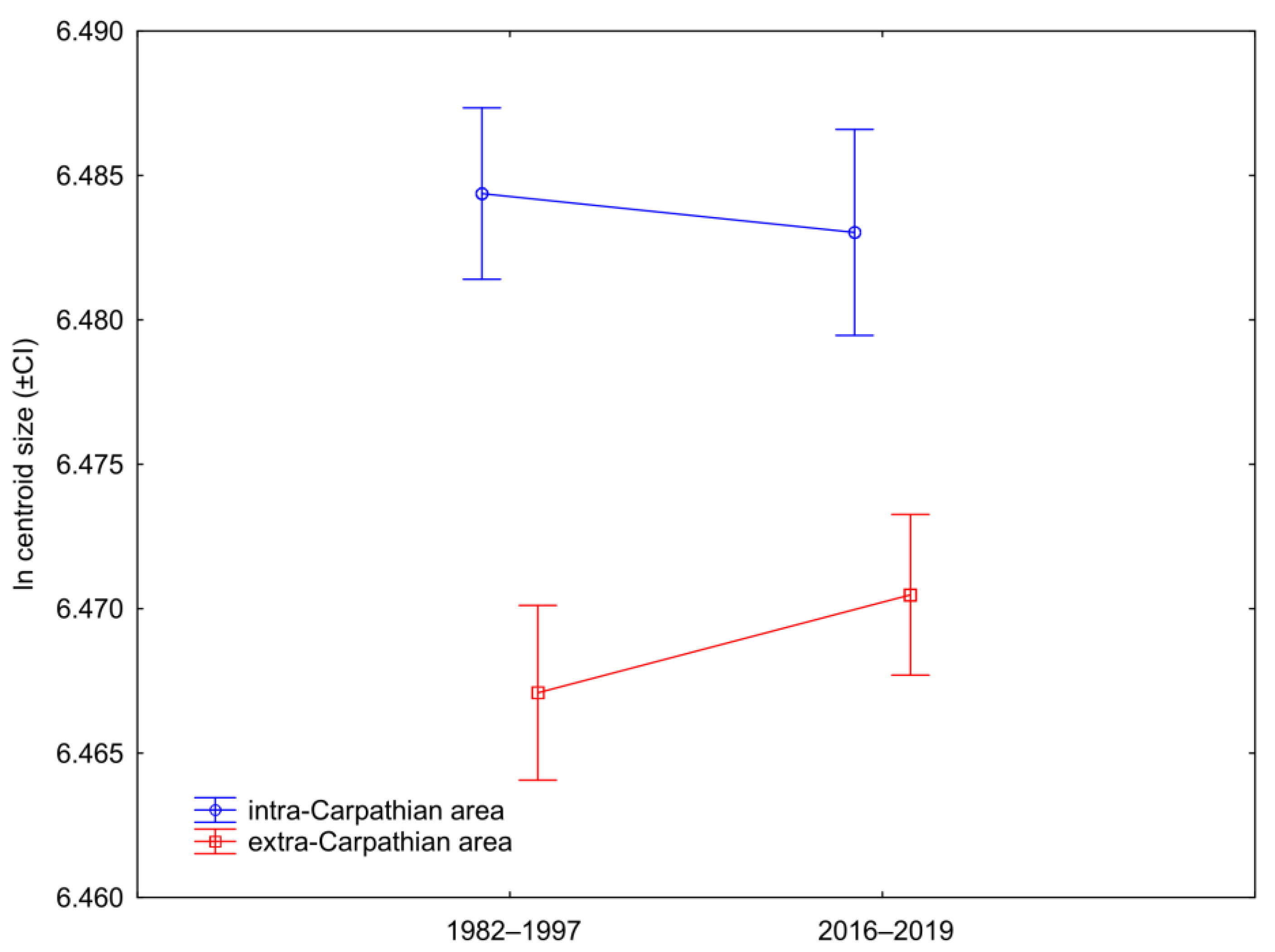
3.2. Wing Size
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gallai, N.; Salles, J.-M.; Settele, J.; Vaissière, B.E. Economic Valuation of the Vulnerability of World Agriculture Confronted with Pollinator Decline. Ecol. Econom. 2009, 68, 810–821. [Google Scholar] [CrossRef]
- Bouga, M.; Alaux, C.; Bienkowska, M.; Büchler, R.; Carreck, N.L.; Cauia, E.; Chlebo, R.; Dahle, B.; Dall’Olio, R.; De la Rúa, P.; et al. A Review of Methods for Discrimination of Honey Bee Populations as Applied to European Beekeeping. J. Apic. Res. 2011, 50, 51–84. [Google Scholar] [CrossRef]
- Engel, M.S. The Taxonomy of Recent and Fossil Honey Bees (Hymenoptera: Apidae; Apis). J. Hymenopt. Res. 1999, 8, 165–196. [Google Scholar]
- Meixner, M.D.; Costa, C.; Kryger, P.; Hatjina, F.; Bouga, M.; Ivanova, E.; Büchler, R. Conserving Diversity and Vitality for Honey Bee Breeding. J. Apic. Res. 2010, 49, 85–92. [Google Scholar] [CrossRef]
- Meixner, M.D.; Pinto, M.A.; Bouga, M.; Kryger, P.; Ivanova, E.; Fuchs, S. Standard Methods for Characterising Subspecies and Ecotypes of Apis Mellifera. J. Apic. Res. 2013, 52, 1–28. [Google Scholar] [CrossRef]
- Ruttner, F. Biogeography and Taxonomy of Honeybees; Springer: Berlin, Germany, 1988. [Google Scholar]
- Franck, P.; Garnery, L.; Loiseau, A.; Oldroyd, B.P.; Hepburn, H.R.; Solignac, M.; Cornuet, J.-M. Genetic Diversity of the Honeybee in Africa: Microsatellite and Mitochondrial Data. Heredity 2001, 86, 420–430. [Google Scholar] [CrossRef]
- Alburaki, M.; Moulin, S.; Legout, H.; Alburaki, A.; Garnery, L. Mitochondrial Structure of Eastern Honeybee Populations from Syria, Lebanon and Iraq. Apidologie 2011, 42, 628. [Google Scholar] [CrossRef]
- De la Rúa, P.; Jaffé, R.; Dall’Olio, R.; Muñoz, I.; Serrano, J. Biodiversity, Conservation and Current Threats to European Honeybees. Apidologie 2009, 40, 263–284. [Google Scholar] [CrossRef]
- Oleksa, A.; Chybicki, I.; Tofilski, A.; Burczyk, J. Nuclear and Mitochondrial Patterns of Introgression into Native Dark Bees (Apis Mellifera Mellifera) in Poland. J. Apic. Res. 2011, 50, 116–129. [Google Scholar] [CrossRef]
- Requier, F.; Garnery, L.; Kohl, P.L.; Njovu, H.K.; Pirk, C.W.W.; Crewe, R.M.; Steffan-Dewenter, I. The Conservation of Native Honey Bees Is Crucial. Trends Ecol. Evol. 2019, 34, 789–798. [Google Scholar] [CrossRef]
- Gregorc, Ă.; Lokar, V.; Škerl, M.I.S. Testing of the Isolation of the Rog-Ponikve Mating Station for Carniolan (Apis Mellifera Carnica) Honey Bee Queens. J. Apic. Res. 2008, 47, 137–140. [Google Scholar] [CrossRef]
