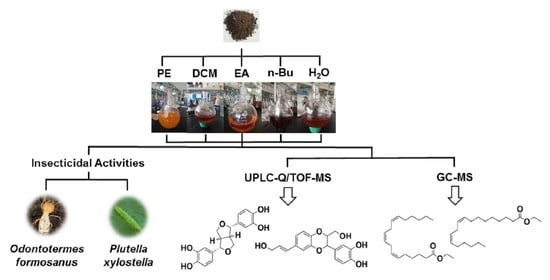
Insecticidal Activities Against Odontotermes formosanus and Plutella xylostella and Corresponding Constituents of Tung Meal from Vernicia fordii
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Rearing of Insects
2.2. Acquisition of Plant Materials and Extracts
2.3. Insecticidal Bioassay
2.3.1. Insecticidal Activity on Forest Pest O. Formosanus
2.3.2. Insecticidal Activity on Agricultural Pest P. xylostella
2.4. Chemical Composition Analysis
2.4.1. UPLC-Q/TOF-MS Analysis of EA Extracts
2.4.2. GC-MS Analysis of PE Extracts
2.5. Statistical Analysis
3. Results and Discussion
3.1. Insecticidal Activity of Tung Meal on Forest Pest O. Formosanus
3.2. Insecticidal Activity of Tung Meal on Agricultural Pest P. xylostella
3.3. Characterization of Chemical Components in EA Extracts by UPLC-Q/TOF-MS
3.3.1. Lignans
3.3.2. Fatty Acids
3.3.3. Others
3.4. Identification of Volatile Constituents in PE Extracts by GC-MS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cui, P.; Lin, Q.; Fang, D.; Zhang, L.; Li, R.; Cheng, J.; Gao, F.; Shockey, J.; Hu, S.; Lü, S. Tung tree (Vernicia fordii, Hemsl.) genome and transcriptome sequencing reveals co-ordinate up-regulation of fatty acid β-oxidation and triacylglycerol biosynthesis pathways during eleostearic acid accumulation in seeds. Plant Cell Physiol. 2018, 59, 1990–2003. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Zhao, J.; Li, G.; Huang, Y.; Yang, Z.; Yuan, T. Facile synthesis and characterization of novel multi-functional bio-based acrylate prepolymers derived from tung oil and its application in UV-curable coatings. Ind. Crop. Prod. 2019, 138, 111585. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, J.; Wu, F.; Qu, S. Extraction and composition characterisation of amino acids from tung meal. Nat. Prod. Res. 2016, 30, 849–852. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Martinez-Herrera, J.; Becker, K. Variations in seed number per fruit, seed physical parameters and contents of oil, protein and phorbol ester in toxic and non-toxic genotypes of Jatropha curcas. J. Plant Sci. 2008, 3, 260–265. [Google Scholar] [CrossRef]
- Mann, G.E.; Hoffman, W.H.; Ambrose, A.M. Oilseed processing, detoxification and toxicological studies of tung meal. J. Agric. Food Chem. 1954, 2, 258–263. [Google Scholar] [CrossRef]
