Effects on Brood Development in the Carpenter Ant Camponotus vicinus Mayr after Exposure to the Yeast Associate Schwanniomyces polymorphus Kloecker
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Artificial Diet Preparation
2.2. Effects of Diets Supplemented with Schwanniomyces polymorphus
- Basal diet (fed basal, complete diet only),
- Basal diet + yeast (fed basal, complete diet + S. polymorphus),
- No B vitamins (fed basal diet minus all B vitamins),
- No B vitamins + yeast (fed basal diet minus all B vitamins + S. polymorphus),
- No B vitamins, no cholesterol (fed basal diet minus all B vitamins and cholesterol),
- No B vitamins, no cholesterol + yeast (fed basal diet minus all B vitamins and cholesterol + S. polymorphus),
- Sugar water (fed sugar water only (0.5 M sucrose)),
- Sugar water + yeast (fed sugar water only + S. polymorphus),
- Heat/chemical basal diet (defaunation then fed basal diet),
- Heat/chemical basal diet + yeast (defaunation then fed basal diet + S. polymorphus),
- Heat/chemical, no B vitamins (defaunation then fed basal diet minus all B vitamins),
- Heat/chemical, no B vitamins + yeast (defaunation then fed basal diet minus B vitamins + S. polymorphus),
- Heat/chemical, no B vitamins, no cholesterol (defaunation then fed basal minus B vitamins, minus cholesterol),
- Heat/chemical, no B vitamins, no cholesterol + yeast (defaunation then fed basal minus B vitamins, minus cholesterol + S. polymorphus),
- Heat/chemical, sugar water (defaunation then fed sugar water only (0.5 M sucrose)),
- Heat/Chemical, sugar water + yeast (defaunation then fed sugar water only + S. polymorphus).
2.3. Yeast Isolations
2.4. Statistical Analysis
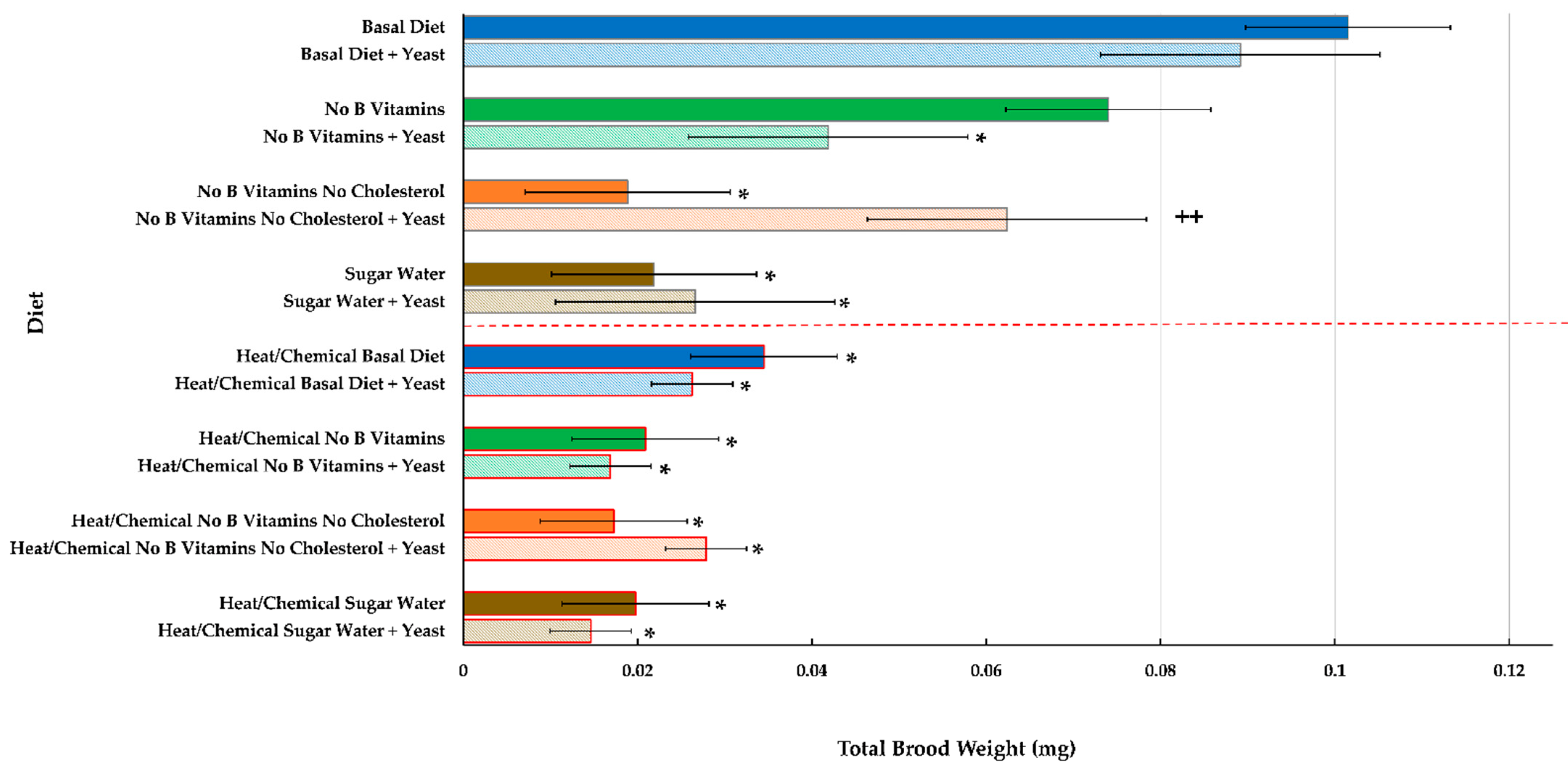
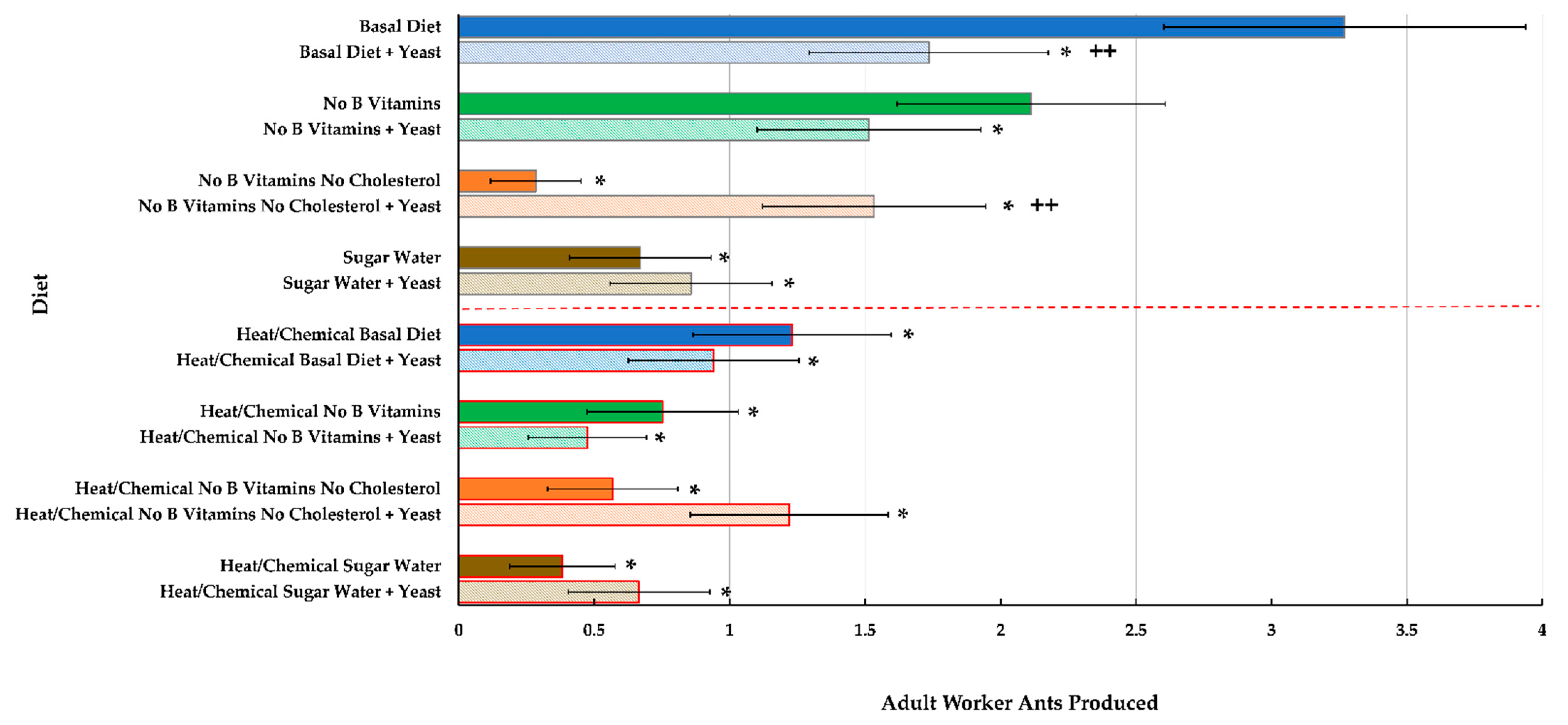
3. Results
3.1. Effects of Diets on Ants Exposed to Schwanniomyces polymorphus
Brood Development and End Weight
3.2. Yeast Isolations
Adult Number
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hölldobler, B.; Wilson, E.O. The Ants; Harvard University Press: Cambridge, MA, USA, 1990. [Google Scholar]
- Hansen, L.D.; Klotz, J.H. Carpenter Ants of the United States and Canada; Cornell University Press: Ithaca, NY, USA, 2005; p. 204. [Google Scholar]
- Mankowski, M.E.; Morrell, J.J. Incidence of wood-destroying organisms in Oregon residential structures. For. Prod. J. 2000, 50, 49–52. [Google Scholar]
- Forbes, J. Anatomy and histology of the worker of Camponotus herculeanus pennsylvanicus DeGeer (Formicidae, Hymenoptera). Ann. Entomol. Soc. Am. 1938, 31, 181–195. [Google Scholar] [CrossRef]
