Mind the Wound!—Fruit Injury Ranks Higher than, and Interacts with, Heterospecific Cues for Drosophila suzukii Oviposition
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fly Species and Culture Conditions
2.2. Host Fruit Categories and General Experimental Design
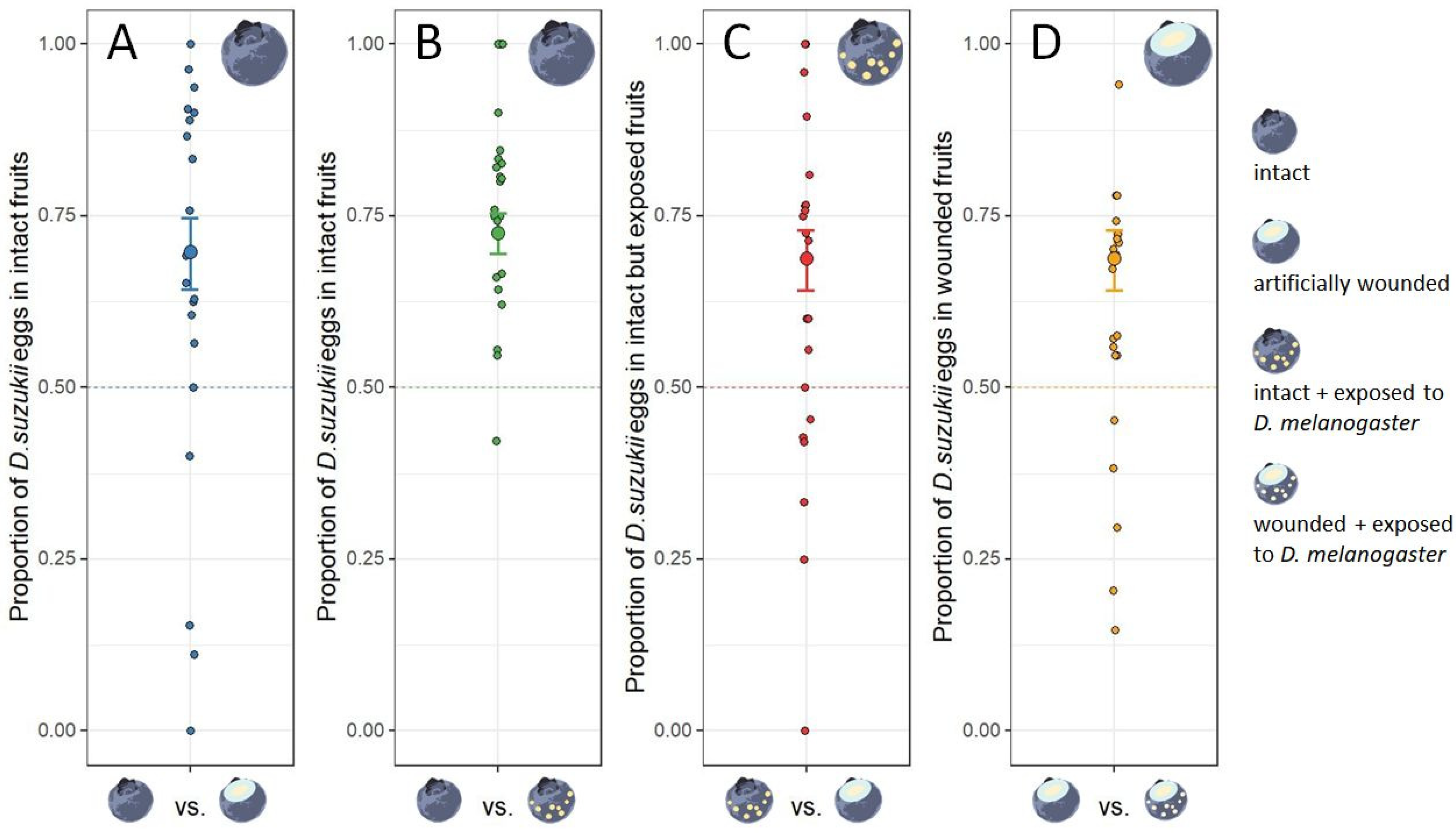
2.3. Experiment 1: Drosophila suzukii Egg-Laying Response to Fruits with Heterospecific Egg-Laying Cues
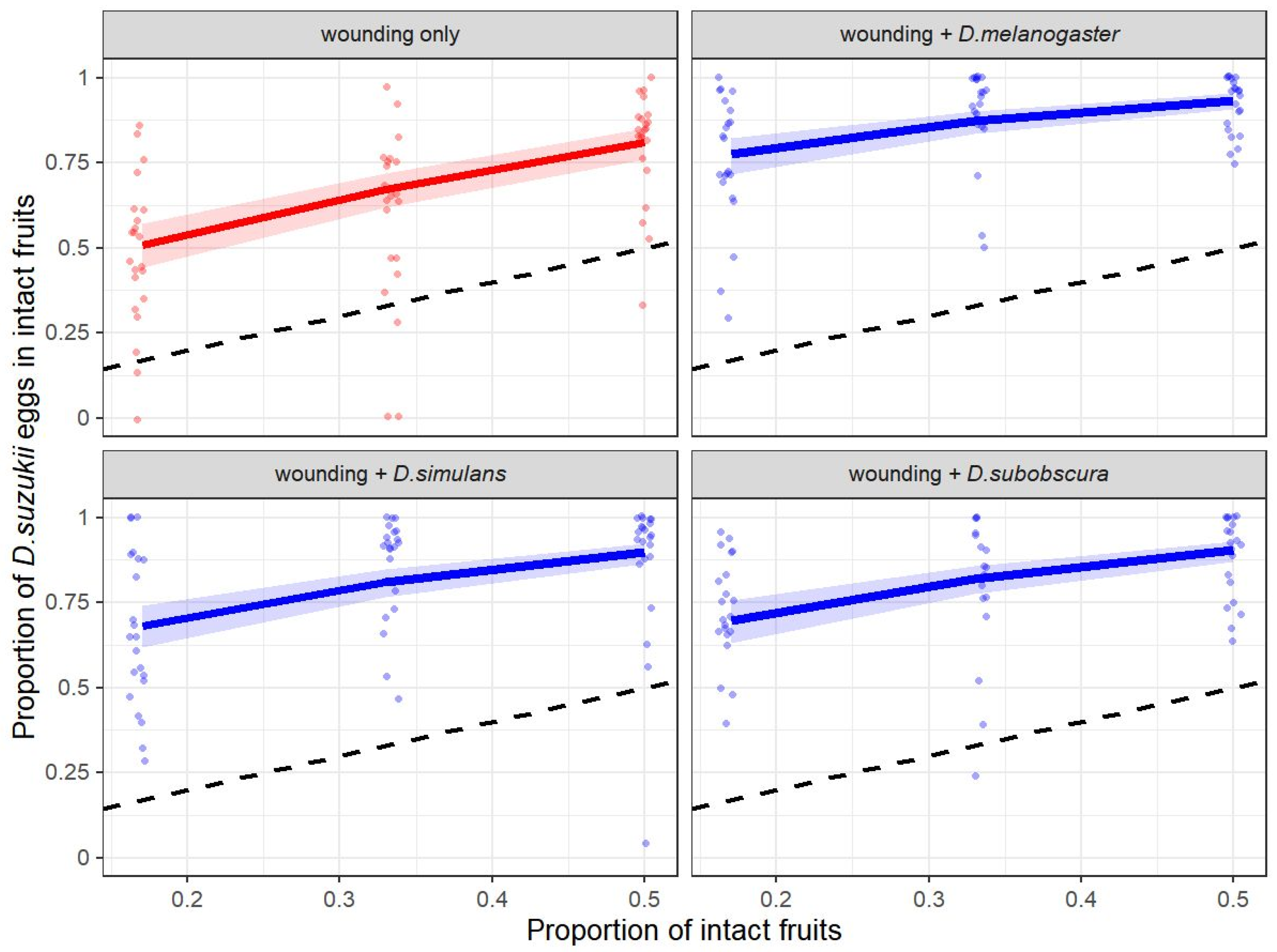
2.4. Experiment 2: Drosophila suzukii Egg-Laying Response to Heterospecific and Fruit-Wounding Cues
2.5. Statistical Analyses
3. Results
3.1. Experiment 1: Drosophila suzukii Egg-Laying Response to Fruits with Heterospecific Egg-Laying Cues
3.2. Experiment 2: Drosophila suzukii Egg-Laying Response to Heterospecific and Fruit-Wounding Cues
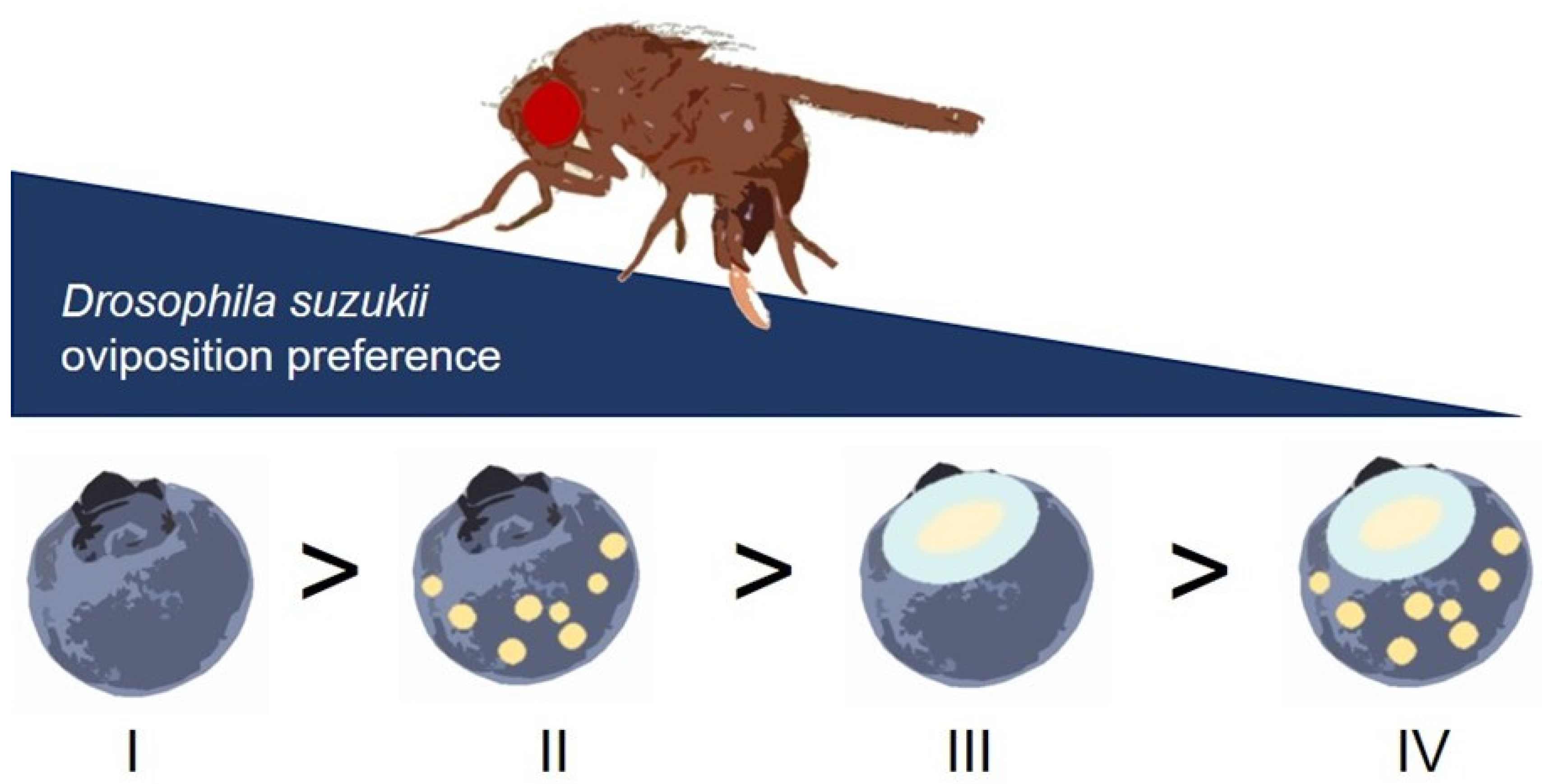
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Explanatory Treatment Variables | Estimate | Std. Error | t-Value | p-Value | |
|---|---|---|---|---|---|
| logit (1/6) 2 intact vs. 10 treated fruits | wounding only | 1.562 | 0.173 | 9.04 | <0.001 |
| D. melanogaster | 2.818 | 0.207 | 13.64 | <0.001 | |
| D. simulans | 2.206 | 0.191 | 11.53 | <0.001 | |
| D. subobscura | 2.641 | 0.180 | 14.65 | <0.001 | |