- Zan, L.; Song, W.; Liu, J.; Guo, S.; Li, H.; Wang, Y. Study on toxicity of water extract from tung meal on aboveground and underground pest. Nonwood Forest Res. 2018, 36, 75–78. [Google Scholar]
- Roongtawan, M.; Anuluck, J.; Udom, C.; Atchariya, J.; Doungrat, R.; Anchalee, W.; Pradya, S.; Benjawan, B. Natural larvicides of botanical origin against dengue vector aedes aegypti (Diptera: Culicidae). Southeast Asian J. Trop. Med. 2018, 49, 227–239. [Google Scholar]
- Santos, A.A.; Santos de Oliveira, B.M.; Melo, C.R.; Santana Lima, A.P.; Rabelo Santana, E.D.; Blank, A.F.; Picanco, M.C.; Araujo, A.P.A.; Cristaldo, P.F.; Bacci, L. Sub-lethal effects of essential oil of lippia sidoides on drywood termite Cryptotermes brevis (Blattodea: Termitoidea). Ecotoxicol. Environ. Saf. 2017, 145, 436–441. [Google Scholar] [CrossRef]
- Rust, M.K.; Su, N.-Y. Managing social insects of urban importance. Annu. Rev. Entomol. 2012, 57, 355–375. [Google Scholar] [CrossRef]
- Sarfraz, M.; Keddie, B.A. Conserving the efficacy of insecticides against Plutella xylostella (L.) (Lep., Plutellidae). J. Appl. Entomol. 2005, 129, 149–157. [Google Scholar] [CrossRef]
- Abro, G.H.; Syed, T.S.; Kalhoro, A.N.; Sheikh, G.H.; Awan, M.S.; Jessar, R.D.; Shelton, A.M. Insecticides for control of the diamondback moth, Plutella xylostella (L.) (Lepidoptera: Plutellidae) in pakistan and factors that affect their toxicity. Crop. Prot. 2013, 52, 91–96. [Google Scholar] [CrossRef]
- Furlong, M.J.; Wright, D.J.; Dosdall, L.M. Diamondback moth ecology and management: Problems, progress, and prospects. Annu. Rev. Entomol. 2013, 58, 517–541. [Google Scholar] [CrossRef]
- Sparks, T.C.; Wessels, F.J.; Lorsbach, B.A.; Nugent, B.M.; Watson, G.B. The new age of insecticide discovery-the crop protection industry and the impact of natural products. Pestic. Biochem. Phys. 2019, 161, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Domingues, P.M.; Santos, L. Essential oil of pennyroyal (Mentha pulegium): Composition and applications as alternatives to pesticides-New tendencies. Ind. Crop. Prod. 2019, 139, 111534. [Google Scholar] [CrossRef]
- Sharma, A.; Shukla, A.; Attri, K.; Kumar, M.; Kumar, P.; Suttee, A.; Singh, G.; Barnwal, R.P.; Singla, N. Global trends in pesticides: A looming threat and viable alternatives. Ecotoxicol. Environ. Saf. 2020, 201, 110812. [Google Scholar] [CrossRef] [PubMed]