- Eisner, T.; Happ, G.M. The infrabuccal pocket of a Formicine ant: A social filtration device. Psyche 1962, 69, 107–116. [Google Scholar] [CrossRef]
- Febvay, G.; Kermarrac, A. Digestive Physiology of Leaf-Cutting Ants. In Fire Ants and Leaf-Cutting Ants: Biology and Management; Lofgren, C.S., Vander Meer, R.K., Eds.; West View Press: New York, NY, USA, 1986; pp. 274–288. [Google Scholar]
- Cannon, C.A.; Fell, R.D. Patterns of Macronutrient Collection in the Black Carpenter Ant, Camponotus pennsylvanicus (De Geer) (Hymenoptera: Formicidae). Environ. Entomol. 2002, 31, 977–981. [Google Scholar] [CrossRef]
- Wang, C.; Billen, J.; Wei, C.; He, H. Morphology and ultrastructure of the infrabuccal pocket in Camponotus japonicus Mayr (Hymenoptera: Formicidae). Insectes Soc. 2019, 66, 637–646. [Google Scholar] [CrossRef]
- Hansen, L.D.; Spangenburg, W.J.; Gaver, M.M. The Infrabuccal chamber of Camponotus modoc (Hymenoptera:Formicidae): Ingestion, digestion, and survey of bacteria. In Proceedings of the 3rd International Conference of Urban Pests, Prague, Czech Republic, 19–22 July 1999; Robinson, W.H., Rettich, F., Rambo, G.W., Eds.; pp. 211–219. [Google Scholar]
- Mankowski, M.E.; Morrell, J.J. Yeasts associated with the infrabuccal pocket and colonies of the carpenter ant Camponotus vicinus. Mycologia 2004, 96, 226–231. [Google Scholar] [CrossRef]
- Buchner, P. Endosymbiosis of Animals with Plant Microorganisms; Interscience Publishers: New York, NY, USA, 1965; p. 909. [Google Scholar]
- Zientz, E.; Feldhaar, H.; Stoll, S.; Gross, R. Insights into the microbial world associated with ants. Arch. Microbiol. 2005, 184, 199–206. [Google Scholar] [CrossRef]
- Cook, S.C.; Davidson, D.W. Nutritional and functional biology of exudate-feeding ants. Entomol. Exp. Appl. 2006, 118, 1–10. [Google Scholar] [CrossRef]
- Sauer, C.; Dudaczek, D.; Hölldobler, B.; Gross, R. Tissue localization of the endosymbiotic bacterium “Candidatus Blochmannia floridanus” in adults and larvae of the carpenter ant Camponotus floridanus. App. Environ. Microbiol. 2002, 68, 4187–4193. [Google Scholar] [CrossRef]
- de Souza, D.J.; Bezier, A.; Depoix, D.; Drezen, J.M.; Lenoir, A. Blochmania endosymbionts improve colony growth and immune defense in the ant Camponotus fellah. BMC Microbiol. 2009, 9, 29. [Google Scholar] [CrossRef]
- Golubev, V.I.; Bab’eva, I.P. Debaryomyces formicarius sp. n. and Debaryomyces cantarellii associated with the ants of the group Formica rufa L. J. Gen. Appl. Microbiol. 1972, 18, 249–254. [Google Scholar] [CrossRef][Green Version]