| logit (1/3) 4 intact vs. 8 treated fruits | wounding only | 1.350 | 0.188 | 7.25 | <0.001 |
| D. melanogaster | 2.709 | 0.304 | 8.93 | <0.001 | |
| D. simulans | 2.466 | 0.255 | 9.66 | <0.001 | |
| D. subobscura | 2.098 | 0.266 | 7.88 | <0.001 | |
| logit (1/2) 6 intact vs. 6 treated fruits | wounding only | 1.661 | 0.182 | 9.11 | <0.001 |
| D. melanogaster | 2.528 | 0.222 | 11.40 | <0.001 | |
| D. simulans | 2.061 | 0.394 | 5.24 | <0.001 | |
| D. subobscura | 2.044 | 0.254 | 8.05 | <0.001 | |
References
- Forsman, J.T.; Mönkkönen, M.; Korpimäki, E.; Thomson, R.L. Mammalian nest predator feces as a cue in avian habitat selection decisions. Behav. Ecol. 2012, 24, 262–266. [Google Scholar] [CrossRef]
- Nersesian, C.L.; Banks, P.B.; McArthur, C. Behavioural responses to indirect and direct predator cues by a mammalian herbivore, the common brushtail possum. Behav. Ecol. Sociobiol. 2012, 66, 47–55. [Google Scholar] [CrossRef]
- Lecchini, D.; Nakamura, Y. Use of chemical cues by coral reef animal larvae for habitat selection. Aquat. Biol. 2013, 19, 231–238. [Google Scholar] [CrossRef]
- Fader, J.E.; Juliano, S.A. Oviposition habitat selection by container-dwelling mosquitoes: Responses to cues of larval and detritus abundances in the field. Ecol. Entomol. 2014, 39, 245–252. [Google Scholar] [CrossRef]