- Sayed, S.M.; Alotaibi, S.S.; Gaber, N.; Elarrnaouty, S.-A. Evaluation of five medicinal plant extracts on Aphis craccivora (Hemiptera: Aphididae) and its predator, Chrysoperla Carnea (Neuroptera: Chrysopidae) under laboratory conditions. Insects 2020, 11, 398. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Grandon, G.M.; Harte, S.J.; Ewany, J.; Bray, D.; Stevenson, P.C. Additive effect of botanical insecticide and entomopathogenic fungi on pest mortality and the behavioral response of its natural enemy. Plants 2020, 9, 173. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.L.; Auad, A.M.; Magno, M.C.; Resende, T.T.; Fonseca, M.G.; Silva, S.E.B. Insecticidal activity of compounds of plant origin on Mahanarva spectabilis (Hemiptera: Cercopidae). Insects 2019, 10, 360. [Google Scholar] [CrossRef] [PubMed]
- Gu, R.; Morcol, T.; Liu, B.; Shi, M.; Kennelly, E.J.; Long, C. GC-MS, UPLC-QTOF-MS, and bioactivity characterization of Acer truncatum seeds. Ind. Crop. Prod. 2019, 138, 111480. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Q.; Zhong, C.; Guo, M. Volatile fingerprints and biomarkers of three representative kiwifruit cultivars obtained by headspace solid-phase microextraction gas chromatography mass spectrometry and chemometrics. Food Chem. 2019, 271, 211–215. [Google Scholar] [CrossRef]
- Rampadarath, S.; Puchooa, D.; Jeewon, R. Jatropha curcas L: Phytochemical, antimicrobial and larvicidal properties. Asian Pac. J. Trop. Bio. 2016, 6, 858–865. [Google Scholar] [CrossRef]
- Xing, S.; Peng, C.; Wang, M.; Peng, Y.; Li, X. Ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry analysis of In vitro metabolites of lignans from fructus forsythiae by Human Fecal Flora. Pharmacogn. Mag. 2018, 14, 364. [Google Scholar] [CrossRef]
- Chen, G.; Zhao, W.; Li, Y.; Zhou, D.; Ding, J.; Lin, B.; Li, W.; Yang, Y.; Liu, J.; Hou, Y.; et al. Bioactive chemical constituents from the seed testa of Vernicia fordii as potential neuroinflammatory inhibitors. Phytochemistry 2020, 171, 112233. [Google Scholar] [CrossRef] [PubMed]
- Petrillo, A.D.; González-Paramás, A.M.; Rosa, A.; Ruggiero, V.; Boylan, F.; Kumar, A.; Pintus, F.; Santos-Buelga, C.; Fais, A.; Era, B. Chemical composition and enzyme inhibition of Phytolacca dioica L. seeds extracts. J. Enzym. Inhib. Med. Chem. 2019, 34, 519–527. [Google Scholar] [CrossRef]
