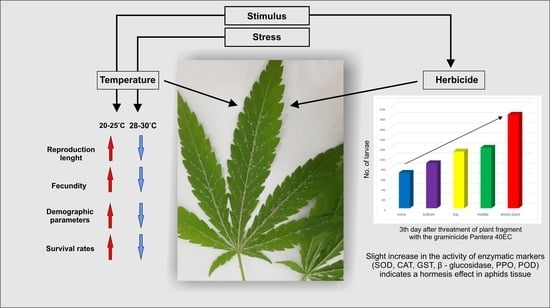
Mild Abiotic Stress Affects Development and Stimulates Hormesis of Hemp Aphid Phorodon cannabis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Host Plants
2.3. Effect of Temperature on Survival, Development, Fecundity and Demographic Parameters of Phorodon cannabis
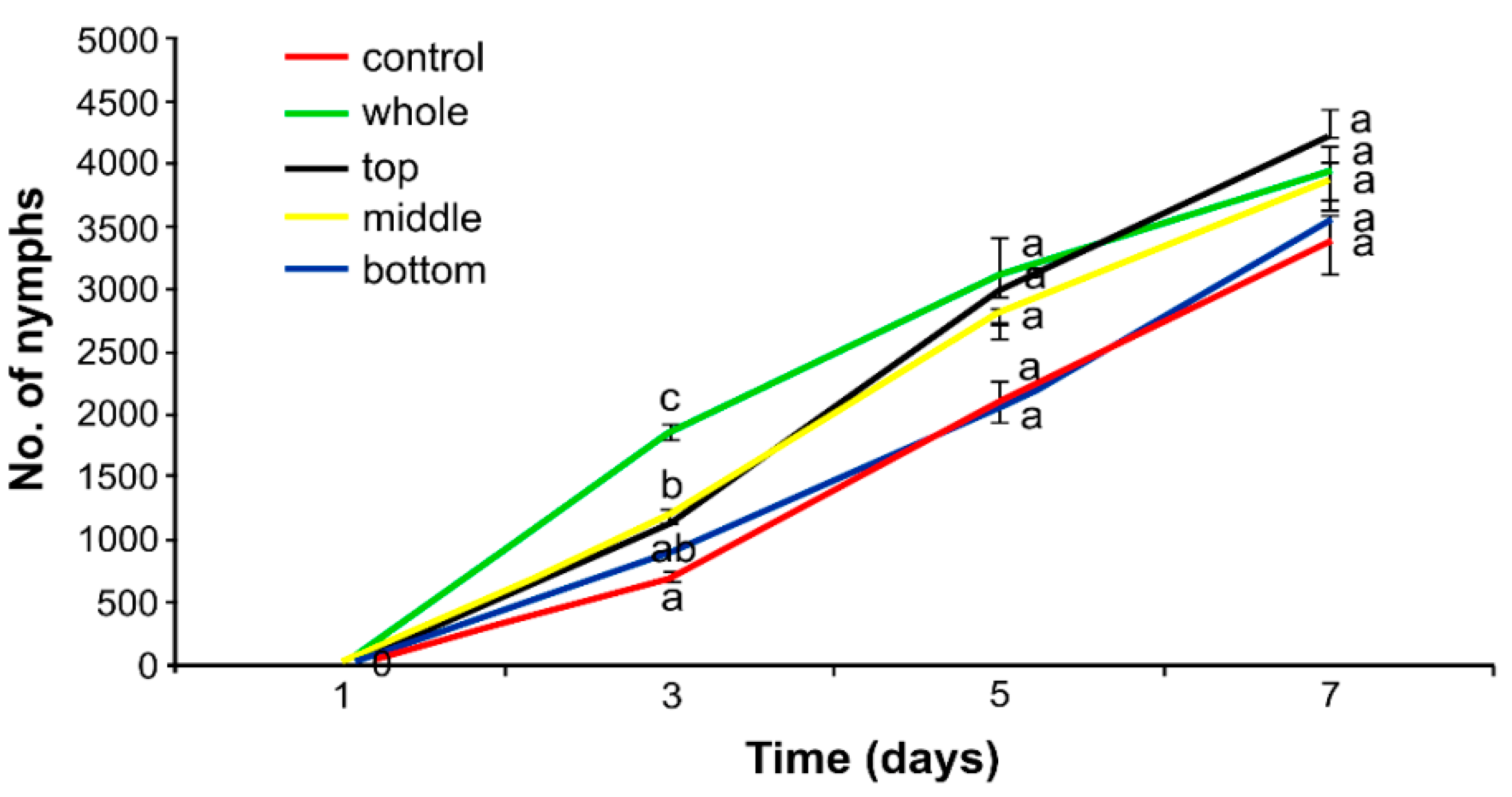
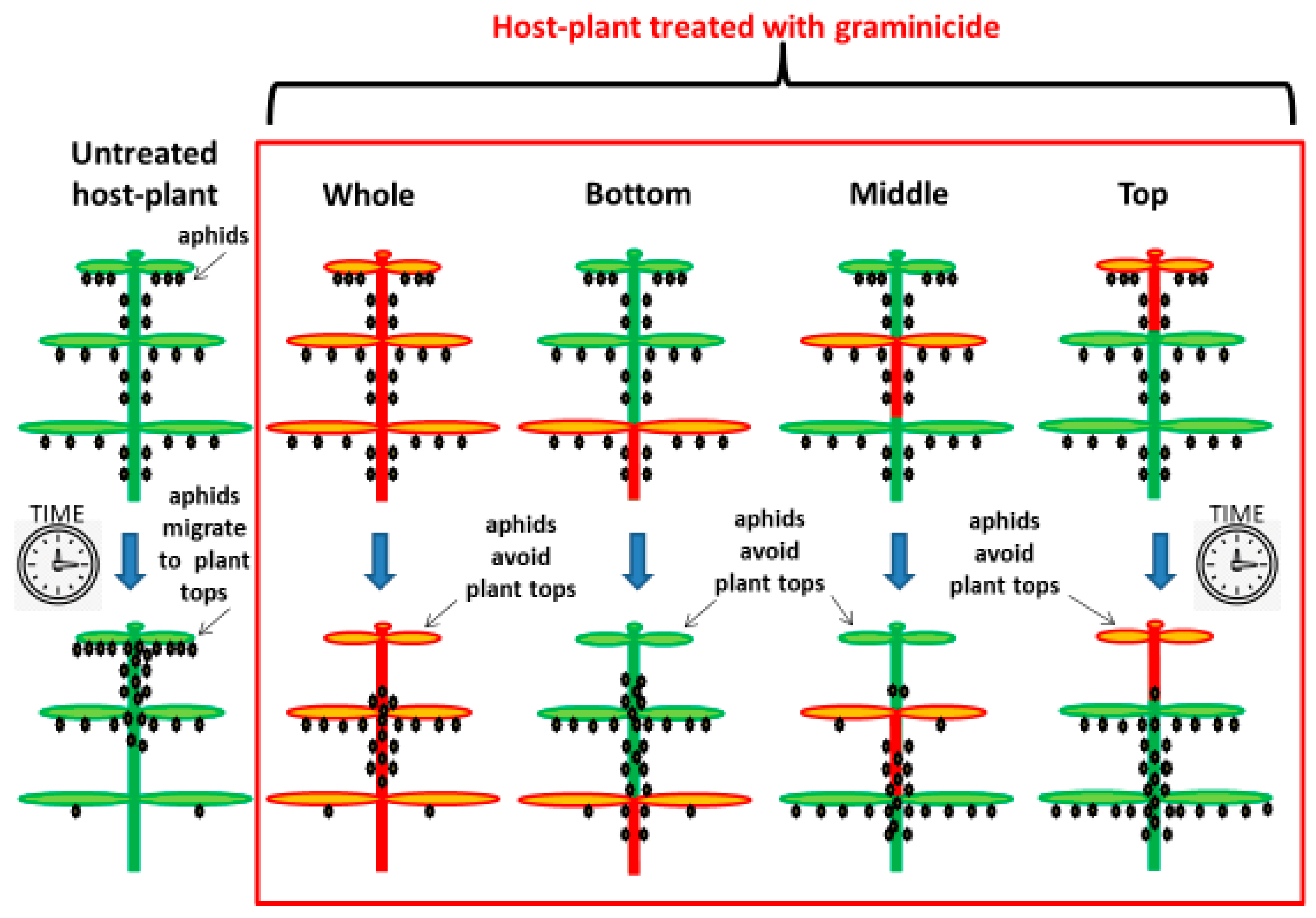
2.4. Effect of Herbicide on the Aphid Population
2.5. Effect of Herbicide on the Enzymatic Activity in Aphid Bodies
2.6. Statistical Analyses
3. Results
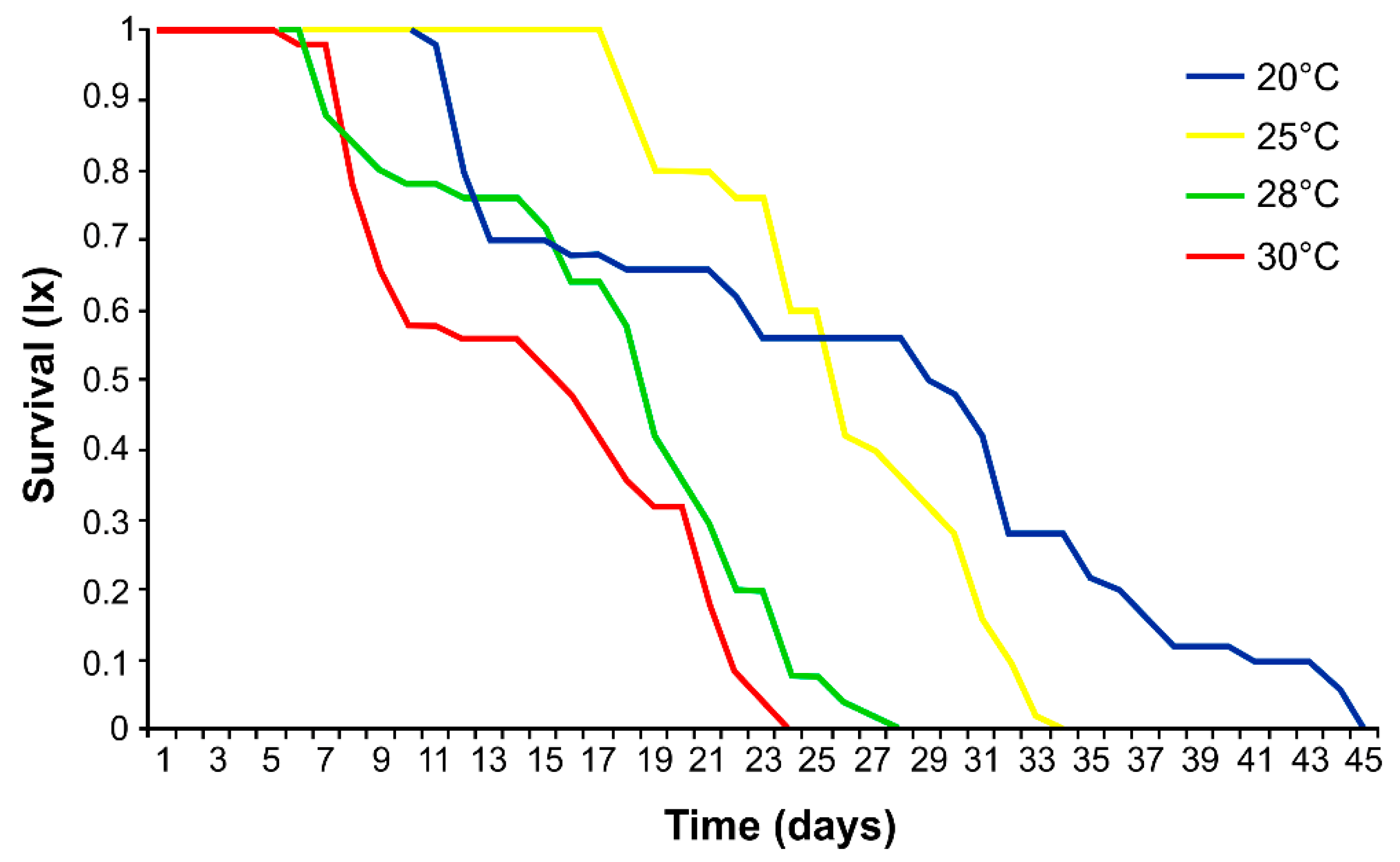
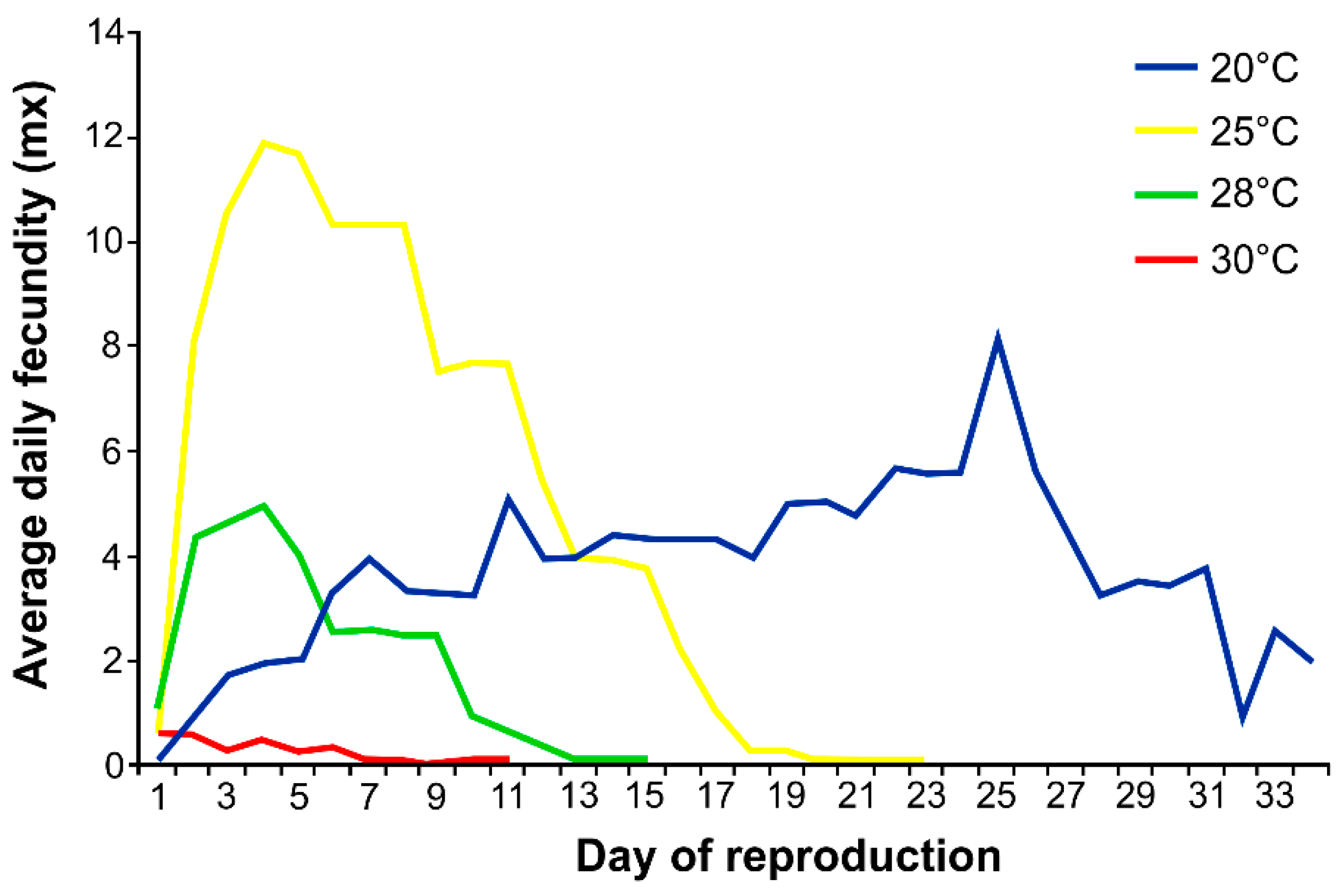
3.1. Effects of Temperature on Aphid Development, Fecundity and Mortality
3.2. Effect of Herbicide on the Aphid Population
3.3. Effect of Herbicide on the Aphid Enzyme Activity
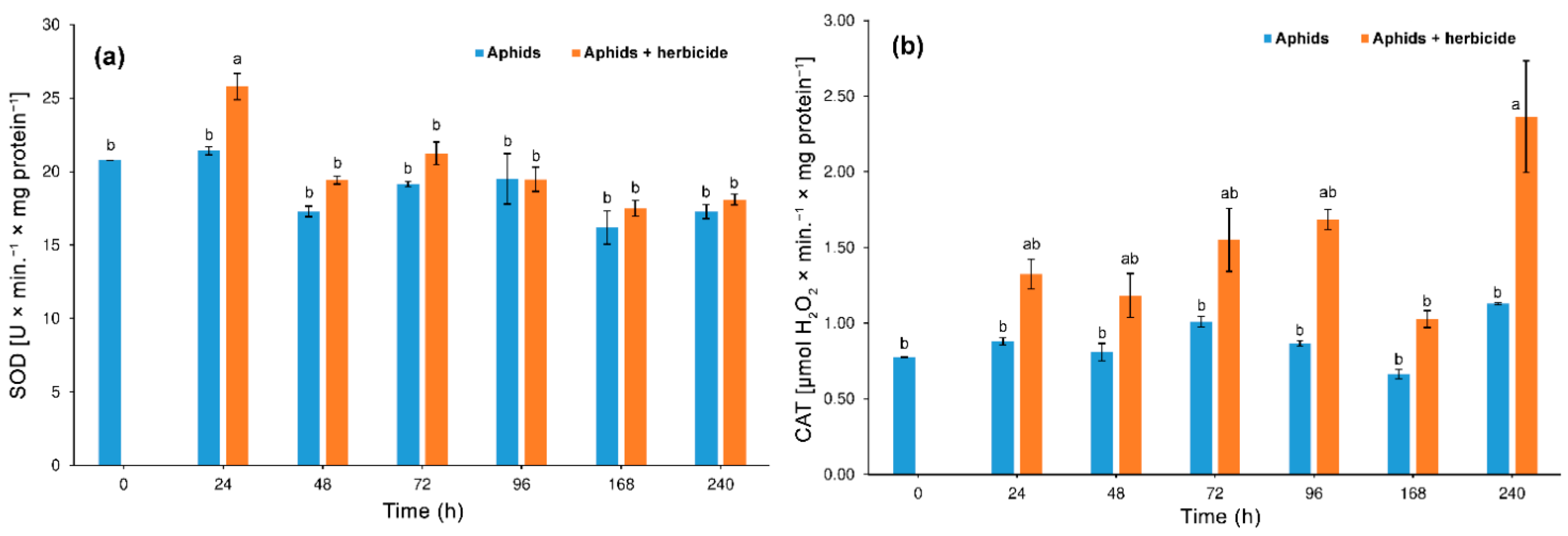
3.3.1. Superoxide Dismutase (SOD) and Catalase (CAT) Activity
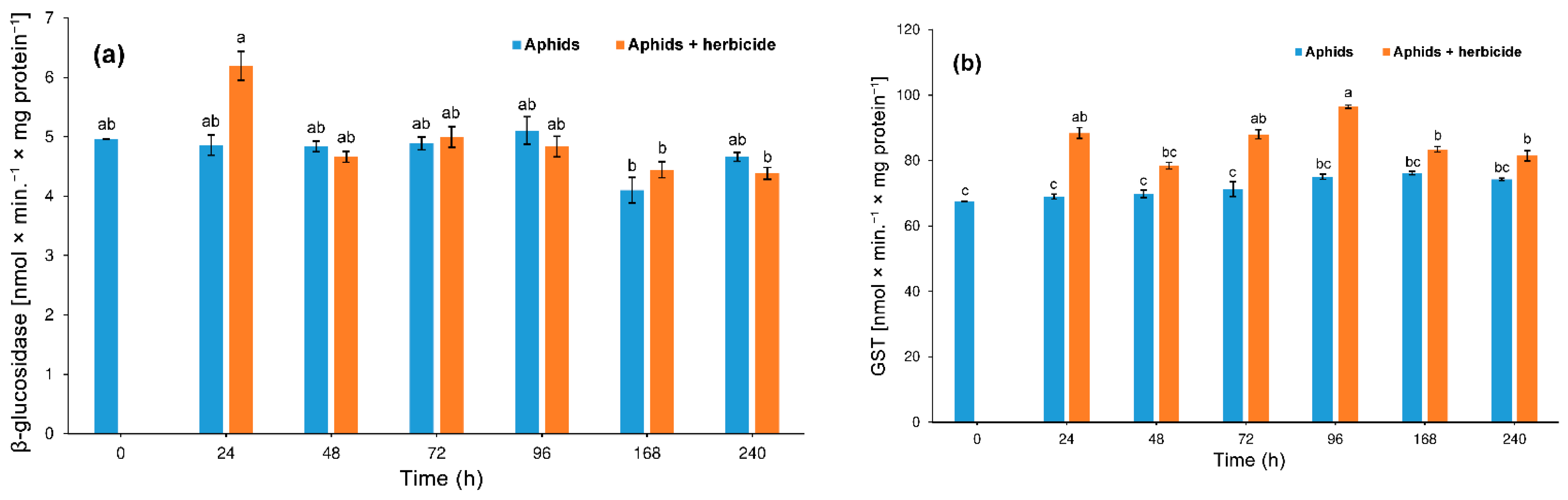
3.3.2. β-Glucosidase and S-Glutathione Transferase (GST)
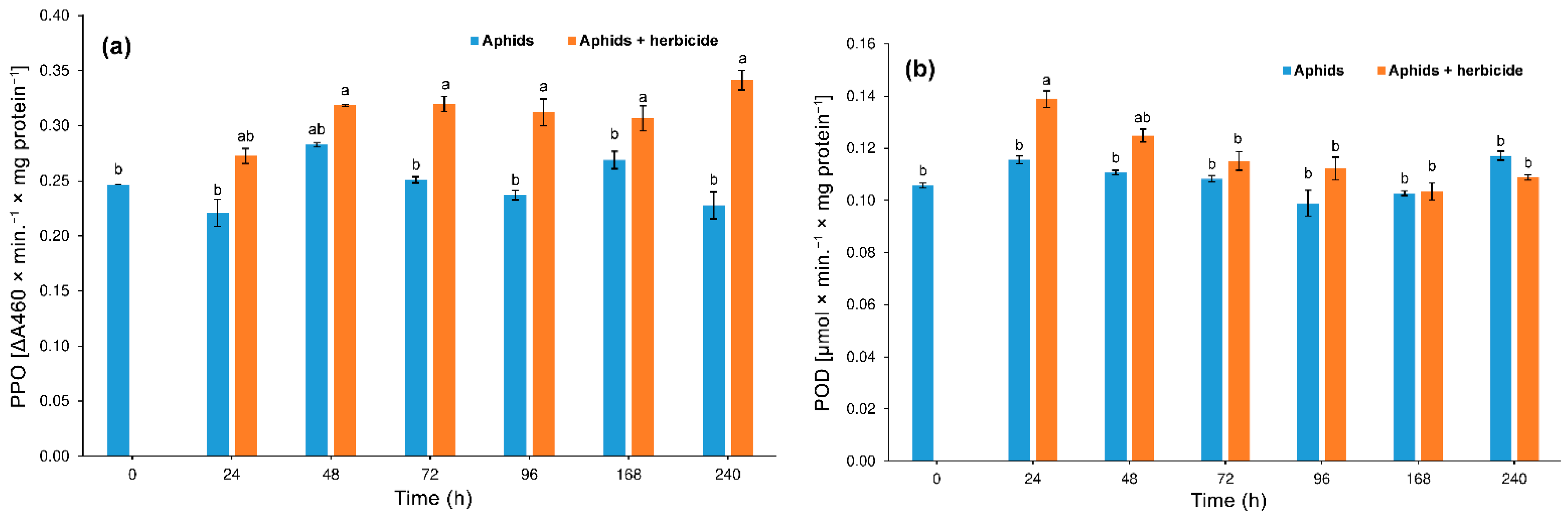
3.3.3. Polyphenol Oxidase (PPO) and Peroxidase (POD)
4. Discussion
4.1. Effect of Temperature on Survival, Development, Fecundity and Demographic Parameters of Phorodon cannabis
4.2. Hormetic Effect of the Herbicide on Hemp Aphid Population
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jaworski, T.; Hilszczański, J. The effect of temperature and humidity changes on insects development their impact on forest ecosystems in the expected climate change. For. Res. Pap. 2013, 74, 345–355. [Google Scholar] [CrossRef]
- Bale, J.S.; Masters, G.J.; Hodkinson, I.D.; Awmack, C.; Bezemer, T.M.; Brown, V.K.; Butterfield, J.; Buse, A.; Coulson, J.C.; Farrar, J.; et al. Herbivory in global climate change research: Direct effects of rising temperature on insect herbivores. Glob. Chang. Biol. 2002, 8, 1–16. [Google Scholar] [CrossRef]
- Dixon, A.F.G. Parthenogenetic reproduction and the rate of increase in aphids. In Aphids Their Biology, Natural Enemies and Control; Minks, A.K., Harrewijn, P., Eds.; Elsevier: Amsterdam, The Netherland, 1987; Volume A, pp. 269–287. [Google Scholar]
- Mehrparvar, M.; Hatami, B. Effect of temperature on some biological parameters of an Iranian population of the rose aphid, Macrosiphum rosae (Hemiptera: Aphididae). Eur. J. Entomol. 2007, 104, 631–634. [Google Scholar] [CrossRef]