- Golubev, V.I.; Bab’eva, I.P. Yeasts of the genus Debaryomyces Klock in the nests of ants of the group Formica rufa L. Sov. J. Ecol. 1972, 3, 59–62. [Google Scholar] [PubMed]
- Gusteleva, L.A. Biosynthesis of vitamins of the B group by yeasts symbiotic on xylophageous insects. Microbiology 1975, 44, 36–38. [Google Scholar]
- Maksimova, I.A.; Glushakova, A.M.; Kachalkin, A.V.; Chernov, I.Y.; Panteleeva, S.N.; Reznikova, Z.I. Yeast communities of Formica aquilonia colonies. Microbiology 2016, 85, 124–129. [Google Scholar] [CrossRef]
- Siedlecki, I.; Gorczak, M.; Okrasińska, A.; Wrzosek, M. Chance or Necessity—The Fungi Co− Occurring with Formica polyctena Ants. Insects 2021, 12, 204. [Google Scholar] [CrossRef]
- Ba, A.S.; Guo, D.A.; Norton, R.A.; Phillips, S.A.; Ne, W.D. Developmental differences in the sterol composition of Solenopsis invicta. Arch. Insect Biochem. Physiol. 1995, 29, 1–9. [Google Scholar] [CrossRef]
- Ba, A.S.; Phillips, S.A. Yeast biota of the red imported fire ant. Mycol. Res. 1996, 100, 740–746. [Google Scholar] [CrossRef]
- Ba, A.S.; Phillips, S.A.; Anderson, J.T. Yeasts in the mound soil of the red imported fire ant. Mycol. Res. 2000, 10, 969–973. [Google Scholar] [CrossRef]
- Dadd, R.H. Qualitative requirements and utilization of nutrients: Insects. In CRC Handbook Series in Nutrition and Food, Section D: Nutritional Requirements; Rechcigl, M., Jr., Ed.; CRC Press: Cleveland, IL, USA, 1977; Volume 1, pp. 305–346. [Google Scholar]
- Dadd, R.H. Nutrition: Organisms. In Comprehensive Insect Physiology, Biochemistry and Pharmacology; Kerkut, G.A., Gilbert, L.I., Eds.; Pergamon Press: Oxford, UK, 1985; Volume 4, pp. 313–390. [Google Scholar]
- Reinecke, J.P. Nutrition: Artificial Diets. In Comprehensive Insect Physiology, Biochemistry and Pharmacology; Kerkut, G.A., Gilbert, L.I., Eds.; Pergamon Press: Oxford, UK, 1985; Volume 4, pp. 391–419. [Google Scholar]
- Pagnocca, F.C.; Rodrigues, A.; Nagamoto, N.S.; Bacci, M. Yeasts and filamentous fungi carried by the gynes of leaf-cutting ants. Antonie Leeuwenhoek 2008, 94, 517–526. [Google Scholar] [CrossRef]
- Arcuri, S.L.; Pagnocca, F.C.; da Paixão Melo, W.G.; Nagamoto, N.S.; Komura, D.L.; Rodrigues, A. Yeasts found on an ephemeral reproductive caste of the leaf-cutting ant Atta sexdens rubropilosa. Antonie Leeuwenhoek 2014, 106, 475–487. [Google Scholar] [CrossRef]
- Maurer, P.; Debieu, D.; Malosse, C.; Leroux, P.; Riba, G. Sterols and symbiosis in the leaf-cutting ant Acromyrmex octispinosus (Reich) (Hymenoptera, Formicidae: Attini). Arch. Insect Biochem. Physiol. 1992, 20, 13–21. [Google Scholar] [CrossRef]