- Waibel, R.; Benirschke, G.; Benirschke, M.; Achenbach, H. Sesquineolignans and other constituents from the seeds of Joannesia princeps. Phytochemistry 2003, 62, 805–811. [Google Scholar] [CrossRef]
- Yasmeen, F.; Szmigielski, R.; Vermeylen, R.; Gómez-González, Y.; Surratt, J.D.; Chan, A.W.H.; Seinfeld, J.H.; Maenhaut, W.; Claeys, M. Mass spectrometric characterization of isomeric terpenoic acids from the oxidation of α-pinene, β-pinene, d-limonene, and Δ3-carene in fine forest aerosol. J. Mass Spectrom. 2011, 46, 425–442. [Google Scholar] [CrossRef] [PubMed]
- Papazian, S.; Parrot, D.; Burýšková, B.; Weinberger, F.; Tasdemir, D. Surface chemical defence of the eelgrass Zostera marina against microbial foulers. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Attygalle, A.B.; Ruzicka, J.; Varughese, D.; Bialecki, J.B.; Jafri, S. Low-energy collision-induced fragmentation of negative ions derived from ortho-, meta-, and para-hydroxyphenyl carbaldehydes, ketones, and related compounds. J Mass Spectrom. 2007, 42, 1207–1217. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.S.; Gelman, D.B.; Salvucci, M.E.; Chen, Y.P.; Blackburn, M.B. Insecticidal activity of some reducing sugars against the sweet potato whitefly, Bemisia tabaci, biotype B. J. Insect Sci. 2010, 10, 203. [Google Scholar] [CrossRef]
- Shanmugavel, S.; Velayutham, V.; Kamalanathan, T.; Periasamy, M.; Munusamy, A.; Sundaram, J. Isolation and analysis of mannose/trehalose/maltose specific lectin from jack bean with antibruchid activity. Int. J. Biol. Macromol. 2016, 91, 1–14. [Google Scholar] [CrossRef]
- Küçükaydın, S.; Tel-Çayan, G.; Duru, M.E.; Kesdek, M.; Öztürk, M. Chemical composition and insecticidal activities of the essential oils and various extracts of two Thymus species: Thymus cariensis and Thymus cilicicus. Toxin Rev. 2020, 1–11. [Google Scholar] [CrossRef]
- Yang, X.L.; Jiang, M.Y.; Hsieh, K.L.; Liu, J.K. Chemical constituents from the seeds of Morinda citrifolia. Chin. J. Nat. Med. 2009, 7, 119–122. [Google Scholar] [CrossRef]
- de Barros, R.P.; Reis, L.S.; da Costa, J.G.; Cunha, A.L.; Magalhaes, I.C.S.; da Silva, C.G.; dos Santos, A.F.; das Neves, J.D.S.; Duarte, A.G.; de Mello, G.S.V.; et al. Bioactivity and phenolic composition of extracts of noni (Morinda citrifolia L., Rubiaceae) in tomato moth (Tuta absoluta Meyrick, 1917) (Lepidoptera: Gelechiidae). Afr. J. Agric. Res. 2018, 13, 2063–2069. [Google Scholar] [CrossRef]