- Koeniger, G. Reproduction and mating behavior. In Bee Genetics and Breeding; Rinderer, T.E., Ed.; Academic Press: Orlando, FL, USA, 1986; pp. 255–280. [Google Scholar]
- Neumann, P.; van Praagh, J.P.; Moritz, R.F.A.; Dustmann, J.H. Testing Reliability of a Potential Island Mating Apiary Using DNA Microsatellites. Apidologie 1999, 30, 257–276. [Google Scholar] [CrossRef]
- Kraus, F.B.; Neumann, P.; Moritz, R.F.A. Genetic Variance of Mating Frequency in the Honeybee (Apis Mellifera L.). Insectes Soc. 2005, 52, 1–5. [Google Scholar] [CrossRef]
- Jensen, A.B.; Palmer, K.A.; Chaline, N.; Raine, N.E.; Tofilski, A.; Martin, S.J.; Pedersen, B.V.; Boomsma, J.J.; Ratnieks, F.L.W. Quantifying Honey Bee Mating Range and Isolation in Semi-Isolated Valleysby DNA Microsatellite Paternity Analysis. Conserv. Genet. 2005, 6, 527–537. [Google Scholar] [CrossRef]
- Szabo, T.I. Mating Distance of the Honeybee in North-Western Alberta Canada. J. Apic. Res. 1986, 25, 227–233. [Google Scholar] [CrossRef]
- Chauzat, M.-P.; Cauquil, L.; Roy, L.; Franco, S.; Hendrikx, P.; Ribière-Chabert, M. Demographics of the European Apicultural Industry. PLoS ONE 2013, 8, e79018. [Google Scholar] [CrossRef] [PubMed]
- Jaffé, R.; Dietemann, V.; Allsopp, M.H.; Costa, C.; Crewe, R.M.; Dall’Olio, R.; de la Rúa, P.; El-Niweiri, M.A.; Fries, I.; Kezic, N.; et al. Estimating the Density of Honeybee Colonies across Their Natural Range to Fill the Gap in Pollinator Decline Censuses. Conserv. Biol. 2010, 24, 583–593. [Google Scholar] [CrossRef]
- Kohl, P.L.; Rutschmann, B. The Neglected Bee Trees: European Beech Forests as a Home for Feral Honey Bee Colonies. PeerJ 2018, 6, e4602. [Google Scholar] [CrossRef]
- Oleksa, A.; Gawroński, R.; Tofilski, A. Rural Avenues as a Refuge for Feral Honey Bee Population. J. Insect Conserv. 2013, 17, 465–472. [Google Scholar] [CrossRef]
- Jensen, A.B.; Palmer, K.A.; Boomsma, J.J.; Pedersen, B.V. Varying Degrees of Apis Mellifera Ligustica Introgression in Protected Populations of the Black Honeybee, Apis Mellifera Mellifera, in Northwest Europe. Mol. Ecol. 2005, 14, 93–106. [Google Scholar] [CrossRef]
- Pinto, M.A.; Henriques, D.; Chávez-Galarza, J.; Kryger, P.; Garnery, L.; van der Zee, R.; Dahle, B.; Soland-Reckeweg, G.; de la Rúa, P.; Olio, R.D.; et al. Genetic Integrity of the Dark European Honey Bee (Apis Mellifera Mellifera) from Protected Populations: A Genome-Wide Assessment Using SNPs and MtDNA Sequence Data. J. Apic. Res. 2014, 53, 269–278. [Google Scholar] [CrossRef]