- Ahn, J.J.; Cho, J.R.; Kim, J.H.; Seo, B.Y. Thermal effects on the population parameters and growth of Acyrthosiphon pisum (Harris) (Hemiptera: Aphididae). Insects 2020, 11, 481. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.; Su, H.Y. Age-stage, two-sex life tables of Aphidius gifuensis (Ashmead) (Hymenoptera: Braconidae) and its host Myzus persicae (Sulzer) (Homoptera: Aphididae) with mathematical proof of the relationship between female fecundity and the net reproductive rate. Environ. Entomol. 2006, 35, 10–21. [Google Scholar] [CrossRef]
- Cividanes, F.J.; Santos-Cividanes, T.M. Predicting the occurrence of alate aphids in Brassicaceae. Pesqui. Agropecu. Bras. 2012, 47, 505–510. [Google Scholar] [CrossRef] [Green Version]
- Dawidziuk, A.; Kaczmarek, J.; Podleśna, A.; Kasprzyk, I.; Jędryczka, M. Influence of meteorological parameters on Leptosphaeria maculans and L. biglobosa spore release in central and eastern Poland. Grana 2012, 51, 240–248. [Google Scholar] [CrossRef]
- Mackoś-Iwaszko, E.; Lubiarz, M.; Karczmarz, K. The impact of urban conditions on the occurrence of aphids on Acer platanoides L. Acta Sci. Pol. Hortorum Cultus 2015, 14, 189–207. [Google Scholar]
- Hullé, M.; d’Acier, A.C.; Bankhead-Dronnet, S.; Harrington, R. Aphids in the face of global changes. Comptes Rendus Biol. 2010, 333, 497–503. [Google Scholar] [CrossRef]
- Borowiak-Sobkowiak, B.; Durak, R.; Wilkaniec, B. Morphology, biology and behavioral aspects of Aphis craccivora (Hemiptera: Aphididae) on Robinia pseudoacacia. Acta Sci. Pol. Hortorum Cultus 2017, 16, 39–49. [Google Scholar]
- Durak, R.; Dampc, J.; Dampc, J. Role of temperature in the interaction between Japanese quince Chaenomeles japonica and herbivorous insect Aphis pomi (Hemiptera: Aphidoidea). Environ. Exp. Bot. 2020, 176, 1041000. [Google Scholar] [CrossRef]
- Dixon, A.F.G. Aphid Ecology: An Optimization Approach, 2nd ed.; Chapman and Hall: London, UK, 1998; pp. 1–300. [Google Scholar]
- Durak, R.; Węgrzyn, E.; Leniowski, K. Do all aphids benefit from climate warming? An effect of temperature increase on a native species of temperate climatic zone Cinara juniperi. Ethol. Ecol. Evol. 2016, 28, 188–201. [Google Scholar] [CrossRef]
- Wachowska, U.; Irzykowski, W.; Jędryczka, M. Agrochemicals: Effect on genetic resistance in yeasts colonizing winter wheat kernels. Ecotoxicol. Environ. Saf. 2018, 162, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Stark, J.D.; Banks, J.E. Population-level effects of pesticides and other toxicants on arthropods. Annu. Rev. Entomol. 2003, 48, 505–519. [Google Scholar] [CrossRef]