- Ishibashi, F.; Taniguchi, E. Synthesis and absolute configuration of the insecticidal sesquilignan (+)-HAEDOXAN a in honour of professor G. H. Neil Towers 75th birthday. Phytochemistry 1998, 49, 613–622. [Google Scholar] [CrossRef]
- Taniguchi, E.; Imamura, K.; Ishibashi, F.; Matsui, T.; Nishio, A. Structure of the novel insecticidal sesquilignan, haedoxan a. Agric. Biol. Chem. 1989, 53, 631–643. [Google Scholar] [CrossRef]
- Chauhan, N.; Kumar, P.; Mishra, S.; Verma, S.; Malik, A.; Sharma, S. Insecticidal activity of Jatropha Curcas extracts against housefly, Musca domestica. Environ. Sci. Pollut. Res. Int. 2015, 22, 14793–14800. [Google Scholar] [CrossRef]
- Silva, G.N.; Faroni, L.R.A.; Sousa, A.H.; Freitas, R.S. Bioactivity of Jatropha Curcas L. to insect pests of stored products. J. Stored. Prod. Res. 2012, 48, 111–113. [Google Scholar] [CrossRef]
- Li, X.; Li, L.; Wang, J.; Wang, T.; Wang, L. Two new lignans with antioxidative activities from Jatropha Curcas. Nat. Prod. Res. 2014, 28, 1985–1991. [Google Scholar] [CrossRef]
- Łyczko, J.; Masztalerz, K.; Lipan, L.; Lech, K.; Carbonell-Barrachina, Á.A.; Szumny, A. Chemical determinants of dried Thai basil (O. basilicum var. thyrsiflora) aroma quality. Ind. Crop. Prod. 2020, 155, 112769. [Google Scholar] [CrossRef]
- Zhang, L.; Jia, B.; Tan, X.; Thammina, C.S.; Long, H.; Liu, M.; Wen, S.; Song, X.; Cao, H. Fatty acid profile and unigene-derived simple sequence repeat markers in tung tree (Vernicia fordii). PLoS ONE 2014, 9, e105298. [Google Scholar] [CrossRef]
- Kumar, S.; Dhillon, M.K.; Singh, M.; Rathi, R.S.; Misra, A.K.; Rana, J.C. Fatty and amino acid compositions of Vernicia fordii: A source of α-eleostearic acid and methionine. Indian J. Exp. Biol. 2017, 55, 734–739. [Google Scholar]
- Cui, S.; Tan, S.; Ouyang, G.; Jiang, S.; Pawliszyn, J. Headspace solid-phase microextraction gas chromatography–mass spectrometry analysis of Eupatorium odoratum extract as an oviposition repellent. J. Chromatogr. B 2009, 877, 1901–1906. [Google Scholar] [CrossRef]