- Maurer, P.; Girault, J.P.; Larchevêque, M.; Lafont, R. 24-Epi-makisterone a (not makisterone A) is the major ecdysteroid in the leaf-cutting ant Acromyrmex octospinosus (reich)(hymenoptera, formicidae: Attini). Arch. Insect Biochem. Physiol. 1993, 23, 29–35. [Google Scholar] [CrossRef]
- Stefanini, I.; Dapporto, L.; Legras, J.L.; Calabretta, A.; Di Paola, M.; De Filippo, C.; Viola, R.; Capretti, P.; Polsinelli, M.; Turillazzi, S.; et al. Role of social wasps in Saccharomyces cerevisiae ecology and evolution. Proc. Natl. Acad. Sci. USA 2012, 109, 13398–13403. [Google Scholar] [CrossRef] [PubMed]
- Stefanini, I.; Dapporto, L.; Berná, L.; Polsinelli, M.; Turillazzi, S.; Cavalieri, D. Social wasps are a Saccharomyces mating nest. Proc. Natl. Acad. Sci. USA 2016, 113, 2247–2251. [Google Scholar] [CrossRef]
- Paludo, C.R.; Menezes, C.; Silva-Junior, E.A.; Vollet-Neto, A.; Andrade-Dominguez, A.; Pishchany, G.; Khadempour, L.; do Nascimento, F.S.; Currie, C.R.; Kolter, R.; et al. Stingless bee larvae require fungal steroid to pupate. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Pant, N.C.; Dang, K. Physiology and elimination of intracellular symbiotes in some stored products beetles. In Insect and Mite Nutrition: Significance and Implications in Ecology and Pest Management; Rodriguez, J.G., Ed.; North-Holland Publishing: Amsterdam, The Netherlands, 1972; pp. 311–322. [Google Scholar]
- Ganter, P.F. Yeast and invertebrate associations. In Biodiversity and Ecophysiology of Yeasts; Rosa, C., Péter, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 303–370. [Google Scholar]
- Gilliam, M. Identification and roles of non-pathogenic microflora associated with honey bees. FEMS Microbiol. Lett. 1997, 155, 1–10. [Google Scholar] [CrossRef]
- Kaufman, M.G.; Walker, E.D.; Odelson, D.A.; Klug, M.J. Microbial Community Ecology and Insect Nutrition. Am. Entomol. 2000, 46, 173–184. [Google Scholar] [CrossRef]
- Stefanini, I. Yeast-insect associations: It takes guts. Yeast 2018, 35, 315–330. [Google Scholar] [CrossRef]
- Barnett, J.A.; Payne, R.W.; Yarrow, D. Yeasts: Characteristics and Identification; Cambridge University Press: Cambridge, UK, 1990; p. 1012. [Google Scholar]
- House, H.L. Nutritional studies with Pseudosarcophaga affinis (Fall.), A dipterous parasite of the spruce budworm, Choristoneura fumiferana (Clem.) I. A chemically defined medium and aseptic-culture technique. Can. J. Zool. 1954, 5, 331–341. [Google Scholar] [CrossRef]
- Dadd, R.H. The nutritional requirements of locusts-IV: Requirements for vitamins of the B complex. J. Insect Physiol. 1961, 6, 1–12. [Google Scholar] [CrossRef]