- Cutler, G.C. Insects, insecticides and hormesis: Evidence and considerations for study. Dose-Response 2013, 11, 154–177. [Google Scholar] [CrossRef]
- Ayyanath, M.-M.; Cutler, G.C.; Scott-Dupree, C.D.; Sibley, P.K. Transgenerational shifts in reproduction hormesis in green peach aphid exposed to low concentrations of imidacloprid. PLoS ONE 2013, 8, e74532. [Google Scholar] [CrossRef]
- Kraus, E.C.; Stout, M.J. Direct and indirect effects of herbicides on insect herbivores in rice, Oryza sativa. Sci. Rep. 2019, 9, 6998. [Google Scholar] [CrossRef] [PubMed]
- Krzyżanowski, R.; Bednarczyk, I.; Cudziło-Abramczuk, J. Effect of chlorophenoxy herbicides residues on probing behavior of cereal aphid. Agron. Sci. 2018, 73, 29–36. [Google Scholar] [CrossRef]
- Gupta, G.; Bhattacharya, A.K. Assessing toxicity of post-emergence herbicides to the Spilarctia obliqua Walker (Lepidoptera: Arctiidae). J. Pest Sci. 2008, 81, 9–15. [Google Scholar] [CrossRef]
- Hill, M.P.; Coetzee, J.A.; Ueckermann, C. Toxic efect of herbicides used for water hyacinth control on two insects released for its biological control in South Africa. Biocontrol Sci. Technol. 2012, 22, 1321–1333. [Google Scholar] [CrossRef] [Green Version]
- Lipok, J. Dual action of phosphonate herbicides in plants affected by herbivore—Model study on black bean aphid Aphis fabae rearing on broad bean Vicia faba plants. Ecotoxicol. Environ. Saf. 2009, 72, 1701–1706. [Google Scholar] [CrossRef]
- Dampc, J.; Kula-Maximenko, M.; Molon, M.; Durak, R. Enzymatic defense response of apple aphid Aphis pomi to increased temperature. Insects 2020, 11, 436. [Google Scholar] [CrossRef] [PubMed]
- Després, L.; David, J.P.; Gallet, C. The evolutionary ecology of insect resistance to plant chemicals. Trends Ecol. Evol. 2007, 22, 298–307. [Google Scholar] [CrossRef]
- Durak, R.; Molon, M.; Durak, T.; Chrzanowski, G. The enzymatic markers of the adaptation of Cinara tujafilina to changing the host plant. Ethol. Ecol. Evol. 2018, 30, 416–429. [Google Scholar] [CrossRef]
- Lopez-Martinez, G.; Elnitsky, M.A.; Benoit, J.B.; Lee, R.E.; Denlinger, D.L. High resistance to oxidative damage in the Antarctic midge Belgica antarctica, and developmentally linked expression of genes encoding superoxide dismutase, catalase and heat shock proteins. Insect Biochem. Mol. Biol. 2008, 38, 796–804. [Google Scholar] [CrossRef]