- Chaubey, M.K. Biological activities of Zingiber officinale (Zingiberaceae) and Piper cubeba (Piperaceae) essential oils against pulse beetle, Callosobruchus chinensis (Coleoptera: Bruchidae). Pak. J. Biol. Sci. 2013, 16, 517–523. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Babu, G.D.K.; Dolma, S.K.; Sharma, M.; Reddy, S.G.E. Chemical composition of essential oil and oleoresins of Zingiber officinale and toxicity of extracts/essential oil against diamondback moth (Plutella xylostella). Toxin Rev. 2020, 39, 226–235. [Google Scholar] [CrossRef]
- Ma, X.; Wang, L.; Yang, M.; Hao, F. Insecticidal activity and active components of Cynanchi auriculati against Plutella xylostella. Agrochemicals 2009, 48, 261–264. [Google Scholar] [CrossRef]


| Extracts | Survival Rate | |
|---|---|---|
| O. formosanus | P. xylostella | |
| DMSO | 97.50 ± 2.50 a | 97.92 ± 2.08 a |
| Control | 97.50 ± 4.33 a | 95.83 ± 4.17 a |
| CE | 47.5 ± 16.01 b | 75.00 ± 8.33 bcd |
| PE | 85 ± 8.66 a | 58.33 ± 8.33 d |
| DCM | 41.25 ± 20.73 bc | 85.42 ± 2.08 ab |
| EA | 23.75 ± 10.23 c | 75.00 ± 4.17 bcd |
| n-Bu | 56.25 ± 12.44 b | 60.42 ± 6.25 cd |
| H2O | 25 ± 9.35 c | 79.17 ± 4.17 abc |
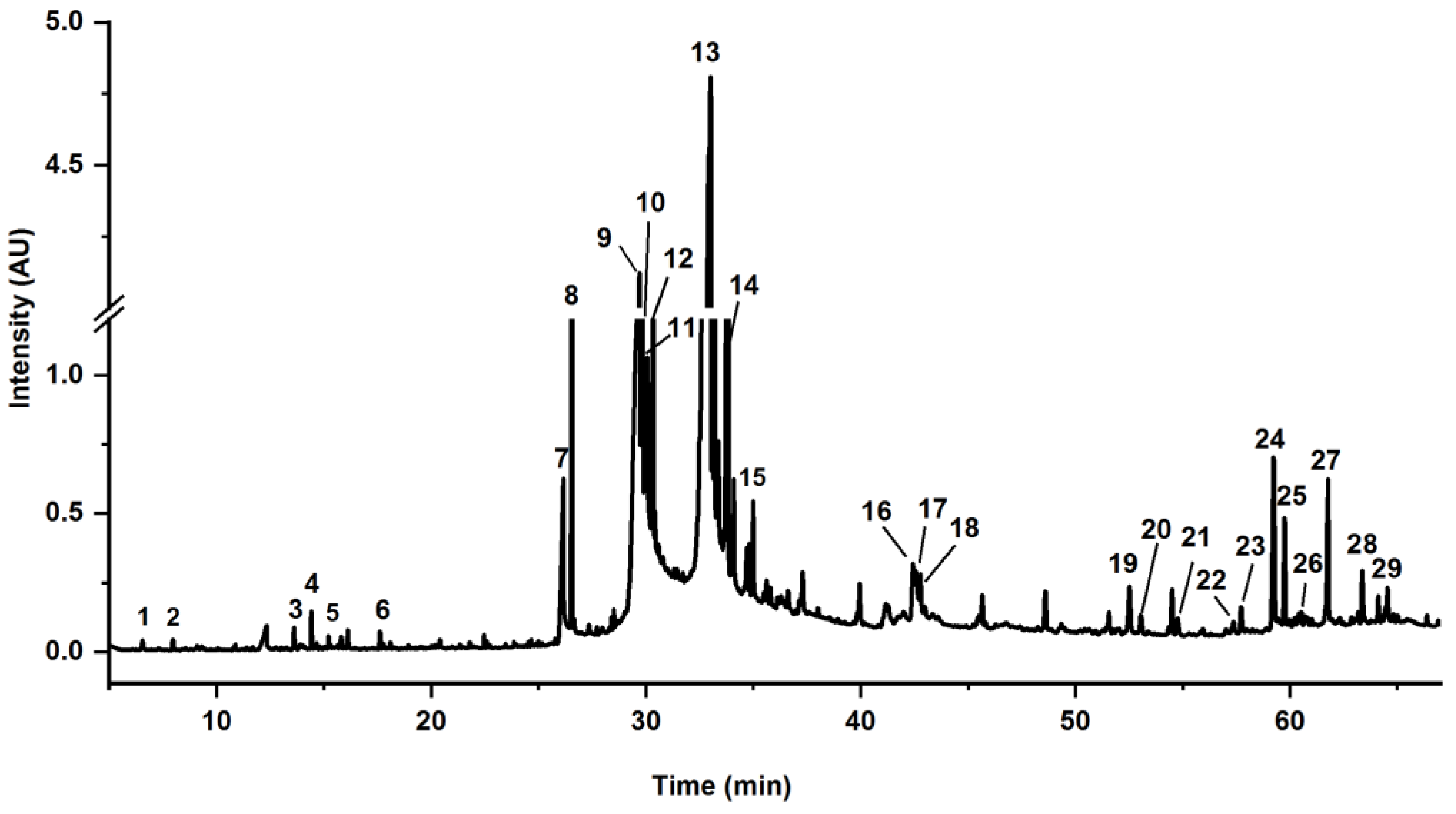
| No. | tR a (Min) | Components | Content b (%) | Formula | Measured Mass (m/z) | MS1 (m/z) | MS/MS (m/z) | Error (ppm) |
|---|---|---|---|---|---|---|---|---|
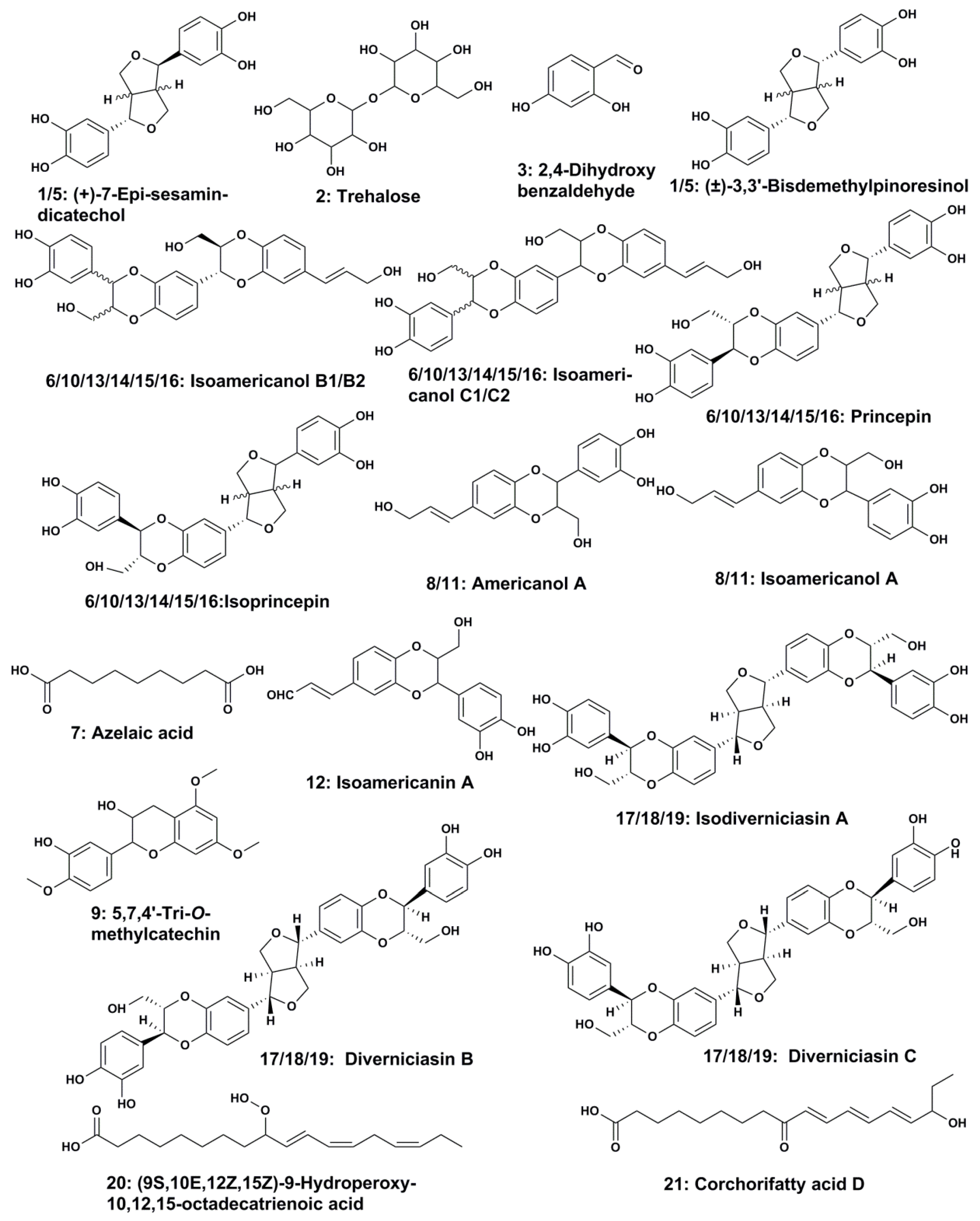
| 1 | 0.68 | (+)-7-Epi-sesamin-dicatechol/(±)-3,3′-Bisdemethylpinoresinol | 1.42 | C18H18O6 | 329.1025 | 329.1043 | 329, 299, 269, 165, 137, 109 | 5.47 |
| 2 | 0.82 | Trehalose | 0.46 | C12H22O11 | 387.1139 | 387.1168 | 341, 179, 161, 143 | 7.49 |
| 3 | 3.65 | 2,4-dihydroxybenzaldehyde | 2.84 | C7H6O3 | 137.0239 | 137.0241 | 137, 119, 109, 108 | 1.46 |
| 4 | 8.55 | Unknown | 5.47 | - | - | 467.1955 | 467, 403, 343, 331, 313, 233, 203, 185 | - |
| 5 | 12.59 | (+)-7-Epi-sesamin-dicatechol/(±)-3,3′-Bisdemethylpinoresinol | 12.16 | C18H18O6 | 329.1025 | 329.1048 | 329, 269, 189, 165, 137, 109 | 6.99 |
| 6 | 13.84 | Isoamericanol B1 or related isomers (B2/C1/C2/(iso)princepin) | 0.54 | C27H26O9 | 493.1499 | 493.1513 | 493, 475, 463, 329, 327, 281, 165, 163 | 2.84 |
| 7 | 15.98 | Azelaic acid | 4.38 | C9H16O4 | 187.0979 | 187.0987 | 187, 169, 143, 125 | 4.28 |
| 8 | 17.06 | (iso)americanol A | 7.51 | C18H18O6 | 659.2129 | 659.2154 | 329, 311, 299, 165, 147, 135 | 3.79 |
| 9 | 17.53 | 5,7,4′-Tri-O-methylcatechin | 1.13 | C18H20O6 | 663.2442 | 663.2473 | 332, 331, 313, 301, 165, 147, 135 | 4.68 |