- Baker, J.E. Vitamin requirements of larvae of Sitophilus oryzae. J. Insect Physiol. 1975, 21, 1337–1342. [Google Scholar] [CrossRef]
- Yazgan, S. A chemically defined synthetic diet and larval nutritional requirements of the endoparasitoid Itoplectis conquisitor (Hymenoptera). J. Insect Physiol. 1972, 18, 2123–2141. [Google Scholar] [CrossRef]
- Wheeler, D.E. Nourishment in ants: Patterns in individuals and societies. In Nourishment and Evolution in Insect Societies; Hunt, J.E., Nalepa, C.A., Eds.; Westview Press: Oxford, UK, 1994; pp. 245–278. [Google Scholar]
- Cassill, D.B.; Tschinkel, W.R. Behavioral and developmental homeostasis in the fire ant, Solenopsis invicta. J. Insect Physiol. 2000, 46, 933–939. [Google Scholar] [CrossRef]
- Vogt, J.T. Attractiveness and effectiveness of an artificial diet fed to hybrid imported fire ants, Solenopsis invicta x richteri (Hymenoptera: Formicidae). Fla. Entomol. 2003, 86, 456–459. [Google Scholar] [CrossRef]
- Straka, J.; Feldhaar, H. Development of a chemically defined diet for ants. Insectes Soc. 2007, 54, 100–104. [Google Scholar] [CrossRef]
- Dussutour, A.; Simpson, S.J. Description of a simple synthetic diet for studying nutritional responses in ants. Insectes Soc. 2008, 55, 329–333. [Google Scholar] [CrossRef]
- Santo Domingo, J.W.; Kaufman, M.G.; Klug, M.J.; Holben, W.E.; Harris, D.; Tiedje, J.M. Influence of diet on the structure and function of the bacterial hindgut community of crickets. Mol. Ecol. 1998, 7, 761–767. [Google Scholar] [CrossRef]
- Mankowski, M.E.; Morrell, J.J. Incidence of Apocephalus horridus (Diptera: Phoridae) in colonies of Camponotus vicinus (Hymenoptera: Formicidae) and the effect of antibiotic/antimycotic mixtures on fly emergence. Sociobiology 2003, 42, 477–484. [Google Scholar]
- Smith, F. Effect of reduced food supply upon the stature of Camponotus ants (Hymenoptera: Formicidae). Entomol. News. 1942, 53, 133–135. [Google Scholar]
- Smith, F. Nutritional requirements of Camponotus ants. Ann. Entomol. Soc. Am. 1944, 37, 410–418. [Google Scholar] [CrossRef]
- Mankowski, M.E.; Morrell, J.J. Effects of B Vitamin Deletion in Chemically Defined Diets on Brood Development in the Carpenter Ant Camponotus vicinus (Mayr) (Hymenoptera: Formicidae). J. Econ. Entomol. 2014, 107, 1299–1306. [Google Scholar] [CrossRef]
- Armstrong, E. The effects of vitamin deficiencies on the growth and mortality of Tribolium castaneum infected with Nosema whitei. J. Inverteb. Pathol. 1978, 31, 303–306. [Google Scholar] [CrossRef]