- Lalouette, L.; Williams, C.M.; Hervant, F.; Sinclair, B.J.; Renault, D. Metabolic rate and oxidative stress in insects exposed to low temperature thermal fluctuations. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2011, 158, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, T.; Matsumoto, H.; Hayakawa, Y. Heat stress hardening of oriental armyworms is induced by a transient elevation of reactive oxygen species during sublethal stress. Arch. Insect Biochem. Physiol. 2017, 96, e21421. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Fu, W.; Li, N.; Zhang, F.; Liu, T.X. Antioxidant responses of Propylaea japonica (Coleoptera: Coccinellidae) exposed to high temperature stress. J. Insect Physiol. 2015, 73, 47–52. [Google Scholar] [CrossRef]
- Krishnan, N.; Kodrík, D.; Turanli, F.; Sehnal, F. Stage-specific distribution of oxidative radicals and antioxidant enzymes in the midgut of Leptinotarsa decemlineata. J. Insect Physiol. 2007, 53, 67–74. [Google Scholar] [CrossRef]
- Łukasik, I.; Goławska, S. Effect of host plant on levels of reactive oxygen species andantioxidants in the cereal aphids Sitobion avenae and Rhopalosiphum padi. Biochem. Syst. Ecol. 2013, 51, 232–239. [Google Scholar] [CrossRef]
- Francis, F.; Vanhaelen, N.; Haubruge, E. Glutathione S-transferases in the adaptation to plant secondary metabolites in the Myzus persicae aphid. Arch. Insect Biochem. Physiol. 2005, 58, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Chrzanowski, G.; Leszczyński, B.; Czerniewicz, P.; Sytykiewicz, H.; Matok, H.; Krzyzanowski, R.; Sempruch, C. Effect of phenolic acids from black currant, sour cherry and walnut on grain aphid (Sitobion avenae F.) development. Crop Prot. 2012, 35, 71–77. [Google Scholar] [CrossRef]
- FAO. Food and agriculture organization of the united nations. In The Future of Food and Agriculture: Trends and Challenges; FAO: Rome, Italy, 2017; p. 180. [Google Scholar]
- Schluttenhofer, C.; Yuan, L. Challenges towards revitalizing hemp: A multifaceted crop. Trends Plants Sci. 2017, 22, 917–929. [Google Scholar] [CrossRef] [Green Version]
- Grotenhermen, F.; Müller-Vahl, K. Medicinal uses of marijuana and cannabinoids. CRC Crit. Rev. Plant Sci. 2016, 35, 378–405. [Google Scholar] [CrossRef]
- Cantele, C.; Bertolino, M.; Bakro, F.; Giordano, M.; Jędryczka, M.; Cardenia, V. Antioxidant effects of hemp (Cannabis sativa L.) inflorescence extract in stripped linseed oil. Antioxidants 2020, 9, 1131. [Google Scholar] [CrossRef] [PubMed]