| 10 | 18.23 | Isoamericanol B1 or related isomers (B2/C1/C2/(iso)princepin) | 1.10 | C27H26O9 | 493.1499 | 493.1534 | 493, 463, 329, 327, 299, 269, 165, 163, 137 | 7.10 |
| 11 | 18.86 | (iso)americanol A | 2.63 | C18H18O6 | 659.2129 | 659.2176 | 329, 311, 299, 165, 147, 135 | 7.13 |
| 12 | 21.39 | Isoamericanin A | 0.93 | C18H16O6 | 327.0869 | 327.0890 | 327, 297, 165, 163, 147, 135 | 6.42 |
| 13 | 23.59 | Isoamericanol B1 or related isomers (B2/C1/C2/(iso)princepin) | 11.87 | C27H26O9 | 493.1499 | 493.1528 | 493, 475, 463, 329, 327, 165, 163, 137 | 5.88 |
| 14 | 25.14 | Isoamericanol B1 or related isomers (B2/C1/C2/(iso)princepin) | 8.67 | C27H26O9 | 493.1499 | 493.1509 | 493, 463, 329, 327, 165, 163 | 2.03 |
| 15 | 26.88 | Isoamericanol B1 or related isomers (B2/C1/C2/(iso)princepin) | 0.69 | C27H26O9 | 493.1499 | 493.1518 | 493, 329, 327, 165, 163 | 3.85 |
| 16 | 27.17 | Isoamericanol B1 or related isomers (B2/C1/C2/(iso)princepin) | 1.14 | C27H26O10 | 493.1499 | 493.1512 | 493, 477, 465, 329, 327, 299, 165, 163, 147, 135 | 2.64 |
| 17 | 31.28 | Isodiverniciasin A/diverniciasin B/diverniciasin C | 2.70 | C36H34O13 | 657.1972 | 657.2005 | 657, 493, 461, 329, 327, 165, 163 | 5.02 |
| 18 | 32.31 | Isodiverniciasin A/diverniciasin B/diverniciasin C | 2.25 | C36H34O13 | 657.1972 | 657.2018 | 657, 493, 461, 329, 327, 165, 163 | 7.00 |
| 19 | 33.40 | Isodiverniciasin A/diverniciasin B/diverniciasin C | 1.28 | C36H34O13 | 657.1972 | 657.1973 | 657, 493, 461, 329, 327, 166, 163 | 0.15 |
| 20 | 38.60 | (9S,10E,12Z,15Z)-9-Hydroperoxyoctadeca-10,12,15-Trienoic acid | 4.25 | C18H30O4 | 309.2066 | 309.2093 | 309, 291, 225, 209, 185 | 8.74 |
| 21 | 39.73 | Corchorifatty acid D | 0.84 | C18H28O4 | 307.1910 | 307.1930 | 307, 289, 265, 223, 185, 137 | 6.51 |
| 22 | 44.54 | Unknown | 10.25 | - | 339.2374 | - | 339, 183, 163, 147 | - |
| No. | tR a (min) | Content (%) | Components b | Formula | SC c |
|---|---|---|---|---|---|
| 1 | 6.56 | 0.06 | Octanoic acid | C8H16O2 | 93 |
| 2 | 7.97 | 0.04 | 2,4-Decadienal | C10H16O | 91 |
| 3 | 13.61 | 0.11 | Caryophyllene | C15H24 | 99 |
| 4 | 14.42 | 0.14 | (-)-α-Gurjunene | C15H24 | 91 |
| 5 | 15.21 | 0.08 | β-Copaene | C15H24 | 93 |
| 6 | 17.62 | 0.07 | (-)-Spathulenol | C15H24O | 90 |
| 7 | 26.15 | 2.09 | Palmitic acid | C16H32O2 | 93 |
| 8 | 26.56 | 3.82 | Ethyl palmitate | C18H36O2 | 99 |