- Littell, R.C.; Milliken, G.A.; Stroup, W.W.; Wolfinger, R.D.; Schabenberger, O. SAS® for Mixed Models, 2nd ed.; SAS Institute, Inc.: Cary, NC, USA, 2006; 814p. [Google Scholar]
- Stroup, W.W.; Milliken, G.A.; Claassen, E.A.; Wolfinger, R.D. SAS® for Mixed Models: Introduction and Basic Applications; SAS Institute Inc.: Cary, NC, USA, 2018; 594p. [Google Scholar]
- SAS Institute, Inc. SAS/STAT® 14.1 User’s Guide; SAS Publishing. SAS Institute Inc.: Cary, NC, USA, 2015. [Google Scholar]
- Friend, W.G.; Dadd, R.H. Insect Nutrition A Comparative Perspective. In Advances in Nutritional Research; Draper, H.H., Ed.; Plenum Press: New York, NY, USA, 1982; Volume 4, pp. 205–247. [Google Scholar]
- Fan, Y.; Wernegreen, J.J. Can’t take the heat: High temperature depletes bacterial endosymbionts of ants. Microb. Ecol. 2013, 66, 727–733. [Google Scholar] [CrossRef][Green Version]
- Feldhaar, H.; Straka, J.; Krischke, M.; Berthold, K.; Stoll, S.; Mueller, M.J.; Gross, R. Nutritional upgrading for omnivorous carpenter ants by the endosymbiont Blochmannia. BMC Biol. 2007, 5, 48. [Google Scholar] [CrossRef]
- Mankowski, M.E.; Morrell, J.J. Role of relative humidity in colony founding and queen survivorship in two carpenter ant species. J. Econ. Entomol. 2011, 104, 740–744. [Google Scholar] [CrossRef] [PubMed]
- Álvaro-Benito, M.; Polo, A.; González, B.; Fernández-Lobato, M.; Sanz-Aparicio, J. Structural and kinetic analysis of Schwanniomyces occidentalis invertase reveals a new oligomerization pattern and the role of its supplementary domain in substrate binding. J. Biol. Chem. 2010, 285, 13930–13941. [Google Scholar] [CrossRef] [PubMed]
- Starmer, W.T.; Barker, J.S.F.; Phaff, H.J.; Fogleman, J.C. Adaptations of Drosophila and yeasts: Their interactions with the volatile 2-propanol in the cactus-microorganism-Drosophila model system. Aust. J. Biol. Sci. 1986, 39, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Leufven, A.; Bergstrom, G.; Falsen, E. Interconversion of verbenols and verbenone by identified yeasts isolated from the spruce bark beetle Ips typographus. J. Chem. Ecol. 1984, 10, 1349–1361. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.S.; Hofstetter, R.W.; Foster, J.T.; Foote, N.E.; Keim, P. Interactions between the yeast Ogataea pini and filamentous fungi associated with the western pine beetle. Microb. Ecol. 2011, 61, 626–634. [Google Scholar] [CrossRef] [PubMed]
| Amino Acids | Water Soluble Vitamins | Fat Soluble Vitamins | Inorganic Salts | Other | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Component | mg/ 100 mL | Component | mg/ 100 mL | Component | mg/ 100 mL | Component | mg/ 100 mL | Component | mg/ 100 mL | Component | mg/ 100 mL |