- Bakro, F.; Jedryczka, M.; Wielgusz, K.; Sgorbini, B.; Inchingolo, R.; Cardenia, V. Simultaneous determination of terpenes and cannabidiol in hemp (Cannabis sativa L.) by fast gas chromatography with flame ionization detection. J. Sep. Sci. 2020, 43, 2817–2826. [Google Scholar] [CrossRef] [PubMed]
- McPartland, J.M. Cannabis pests. J. Intl. Hemp Assoc. 1996, 3, 52–55. [Google Scholar]
- Small, E. Cannabis: A Complete Guide; CRC Press: Boca Raton, FL, USA, 2017; pp. 1–597. [Google Scholar]
- Bakro, F.; Wielgusz, K.; Bunalski, M.; Jędryczka, M. An overview of pathogen and insect threats to fibre and oilseed hemp (Cannabis sativa L.) and methods for their biocontrol. Integr. Control Oilseed Crop. IOBC-WPRS Bull. 2018, 136, 9–20. [Google Scholar]
- McPartland, J.M.; Clarke, R.C.; Watson, D.P. Hemp Diseases and Pests Management and Biocontrol; CAB International, CABI Publishing: Wallingford, Oxfordshire, UK, 2000; pp. 1–251. [Google Scholar]
- Cranshaw, W.S.; Schreiner, M.; eBritt, K.; Kuhar, T.P.; McPartland, J.; Grant, J. Developing insect pest management systems for hemp in the United States: A work in progress. J. Integr. Pest Manag. 2019, 10, 26. [Google Scholar] [CrossRef]
- Blackman, R.L.; Eastop, V.F. Aphids on the World’s Herbaceous Plants and Shrubs; Wiley: London, UK, 2006; pp. 1–1456. [Google Scholar]
- Cranshaw, W.; Halbert, S.; Favret, C.; Britt, K.; Miller, G. Phorodon cannabis Passerini (Hemiptera: Aphididae), a newly recognized pest in North America found on industrial hemp. Insecta Mundi 2018, 662, 1–12. [Google Scholar] [CrossRef]
- Heie, O.E. The Aphidoidea (Hemiptera) of Fennoscandia and Denmark. V, Family Aphididae, Part 2 of Tribe Macrosiphini of Subfamily Aphidinae; Brill: Leiden, The Netherlands, 1994; pp. 1–242. [Google Scholar]
- Halbert, S. Phorodon cannabis, hemp aphid, a new Western Hemisphere record. TRI-OLOGY Ent. Sect. 2016, 55, 6–14. [Google Scholar]
- Birch, L.C. The intrinsic rate of natural increase of an insect population. J. Anim. Ecol. 1948, 17, 15–26. [Google Scholar] [CrossRef]
- Wyatt, I.J.; White, P.F. Simple estimation of intrinsic rates for aphids and tetranychid mites. J. Appl. Ecol. 1977, 14, 757–766. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with Folin reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Wang, Y.; Oberley, L.W.; Murhammer, D.W. Antioxidant defense systems of two lepidopteran insect cell lines. Free Radic. Biol. Med. 2001, 30, 1254–1262. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in Vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, C. α-d-Glucosidase in the serum of the american cockroach, Periplaneta americana. Insect Biochem. 1979, 9, 199–204. [Google Scholar] [CrossRef]