- Büchler, R.; Costa, C.; Hatjina, F.; Andonov, S.; Meixner, M.D.; Conte, Y.L.; Uzunov, A.; Berg, S.; Bienkowska, M.; Bouga, M.; et al. The Influence of Genetic Origin and Its Interaction with Environmental Effects on the Survival of Apis Mellifera L. Colonies in Europe. J. Apic. Res. 2014, 53, 205–214. [Google Scholar] [CrossRef]
- Uzunov, A.; Brascamp, E.W.; Büchler, R. The Basic Concept of Honey Bee Breeding Programs. Bee World 2017, 94, 84–87. [Google Scholar] [CrossRef]
- Fontana, P.; Costa, C.; Prisco, G.D.; Ruzzier, E.; Annoscia, D.; Battisti, A.; Caoduro, G.; Carpana, E.; Contessi, A.; Dal, A.; et al. Appeal for Biodiversity Protection of Native Honey Bee Subspecies of Apis Mellifera in Italy (San Michele All’Adige Declaration). Bull. Insectol. 2018, 71, 257–271. [Google Scholar]
- Pinto, M.A.; Rubink, W.L.; Coulson, R.N.; Patton, J.C.; Johnston, J.S. Temporal Pattern of Africanization in a Feral Honeybee Population from Texas Inferred from Mitochondrial Dna. Evolution 2004, 58, 1047–1055. [Google Scholar] [CrossRef]
- Pinto, M.A.; Rubink, W.L.; Patton, J.C.; Coulson, R.N.; Johnston, J.S. Africanization in the United States: Replacement of Feral European Honeybees (Apis Mellifera L.) by an African Hybrid Swarm. Genetics 2005, 170, 1653–1665. [Google Scholar] [CrossRef]
- Rangel, J.; Giresi, M.; Pinto, M.A.; Baum, K.A.; Rubink, W.L.; Coulson, R.N.; Johnston, J.S. Africanization of a Feral Honey Bee (Apis Mellifera) Population in South Texas: Does a Decade Make a Difference? Ecol. Evol. 2016, 6, 2158–2169. [Google Scholar] [CrossRef]
- Delaney, D.A.; Meixner, M.D.; Schiff, N.M.; Sheppard, W.S. Genetic Characterization of Commercial Honey Bee (Hymenoptera: Apidae) Populations in the United States by Using Mitochondrial and Microsatellite Markers. Ann. Entomol. Soc. Am. 2009, 102, 666–673. [Google Scholar] [CrossRef]
- Page, R.E.J.; Metcalf, R.A. A Population Estimate of Mdh Allozyme Frequencies for the Honey Bee, Apis Mellifera L. (Hymenoptera: Apidae). Pan Pac. Entomol. 1988, 64, 285–289. [Google Scholar]
- Cridland, J.M.; Ramirez, S.R.; Dean, C.A.; Sciligo, A.; Tsutsui, N.D. Genome Sequencing of Museum Specimens Reveals Rapid Changes in the Genetic Composition of Honey Bees in California. Genome Biol. Evol. 2018, 10, 458–472. [Google Scholar] [CrossRef]
- Mikheyev, A.S.; Tin, M.M.Y.; Arora, J.; Seeley, T.D. Museum Samples Reveal Rapid Evolution by Wild Honey Bees Exposed to a Novel Parasite. Nat. Commun. 2015, 6, 7991. [Google Scholar] [CrossRef] [PubMed]