- Shaw, B.; Brain, P.; Wijnen, H.; Fountain, M.T. Reducing Drosophila suzukii emergence through inter-species competition. Pest Manag. Sci. 2018, 74, 1466–1471. [Google Scholar] [CrossRef] [PubMed]
- Kidera, H.; Takahashi, K.H. Chemical cues from competitors change the oviposition preference of Drosophila suzukii. Entomol. Exp. et Appl. 2020, 168, 304–310. [Google Scholar] [CrossRef]
- Poyet, M.; Le Roux, V.; Gibert, P.; Meirland, A.; Prévost, G.; Eslin, P.; Chabrerie, O. The Wide potential trophic niche of the asiatic fruit fly Drosophila suzukii: The key of its invasion success in temperate Europe? PLoS ONE 2015, 10, e0142785. [Google Scholar] [CrossRef] [PubMed]
- Atallah, J.; Teixeira, L.; Salazar, R.; Zaragoza, G.; Kopp, A. The making of a pest: The evolution of a fruit-penetrating ovipositor in Drosophila suzukii and related species. Proc. R. Soc. B Boil. Sci. 2014, 281, 20132840. [Google Scholar] [CrossRef]
- Dos Santos, L.A.; Mendes, M.F.; Krüger, A.P.; Blauth, M.L.; Gottschalk, M.S.; Garcia, F.R.M. Global potential distribution of Drosophila suzukii (Diptera, Drosophilidae). PLoS ONE 2017, 12, e0174318. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, D.; Larsson, M.C.; Anderson, P. Insect host plant selection in complex environments. Curr. Opin. Insect Sci. 2015, 8, 1–7. [Google Scholar] [CrossRef]
- Kienzle, R.; Groß, L.B.; Caughman, S.; Rohlfs, M. Resource use by individual Drosophila suzukii reveals a flexible preference for oviposition into healthy fruits. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Wajnberg, E. Time allocation strategies in insect parasitoids: From ultimate predictions to proximate behavioral mechanisms. Behav. Ecol. Sociobiol. 2006, 60, 589–611. [Google Scholar] [CrossRef]
- Mitsui, H.; Takahashi, K.H.; Kimura, M.T. Spatial distributions and clutch sizes of Drosophila species ovipositing on cherry fruits of different stages. Popul. Ecol. 2006, 48, 233–237. [Google Scholar] [CrossRef]
- Trienens, M.; Keller, N.P.; Rohlfs, M. Fruit, flies and filamentous fungi-experimental analysis of animal-microbe competition using Drosophila melanogaster and Aspergillus mould as a model system. Oikos 2010, 119, 1765–1775. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development for R. RStudio; PBC: Boston, MA, USA, 2020; Available online: http://www.rstudio.com/ (accessed on 21 April 2021).