- Leszczynski, B.; Dixon, A.F.G. Resistance of cereals to aphids: The interaction between hydroxamic acids and glutathione S-transferases in the grain aphid Sitobion avenae (F.) (Hom., Aphididae). J. Appl. Entomol. 1992, 113, 61–67. [Google Scholar] [CrossRef]
- Miles, P.W. Studies on the salivary physiology of plant bugs: Oxidase activity in the salivary apparatus and saliva. J. Insect Physiol. 1964, 10, 121–129. [Google Scholar] [CrossRef]
- Laurema, S.; Varis, A.L.; Miettinen, H. Studies on enzymes in the salivary glands of Lygus rugulipennis (Hemiptera, miridae). Insect Biochem. 1985, 15, 211–224. [Google Scholar] [CrossRef]
- Fehrman, H.; Dimond, A.E. Peroxidase activity and phytophthora resistance in different organs of the potato plant. Phytopathology 1969, 57, 69–72. [Google Scholar]
- Szelegiewicz, H. Mszyce—Aphidodea. In Katalog Fauny Polski; PWN: Warszawa, Poland, 1968; Volume 21, pp. 1–316. [Google Scholar]
- Wilkaniec, A.; Borowiak-Sobkowiak, B.; Irzykowska, L.; Breś, W.; Świerk, D.; Pardela, Ł.; Durak, R.; Środulska-Wielgus, J.; Wielgus, K. Biotic and abiotic factors causing the collapse of Robinia pseudoacacia L. veteran trees in urban environments. PLoS ONE 2021, 16, e0245398. [Google Scholar] [CrossRef]
- Chown, S.L.; Nicolson, S. Insect Physiological Ecology: Mechanisms and Patterns; Oxford University Press: New York, NY, USA, 2004; pp. 1–254. [Google Scholar]
- Kellermann, V.; van Heerwaarden, B.; Sgrò, C.M.; Hoffmann, A.A. Fundamental evolutionary limits in ecological traits drive Drosophila species distributions. Science 2009, 325, 1244–1246. [Google Scholar] [CrossRef] [Green Version]
- Lubawy, J.; Daburon, V.; Chowański, S.; Słocińska, M.; Colinet, H. Thermal stress causes DNA damage and mortality in a tropical insect. J. Exp. Biol. 2019, 222, jeb213744. [Google Scholar] [CrossRef] [PubMed]
- Ceballos, G.; Ehrlich, P.R.; Barnosky, A.D.; Garcia, A.; Pringle, R.M.; Palmer, T.M. Accelerated modern human-induced species losses: Entering the sixth mass extinction. Sci. Adv. 2015, 1, e1400253. [Google Scholar] [CrossRef] [Green Version]
- Durak, R. The overwintering strategy of the anholocyclic aphid Cinara tujafilina. Physiol. Entomol. 2014, 39, 313–321. [Google Scholar] [CrossRef]
- Shrestha, S. Effects of climate change in agricultural insect pest. Acta Sci. Agric. 2019, 3, 74–80. [Google Scholar] [CrossRef]