- De la Rúa, P.; Galián, J.; Pedersen, B.V.; Serrano, J. Molecular Characterization and Population Structure of Apis Mellifera from Madeira and the Azores. Apidologie 2006, 37, 699–708. [Google Scholar] [CrossRef]
- Ferreira, H.; Henriques, D.; Neves, C.J.; Machado, C.A.S.; Azevedo, J.C.; Francoy, T.M.; Pinto, M.A. Historical and Contemporaneous Human-Mediated Processes Left a Strong Genetic Signature on Honey Bee Populations from the Macaronesian Archipelago of the Azores. Apidologie 2020, 51, 316–328. [Google Scholar] [CrossRef]
- Parejo, M.; Wragg, D.; Henriques, D.; Charrière, J.-D.; Estonba, A. Digging into the Genomic Past of Swiss Honey Bees by Whole-Genome Sequencing Museum Specimens. Genome Biol. Evol. 2020, 12, 2535–2551. [Google Scholar] [CrossRef] [PubMed]
- Parejo, M.; Wragg, D.; Gauthier, L.; Vignal, A.; Neumann, P.; Neuditschko, M. Using Whole-Genome Sequence Information to Foster Conservation Efforts for the European Dark Honey Bee, Apis Mellifera Mellifera. Front. Ecol. Evol. 2016, 4. [Google Scholar] [CrossRef]
- Janczyk, A.; Meixner, M.D.; Tofilski, A. Morphometric Identification of the Endemic Maltese Honey Bee (Apis Mellifera Ruttneri). J. Apic. Res. 2021, 60, 157–164. [Google Scholar] [CrossRef]
- Fisteag, J. Cercetări Biometrice La Albinele Româneşti. Ph.D. Thesis, University of Bucharest, Bucharest, Romania, 1937. [Google Scholar]
- Foti, N.; Lungu, M.; Pelimon, P.; Barac, I.; Copaitici, M.; Mirza, E. Studies on the Morphological Characteristics and Biological Traits of the Bee Populations in Romania. Proc. Int. Beekeep. Congr. 1965, 20, 171–176. [Google Scholar]
- Ilyasov, R.; Nikolenko, A.; Tuktarov, V.; Goto, K.; Takahashi, J.-I.; Kwon, H.W. Comparative Analysis of Mitochondrial Genomes of the Honey Bee Subspecies A. m. Caucasica and A. m. Carpathica and Refinement of Their Evolutionary Lineages. J. Apic. Res. 2019, 58, 567–579. [Google Scholar] [CrossRef]
- Mărghitaş, L.A.; Coroian, C.; Dezmirean, D.; Stan, L.; Furdui, E. Genetic Diversity of Honeybees from Moldova (Romania) Based on MtDNA Analysis. Bull. Univ. Agric. Sci. Vet. Med. Cluj Napoca Anim. Sci. Biotechnol. 2010, 67. [Google Scholar] [CrossRef]
- Mărghitas, L.A.; Dezmirean, D.; Teleky, O.; Furdui, E.; Moise, A.; Stan, L.; Mihai, C.; Pece, A.; Coroian, C. Biodiversity Testing of Transylvanian Honeybee Populations Using MtDNA Markers. Bull. Univ. Agric. Sci. Vet. Med. Cluj Napoca Anim. Sci. Biotechnol. 2009, 66. [Google Scholar] [CrossRef]