- Keesey, I.W.; Koerte, S.; Retzke, T.; Haverkamp, A.; Hansson, B.S.; Knaden, M. Adult frass provides a pheromone signature for Drosophila feeding and aggregation. J. Chem. Ecol. 2016, 42, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Belloni, V.; Galeazzi, A.; Bernini, G.; Mandrioli, M.; Versace, E.; Haase, A. Evolutionary compromises to metabolic toxins: Ammonia and urea tolerance in Drosophila suzukii and Drosophila melanogaster. Physiol. Behav. 2018, 191, 146–154. [Google Scholar] [CrossRef]
- Sato, A.; Tanaka, K.M.; Yew, J.Y.; Takahashi, A. Drosophila suzukii avoidance of microbes in oviposition choice. R. Soc. Open Sci. 2021, 8, 201601. [Google Scholar] [CrossRef] [PubMed]
- Karageorgi, M.; Bräcker, L.B.; Lebreton, S.; Minervino, C.; Cavey, M.; Siju, K.; Kadow, I.C.G.; Gompel, N.; Prud’homme, B. Evolution of multiple sensory systems drives novel egg-laying behavior in the fruit pest Drosophila suzukii. Curr. Biol. 2017, 27, 847–853. [Google Scholar] [CrossRef]
- Guo, L.; Zhou, Z.; Mao, F.; Fan, X.; Liu, G.; Huang, J.; Qiao, X. Identification of potential mechanosensitive ion channels involved in texture discrimination during Drosophila suzukii egg-laying behaviour. Insect Mol. Biol. 2020, 29, 444–451. [Google Scholar] [CrossRef]
- Myung, K.; Hamilton-Kemp, T.R.; Archbold, D.D. Biosynthesis of trans-2-hexenal in response to wounding in strawberry fruit. J. Agric. Food Chem. 2006, 54, 1442–1448. [Google Scholar] [CrossRef] [PubMed]
- Alkema, J.T.; Dicke, M.; Wertheim, B. Context-Dependence and the development of push-pull approaches for integrated management of Drosophila suzukii. Insects 2019, 10, 454. [Google Scholar] [CrossRef] [PubMed]
- Stewart, T.J.; Wang, X.-G.; Molinar, A.; Daane, K.M. Factors limiting peach as a potential host for Drosophila suzukii (Diptera: Drosophilidae). J. Econ. Entomol. 2014, 107, 1771–1779. [Google Scholar] [CrossRef] [PubMed]
- Weißinger, L.; Samuel, N.; Breuer, M.; Müller, C. Effects of variety and grape berry condition of Vitis vinifera on preference behavior and performance of Drosophila suzukii. Insects 2019, 10, 432. [Google Scholar] [CrossRef] [PubMed]
- Entling, W.; Anslinger, S.; Jarausch, B.; Michl, G.; Hoffmann, C. Berry skin resistance explains oviposition preferences of Drosophila suzukii at the level of grape cultivars and single berries. J. Pest Sci. 2019, 92, 477–484. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kienzle, R.; Rohlfs, M. Mind the Wound!—Fruit Injury Ranks Higher than, and Interacts with, Heterospecific Cues for Drosophila suzukii Oviposition. Insects 2021, 12, 424. https://doi.org/10.3390/insects12050424
Kienzle R, Rohlfs M. Mind the Wound!—Fruit Injury Ranks Higher than, and Interacts with, Heterospecific Cues for Drosophila suzukii Oviposition. Insects. 2021; 12(5):424. https://doi.org/10.3390/insects12050424
Chicago/Turabian StyleKienzle, Renate, and Marko Rohlfs. 2021. "Mind the Wound!—Fruit Injury Ranks Higher than, and Interacts with, Heterospecific Cues for Drosophila suzukii Oviposition" Insects 12, no. 5: 424. https://doi.org/10.3390/insects12050424
APA StyleKienzle, R., & Rohlfs, M. (2021). Mind the Wound!—Fruit Injury Ranks Higher than, and Interacts with, Heterospecific Cues for Drosophila suzukii Oviposition. Insects, 12(5), 424. https://doi.org/10.3390/insects12050424