- Alford, L.; Tougeron, K.; Pierre, J.S.; Burel, F.; van Baaren, J. The effect of landscape complexity and microclimate on the thermal tolerance of a pest insect. Insect Sci. 2017, 25, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Saska, P.; Skuhrovec, J.; Lukáš, J.; Chi, H.; Tuan, S.-J.; Honěk, A. Treatment by glyphosate-based herbicide alters life history parameters of the rose-grain aphid Metopolophium dirhodum. Sci. Rep. 2016, 6, 27801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birgücü, A.K.; Turanli, F.; Çelik, Y. The effect of herbicides on Russian wheat aphid, Diuraphis noxia (Kurdjumov) (Hemiptera: Aphididae). J. Kans. Entomol. Soc. 2016, 89, 72–79. [Google Scholar] [CrossRef]
- Łukaszewicz, S.; Borowiak-Sobkowiak, B.; Durak, R.; Dancewicz, K.; Politycka, B. Interaction between Acyrthosiphon pisum and selenium-treated Pisum sativum. Eur. Zool. J. 2021, 88, 58–76. [Google Scholar] [CrossRef]
- Woźniak, A.; Bednarski, W.; Dancewicz, K.; Gabryś, B.; Borowiak-Sobkowiak, B.; Bocianowski, J.; Samardakiewicz, S.; Rucińska-Sobkowiak, R.; Morkunas, I. Oxidative stress links response to lead and Acyrthosiphon pisum in Pisum sativum L. J. Plant Physiol. 2019, 240, 152996. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J. Paradigm lost, paradigm found: The re-emergence of hormesis as a fundamental dose response model in the toxicological sciences. Environ. Pollut. 2006, 138, 379–411. [Google Scholar] [CrossRef]
- Calabrese, E.J. Hormesis: Why it is important to toxicology and toxicologists. Environ. Toxicol. Chem. 2009, 27, 1451–1474. [Google Scholar] [CrossRef]
- Mattson, M.P. Hormesis defined. Ageing Res. Rev. 2008, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Bachmann, K.A.; Bailer, A.J.; Bolger, P.M.; Borak, J.; Cai, L.; Cedergreen, N.; Cherian, M.G.; Chiueh, C.C.; Clarkson, T.W.; et al. Biological stress response terminology: Integrating the concepts of adaptive response and preconditioning stress within a hormetic dose-response framework. Toxicol. Appl. Pharmacol. 2007, 222, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Calabrese, E.J.; Baldwin, L.A. Defining hormesis. Hum. Exp. Toxicol. 2002, 21, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Carelli, G.; Iavicoli, I. Defining hormesis: The necessary tool to clarify experimentally the low dose-response relationship. Hum. Exp. Toxicol. 2002, 21, 103–104. [Google Scholar] [CrossRef]
- Urbańska, A. Occurrence and source of hydrogen peroxide in aphids. EJPAU 2009, 12, 27. [Google Scholar]