- Coroian, C.O.; Muñoz, I.; Schlüns, E.A.; Paniti-Teleky, O.R.; Erler, S.; Furdui, E.M.; Mărghitaş, L.A.; Dezmirean, D.S.; Schlüns, H.; de la Rúa, P.; et al. Climate Rather than Geography Separates Two European Honeybee Subspecies. Mol. Ecol. 2014, 23, 2353–2361. [Google Scholar] [CrossRef]
- Momeni, J.; Parejo, M.; Nielsen, R.O.; Langa, J.; Montes, I.; Papoutsis, L.; Farajzadeh, L.; Bendixen, C.; Căuia, E.; Charrière, J.-D.; et al. Authoritative Subspecies Diagnosis Tool for European Honey Bees Based on Ancestry Informative SNPs. BMC Genom. 2021, 22, 101. [Google Scholar] [CrossRef]
- Căuia, E.; Usurelu, D.; Magdalena, L.M.; Cimponeriu, D.; Apostol, P.; Siceanu, A.; Holban, A.; Gavrila, L. Preliminary Researches Regarding the Genetic and Morphometric Characterization of Honeybees (A. Mellifera L.) from Romania. Sci. Pap. Anim. Sci. Biotechnol. 2008, 41, 278–286. [Google Scholar]
- Syromyatnikov, M.Y.; Borodachev, A.V.; Kokina, A.V.; Popov, V.N. A Molecular Method for the Identification of Honey Bee Subspecies Used by Beekeepers in Russia. Insects 2018, 9, 10. [Google Scholar] [CrossRef]
- Murylev, A.V.; Petukhov, A.V.; Lipatov, V.Y. Adaptations of Honeybees Apis Mellifera Mellifera L. and Apis Mellifera Carpathica to Low Winter Temperatures. Russ. J. Ecol. 2012, 43, 409–411. [Google Scholar] [CrossRef]
- Crane, E. The World History of Beekeeping and Honey Hunting; Routledge: Abingdon, UK, 1999; ISBN 978-0-429-23587-0. [Google Scholar]
- Curcă, D.; Andronie, V.; Andronie, I.C. From the History of the Romanian Apiculture. Econom. Manag. Financ. Markets 2011, 6, 1124–1132. [Google Scholar]
- Pelimon, C. Albina Românească Sau Import de Rase Străine? Rom. Apic. 1948, 23, 6–8. [Google Scholar]
- Barac, I. Preserving the Genetic Pool of Apis Mellifera Carpatica. Proc. Int. Beekeep. Congr. 1977, 26, 270–274. [Google Scholar]
- Foti, N. Date Preliminare Asupra Comportării Albinelor de Proveniență de Stepă, Bănățene Și Transilvănene În Condițiile Zonei de Stepă. Apicultura 1957, 4, 3–9. [Google Scholar]
- Mârza, E. Date Comparative Privind Comportarea Populatiilor de Albine de Stepa Si Munte. Lucr. Stiint. 1965, 6, 23–31. [Google Scholar]
- Sănduleac, E.; Antal, L.; Cîmpeanu, L.; Florea, V.; Iliasa, F.; Mălaiu, A.; Perva, P.; Sabău, A.; Texe, E.; Verșehora, D. Cercetari Pentru Stabilirea Tipului de Cules in Diferite Zone Naturale Din R. P. R. Lucr. Stiint. 1961, 3, 233–242. [Google Scholar]