| Alanine | 100 | Leucine | 150 | Ascorbic acid | 10 | Cholesterol | 50 | CaCl2 | 10 | Sucrose | 10,000 |
| Arginine | 100 | Lycine | 100 | Amino benzoic acid | 2.5 | Linoleic acid | 25 | CoCl2 6H2O | 2 | Ribonucleic acid | 100 |
| Aspartic acid | 100 | Methionine | 50 | Biotin | 0.2 | Linolenic acid | 20 | CuSO4 5H2O | 2 | KOH | 1.5 |
| Cysteine | 30 | Phenylalanine | 100 | Calcium Pantothenate | 5 | Oleic acid | 60 | FeCl3 6H2O | 8 | K2PO4 | 1.5 |
| Glutamic acid | 200 | Proline | 150 | Choline Chloride | 125 | Palmitic acid | 60 | K2PO4 | 50 | ||
| Glutamine | 50 | Serine | 100 | Folic acid | 2.5 | Tween 80 | 500 | MgSO4 7H2O | 60 | ||
| Glycine | 150 | Threonine | 75 | Inositol | 25 | Vitamin A | 0.1 | MnSO4 H2O | 0.5 | ||
| Histidine | 50 | Tryptophan | 75 | Nicotinic acid | 10 | Vitamin E | 1 | NaHPO4 | 5 | ||
| Hydroxyproline | 30 | Tyrosine | 100 | Pyridoxine | 2.5 | ZnCl | 2 | ||||
| Isoleucine | 100 | Valine | 100 | Riboflavin | 0.25 | ||||||
| Thiamine | 2.5 | ||||||||||
| Vitamin B12 | 0.1 | ||||||||||
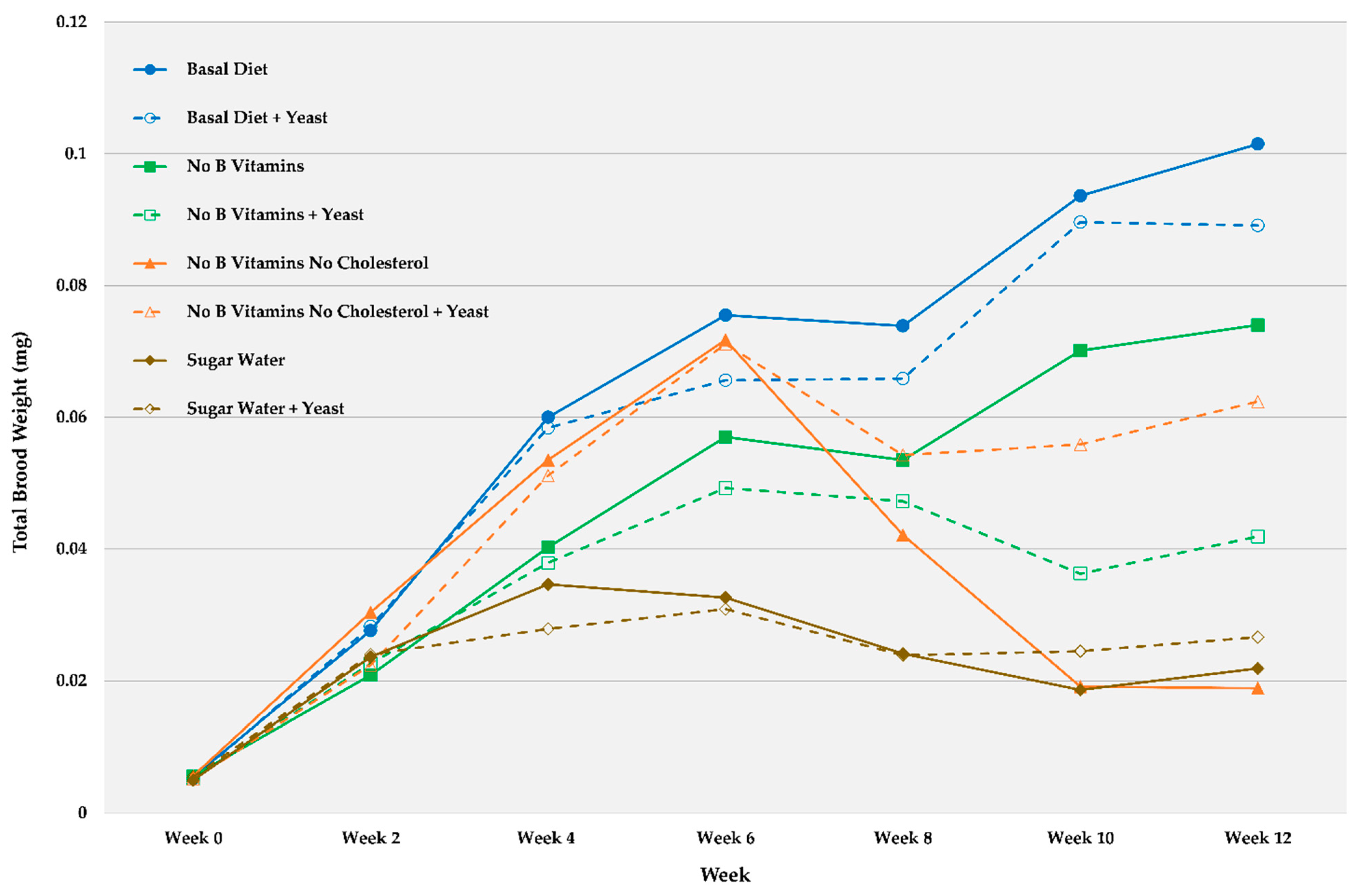
| Week 0 | Week 2 | Week 4 | Week 6 | Week 8 | Week 10 | Week 12 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diet/Treatment | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE |
| Basal Diet | 0.0055 | 0.0004 | 0.0276 | 0.00032 | 0.0600 | 0.0064 | 0.0755 | 0.0114 | 0.0739 | 0.0100 | 0.0936 | 0.0153 | 0.1015 | 0.0211 |
| Basal Diet + Yeast | 0.0053 | 0.0005 | 0.0283 | 0.0049 | 0.0584 | 0.0087 | 0.0656 | 0.0111 | 0.0659 | 0.0116 | 0.0896 | 0.0165 | 0.0891 | 0.0171 |
| No B Vitamins | 0.0056 | 0.0004 | 0.0209 | 0.0050 | 0.0403 | 0.0085 | 0.0570 | 0.0088 | 0.0535 | 0.0110 | 0.0701 | 0.0158 | 0.0740 | 0.0163 |
| No B Vitamins + Yeast | 0.0051 | 0.0003 | 0.0226 | 0.0041 | 0.0379 | 0.0072 | 0.0493 | 0.0141 | 0.0473 | 0.0116 | 0.0363 | 0.0148 | 0.0419 | 0.0160 |
| No B Vitamins No Cholesterol | 0.0056 | 0.0003 | 0.0304 | 0.0047 | 0.0535 | 0.0101 | 0.0718 | 0.0129 | 0.0421 | 0.0127 | 0.0191 | 0.0055 | 0.0189 | 0.0060 |
| No B Vitamins No Cholesterol + Yeast | 0.0051 | 0.0008 | 0.0224 | 0.0038 | 0.0511 | 0.0059 | 0.0711 | 0.0087 | 0.0543 | 0.0088 | 0.0559 | 0.0149 | 0.0624 | 0.0188 |
| Sugar Water | 0.0050 | 0.0003 | 0.0236 | 0.0024 | 0.0346 | 0.0031 | 0.0326 | 0.0044 | 0.0241 | 0.0035 | 0.0186 | 0.0054 | 0.0219 | 0.0061 |
| Sugar Water + Yeast | 0.0055 | 0.0005 | 0.0240 | 0.0037 | 0.0279 | 0.0036 | 0.0309 | 0.0029 | 0.0239 | 0.0040 | 0.0245 | 0.0049 | 0.0266 | 0.0069 |
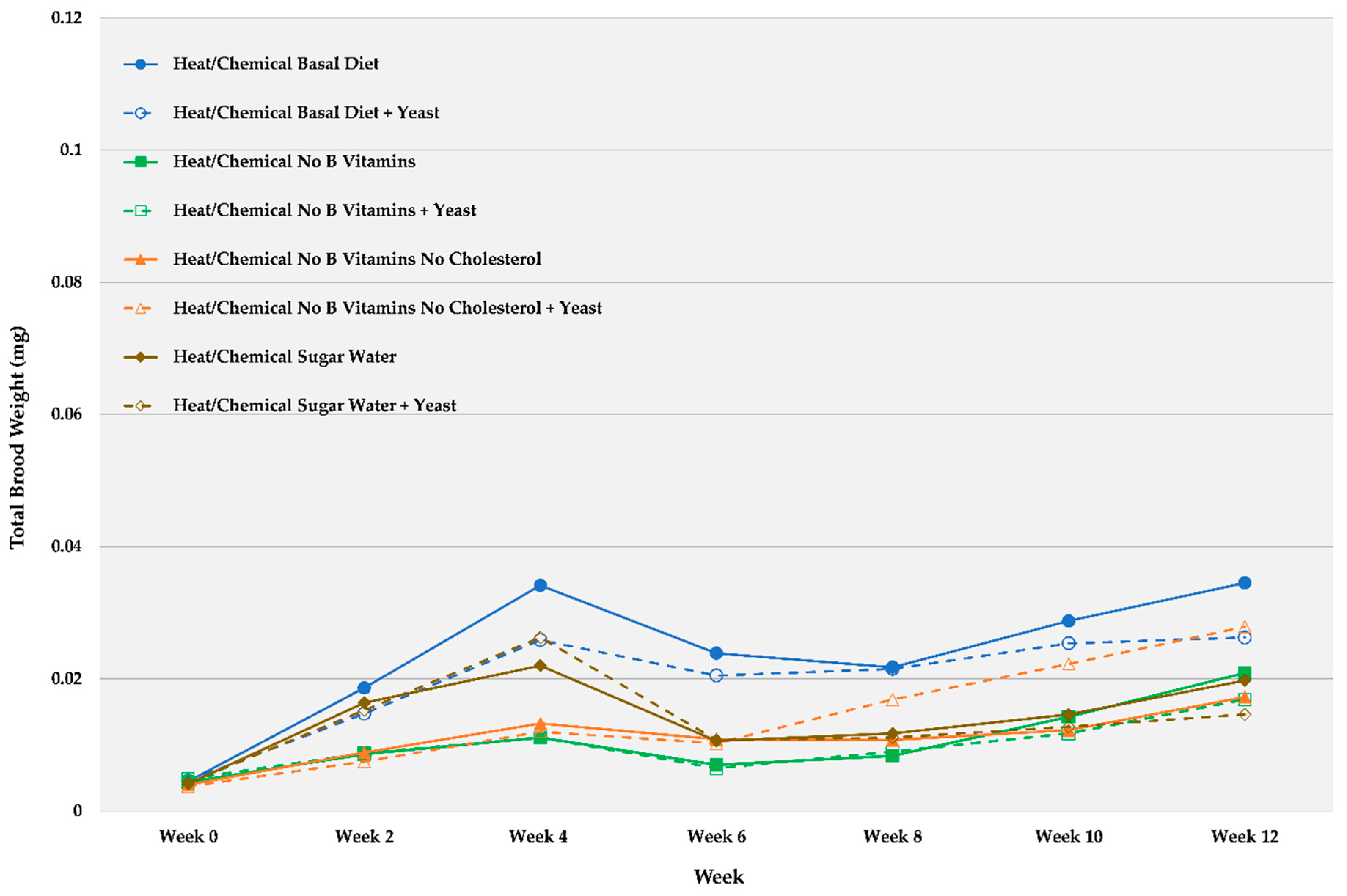