- DeJong, R.J.; Miller, L.M.; Molina-Cruz, A.; Gupta, L.; Kumar, S.; Barillas-Mury, C. Reactive oxygen species detoxification by catalase is a major determinant of fecundity in the mosquito Anopheles gambiae. PNAS 2007, 104, 2121–2126. [Google Scholar] [CrossRef] [Green Version]
- Guedes, N.M.P.; Tolledo, J.; Corrêa, A.S.; Guedes, R.N.C. Insecticide-induced hormesis in an insecticide-resistant strain of the maize weevil, Sitophilus zeamais. J. Appl. Entomol. 2010, 134, 142–148. [Google Scholar] [CrossRef] [Green Version]
| Temperature | Pre-Reproduction | Reproduction | Post-Reproduction | Longevity | Fecundity |
|---|---|---|---|---|---|
| (°C) | Days | Days | Days | Days | Nymphs |
| mean ± SD | mean ± SD | mean ± SD | mean ± SD | mean ± SD | |
| 20 | 11.0 ± 0.2 a | 14.7 ± 11.1 a | 0.0 ± 0.1 a | 25.7 ± 11.2 a | 54.5 ± 48.9 a |
| 25 | 6.4 ± 0.5 b | 15.2 ± 3.4 a | 3.0 ± 2.6 b | 24.6 ± 5.5 a | 111.6 ± 17.7 b |
| 28 | 6.0 ± 0 c | 9.6 ± 3.3 b | 2.6 ± 2.3 b | 18.2 ± 5.1 b | 27.8 ± 10.0 c |
| 30 | 5.4 ± 0.5 d | 2.1 ± 2.2 c | 7.6 ± 4.9 c | 15.1 ± 5.9 b | 2.4 ± 2.9 d |
| Temp. °C | rm | Ro | λ | T | DT |
|---|---|---|---|---|---|
| 20 | 0.153 | 26.708 | 1.165 | 21.470 | 4.530 |
| 25 | 0.355 | 58.037 | 1.426 | 11.440 | 1.953 |
| 28 | 0.244 | 12.243 | 1.276 | 10.266 | 2.841 |
| 30 | 0.019 | 1.168 | 1.017 | 8.196 | 36.481 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durak, R.; Jedryczka, M.; Czajka, B.; Dampc, J.; Wielgusz, K.; Borowiak-Sobkowiak, B. Mild Abiotic Stress Affects Development and Stimulates Hormesis of Hemp Aphid Phorodon cannabis. Insects 2021, 12, 420. https://doi.org/10.3390/insects12050420
Durak R, Jedryczka M, Czajka B, Dampc J, Wielgusz K, Borowiak-Sobkowiak B. Mild Abiotic Stress Affects Development and Stimulates Hormesis of Hemp Aphid Phorodon cannabis. Insects. 2021; 12(5):420. https://doi.org/10.3390/insects12050420
Chicago/Turabian StyleDurak, Roma, Malgorzata Jedryczka, Beata Czajka, Jan Dampc, Katarzyna Wielgusz, and Beata Borowiak-Sobkowiak. 2021. "Mild Abiotic Stress Affects Development and Stimulates Hormesis of Hemp Aphid Phorodon cannabis" Insects 12, no. 5: 420. https://doi.org/10.3390/insects12050420
APA StyleDurak, R., Jedryczka, M., Czajka, B., Dampc, J., Wielgusz, K., & Borowiak-Sobkowiak, B. (2021). Mild Abiotic Stress Affects Development and Stimulates Hormesis of Hemp Aphid Phorodon cannabis. Insects, 12(5), 420. https://doi.org/10.3390/insects12050420