- Oleksa, A.; Kusza, S.; Tofilski, A. Mitochondrial DNA Suggests the Introduction of Honeybees of African Ancestry to East-Central Europe. Insects 2021, 12, 410. [Google Scholar] [CrossRef] [PubMed]
- Janczyk, A.; Tofilski, A. Monthly Changes in Honey Bee Forewings Estimated Using Geometric Morphometrics. J. Apic. Sci. 2021. [Google Scholar] [CrossRef]
- Nawrocka, A.; Kandemir, İ.; Fuchs, S.; Tofilski, A. Computer Software for Identification of Honey Bee Subspecies and Evolutionary Lineages. Apidologie 2018, 49, 172–184. [Google Scholar] [CrossRef]
- Dryden, I.L.; Mardia, K.V. Statistical Shape Analysis; John Wiley and Sons: Chichester, UK, 1998; Volume 4. [Google Scholar]
- Klingenberg, C.P. MorphoJ: An Integrated Software Package for Geometric Morphometrics. Mol. Ecol. Res. 2011, 11, 353–357. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-Km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Puškadija, Z.; Kovačić, M.; Raguž, N.; Lukić, B.; Prešern, J.; Tofilski, A. Morphological Diversity of Carniolan Honey Bee (Apis Mellifera Carnica) in Croatia and Slovenia. J. Apic. Res. 2020, 60, 326–336. [Google Scholar] [CrossRef]

| Groups | Intra-Carpathian 1982–1997 | Extra-Carpathian 1982–1997 | Intra-Carpathian 2016–2019 | Extra-Carpathian 2016–2019 | A. m. carnica | A. m. macedonica |
|---|---|---|---|---|---|---|
| intra-Carpathian 1982–1997 | *** | *** | *** | *** | *** | |
| extra-Carpathian 1982–1997 | 9.28 | *** | *** | *** | *** | |
| intra-Carpathian 2016–2019 | 6.92 | 11.59 | *** | *** | *** | |
| extra-Carpathian 2016–2019 | 15.68 | 7.79 | 8.00 | *** | *** | |
| A. m. carnica | 81.03 | 96.22 | 85.33 | 104.45 | *** | |
| A. m. macedonica | 151.34 | 135.31 | 140.40 | 136.41 | 92.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tofilski, A.; Căuia, E.; Siceanu, A.; Vișan, G.O.; Căuia, D. Historical Changes in Honey Bee Wing Venation in Romania. Insects 2021, 12, 542. https://doi.org/10.3390/insects12060542
Tofilski A, Căuia E, Siceanu A, Vișan GO, Căuia D. Historical Changes in Honey Bee Wing Venation in Romania. Insects. 2021; 12(6):542. https://doi.org/10.3390/insects12060542
Chicago/Turabian StyleTofilski, Adam, Eliza Căuia, Adrian Siceanu, Gabriela Oana Vișan, and Dumitru Căuia. 2021. "Historical Changes in Honey Bee Wing Venation in Romania" Insects 12, no. 6: 542. https://doi.org/10.3390/insects12060542
APA StyleTofilski, A., Căuia, E., Siceanu, A., Vișan, G. O., & Căuia, D. (2021). Historical Changes in Honey Bee Wing Venation in Romania. Insects, 12(6), 542. https://doi.org/10.3390/insects12060542