| 9 | 29.69 | 14.36 | Ethyl linolate | C20H36O2 | 99 |
| 10 | 29.83 | 6.34 | Ethyl oleate | C20H38O2 | 97 |
| 11 | 30.06 | 2.38 | Stearic acid | C18H36O2 | 99 |
| 12 | 30.33 | 3.09 | Ethyl stearate | C20H40O2 | 99 |
| 13 | 33.00 | 38.44 | Ethyl α-linolenateor Ethyl γ-linolenate | C20H34O2 | 98 |
| 14 | 33.82 | 8.93 | Ethyl α-linolenate or Ethyl γ-linolenate | C20H34O2 | 98 |
| 15 | 34.99 | 0.7 | 2,2′-Methylenebis(4-methyl-6-tert-butylphenol) | C23H32O2 | 94 |
| 16 | 42.44 | 0.69 | 2-Linoleoylglycerol | C21H38O4 | 90 |
| 17 | 42.57 | 0.48 | 2-Monoolein | C21H40O4 | 97 |
| 18 | 42.78 | 0.31 | Heptacosane | C27H56 | 91 |
| 19 | 52.52 | 0.58 | γ-Tocopherol | C28H48O2 | 99 |
| 20 | 53.04 | 0.25 | Stigmasta-3,5-diene | C29H48 | 95 |
| 21 | 54.75 | 0.18 | Vitamin E | C29H50O2 | 99 |
| 22 | 57.36 | 0.14 | Dotriacontane | C32H6O6 | 96 |
| 23 | 57.72 | 0.23 | Stigmasterol | C29H48O | 99 |
| 24 | 59.23 | 1.58 | β-Sitosterol | C29H50O | 92 |
| 25 | 59.74 | 0.73 | Tritriacontane | C33H68 | 99 |
| 26 | 60.52 | 0.09 | 4,22-stigmastadiene-3-one | C29H46O | 96 |
| 27 | 61.76 | 0.96 | Stigmast-4-en-3-one | C29H48O | 98 |
| 28 | 63.36 | 0.34 | Pentatriacontane | C35H72 | 97 |
| 29 | 64.53 | 0.36 | Stigmastane-3,6-dione | C29H48O2 | 91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Chen, G.; Lü, S.; Zhang, L.; Guo, M. Insecticidal Activities Against Odontotermes formosanus and Plutella xylostella and Corresponding Constituents of Tung Meal from Vernicia fordii. Insects 2021, 12, 425. https://doi.org/10.3390/insects12050425
Zhang H, Chen G, Lü S, Zhang L, Guo M. Insecticidal Activities Against Odontotermes formosanus and Plutella xylostella and Corresponding Constituents of Tung Meal from Vernicia fordii. Insects. 2021; 12(5):425. https://doi.org/10.3390/insects12050425
Chicago/Turabian StyleZhang, Hui, Guilin Chen, Shiyou Lü, Lin Zhang, and Mingquan Guo. 2021. "Insecticidal Activities Against Odontotermes formosanus and Plutella xylostella and Corresponding Constituents of Tung Meal from Vernicia fordii" Insects 12, no. 5: 425. https://doi.org/10.3390/insects12050425
APA StyleZhang, H., Chen, G., Lü, S., Zhang, L., & Guo, M. (2021). Insecticidal Activities Against Odontotermes formosanus and Plutella xylostella and Corresponding Constituents of Tung Meal from Vernicia fordii. Insects, 12(5), 425. https://doi.org/10.3390/insects12050425