| Week 0 | Week 2 | Week 4 | Week 6 | Week 8 | Week 10 | Week 12 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diet/Treatment | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE |
| Heat/Chemical Basal Diet | 0.0045 | 0.0005 | 0.0186 | 0.0048 | 0.0341 | 0.0078 | 0.0239 | 0.0080 | 0.0218 | 0.0048 | 0.0288 | 0.0082 | 0.0345 | 0.0106 |
| Heat/Chemical Basal Diet + Yeast | 0.0046 | 0.0002 | 0.0148 | 0.0032 | 0.0259 | 0.0041 | 0.0205 | 0.0048 | 0.0215 | 0.0032 | 0.0254 | 0.0041 | 0.0263 | 0.0074 |
| Heat/Chemical No B Vitamins | 0.0044 | 0.0004 | 0.0086 | 0.0015 | 0.0111 | 0.0025 | 0.0070 | 0.0016 | 0.0084 | 0.0020 | 0.0143 | 0.0051 | 0.0209 | 0.0091 |
| Heat/Chemical No B Vitamins + Yeast | 0.0049 | 0.0004 | 0.0088 | 0.0013 | 0.0111 | 0.0020 | 0.0065 | 0.0007 | 0.0090 | 0.0016 | 0.0118 | 0.0029 | 0.0169 | 0.0048 |
| Heat/Chemical No B Vitamins No Cholesterol | 0.0040 | 0.0002 | 0.0089 | 0.0008 | 0.0133 | 0.0023 | 0.0109 | 0.0037 | 0.0108 | 0.0020 | 0.0123 | 0.0034 | 0.0173 | 0.0069 |
| Heat/Chemical No B Vitamins No Cholesterol + Yeast | 0.0038 | 0.0003 | 0.0075 | 0.0010 | 0.0120 | 0.0014 | 0.0103 | 0.0028 | 0.0169 | 0.0048 | 0.0223 | 0.0084 | 0.0279 | 0.0109 |
| Heat/Chemical Fed Sugar Water | 0.0041 | 0.0004 | 0.0164 | 0.0019 | 0.0220 | 0.0028 | 0.0106 | 0.0045 | 0.0118 | 0.0054 | 0.0146 | 0.0063 | 0.0198 | 0.0087 |
| Heat/Chemical Fed Sugar Water + Yeast | 0.0043 | 0.0004 | 0.0153 | 0.0019 | 0.0263 | 0.0031 | 0.0108 | 0.0040 | 0.0111 | 0.0047 | 0.0128 | 0.0044 | 0.0146 | 0.0052 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mankowski, M.E.; Morrell, J.J.; Lebow, P.K. Effects on Brood Development in the Carpenter Ant Camponotus vicinus Mayr after Exposure to the Yeast Associate Schwanniomyces polymorphus Kloecker. Insects 2021, 12, 520. https://doi.org/10.3390/insects12060520
Mankowski ME, Morrell JJ, Lebow PK. Effects on Brood Development in the Carpenter Ant Camponotus vicinus Mayr after Exposure to the Yeast Associate Schwanniomyces polymorphus Kloecker. Insects. 2021; 12(6):520. https://doi.org/10.3390/insects12060520
Chicago/Turabian StyleMankowski, Mark E., Jeffrey J. Morrell, and Patricia K. Lebow. 2021. "Effects on Brood Development in the Carpenter Ant Camponotus vicinus Mayr after Exposure to the Yeast Associate Schwanniomyces polymorphus Kloecker" Insects 12, no. 6: 520. https://doi.org/10.3390/insects12060520
APA StyleMankowski, M. E., Morrell, J. J., & Lebow, P. K. (2021). Effects on Brood Development in the Carpenter Ant Camponotus vicinus Mayr after Exposure to the Yeast Associate Schwanniomyces polymorphus Kloecker. Insects, 12(6), 520. https://doi.org/10.3390/insects12060520






