Border Habitat Effects on Captures of Halyomorpha halys (Hemiptera: Pentatomidae) in Pheromone Traps and Fruit Injury at Harvest in Apple and Peach Orchards in the Mid-Atlantic, USA
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Orchard Sites
2.2. Halyomorpha halys Monitoring
2.3. Fruit Injury Assessments
2.4. Statistical Analysis
3. Results
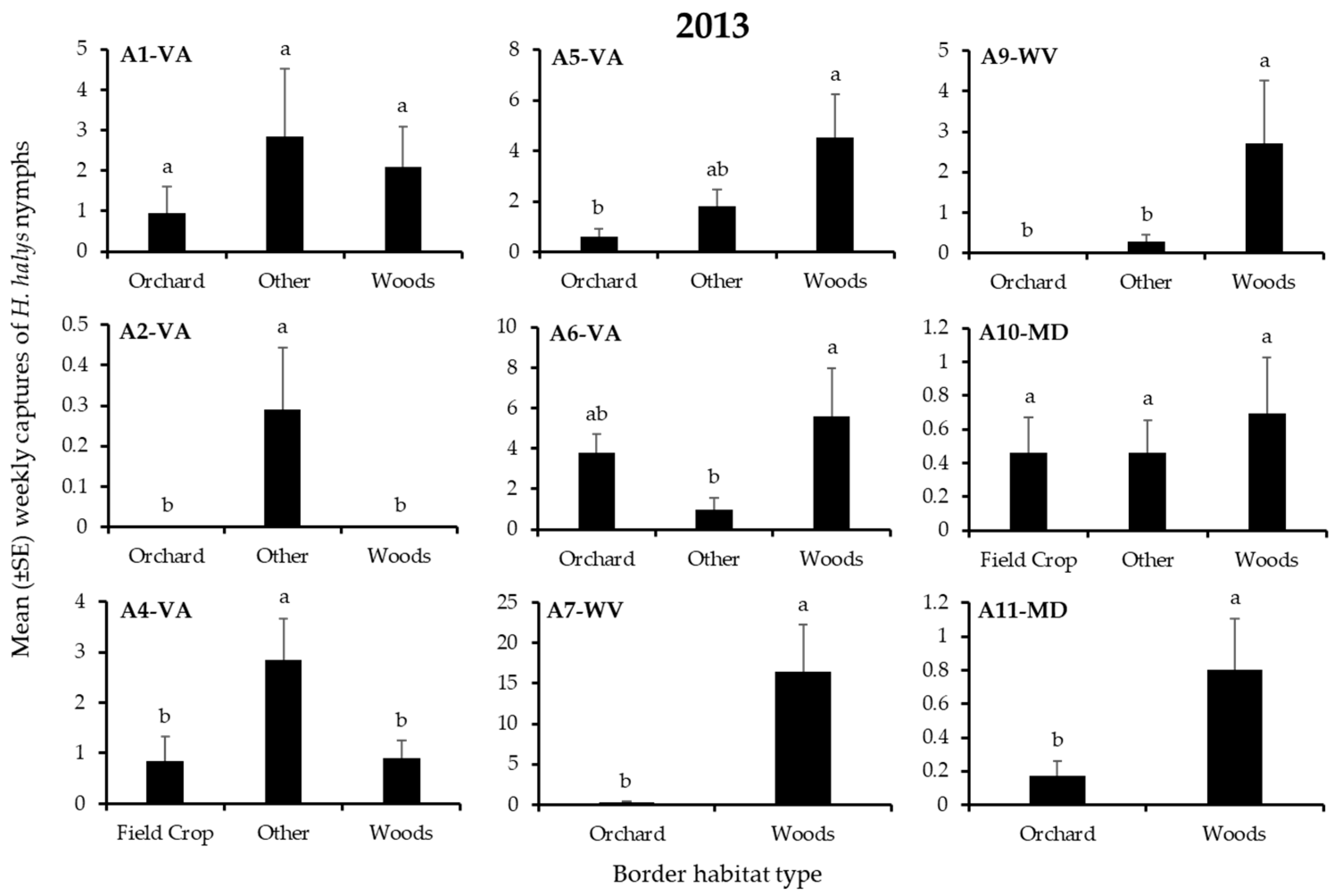
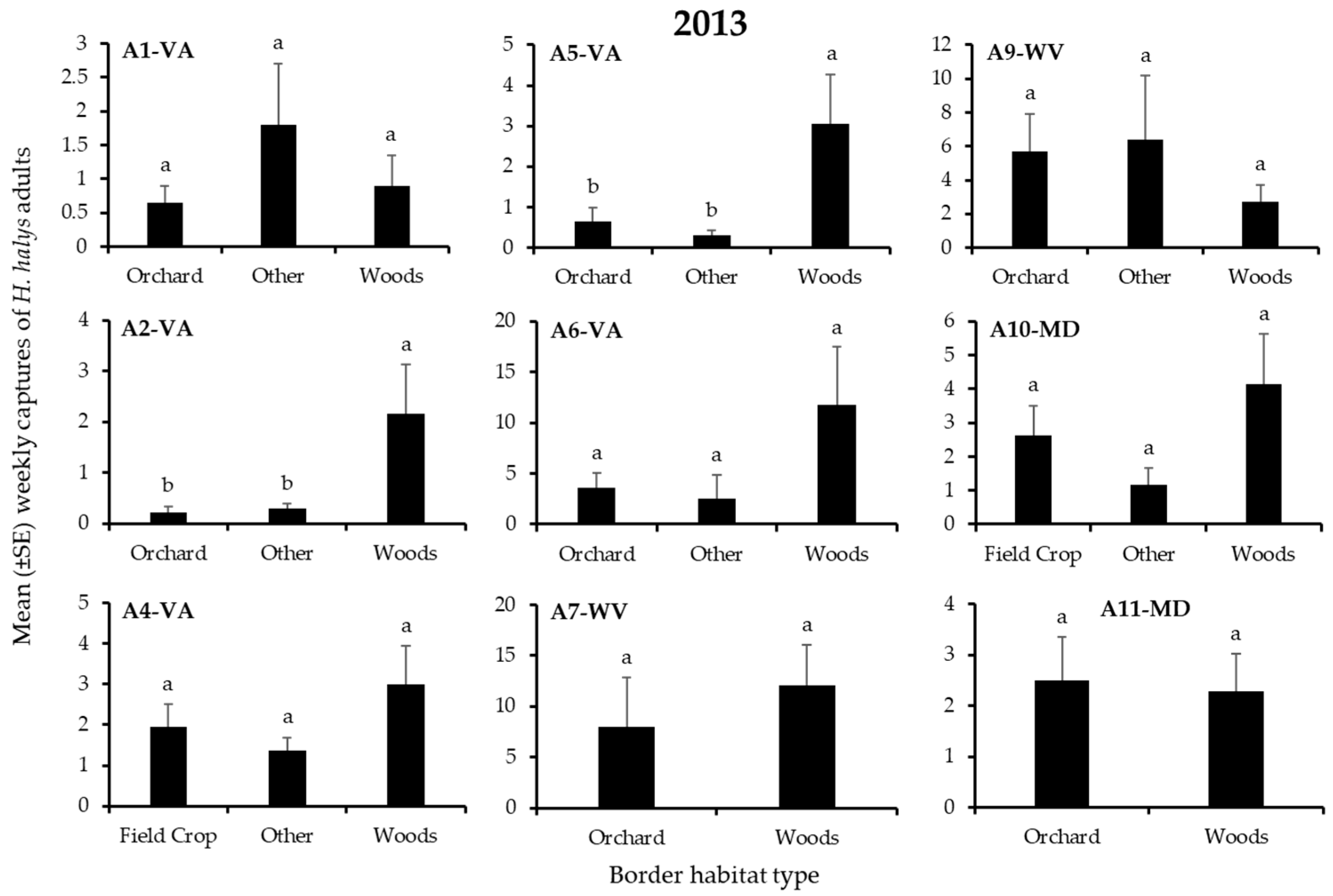
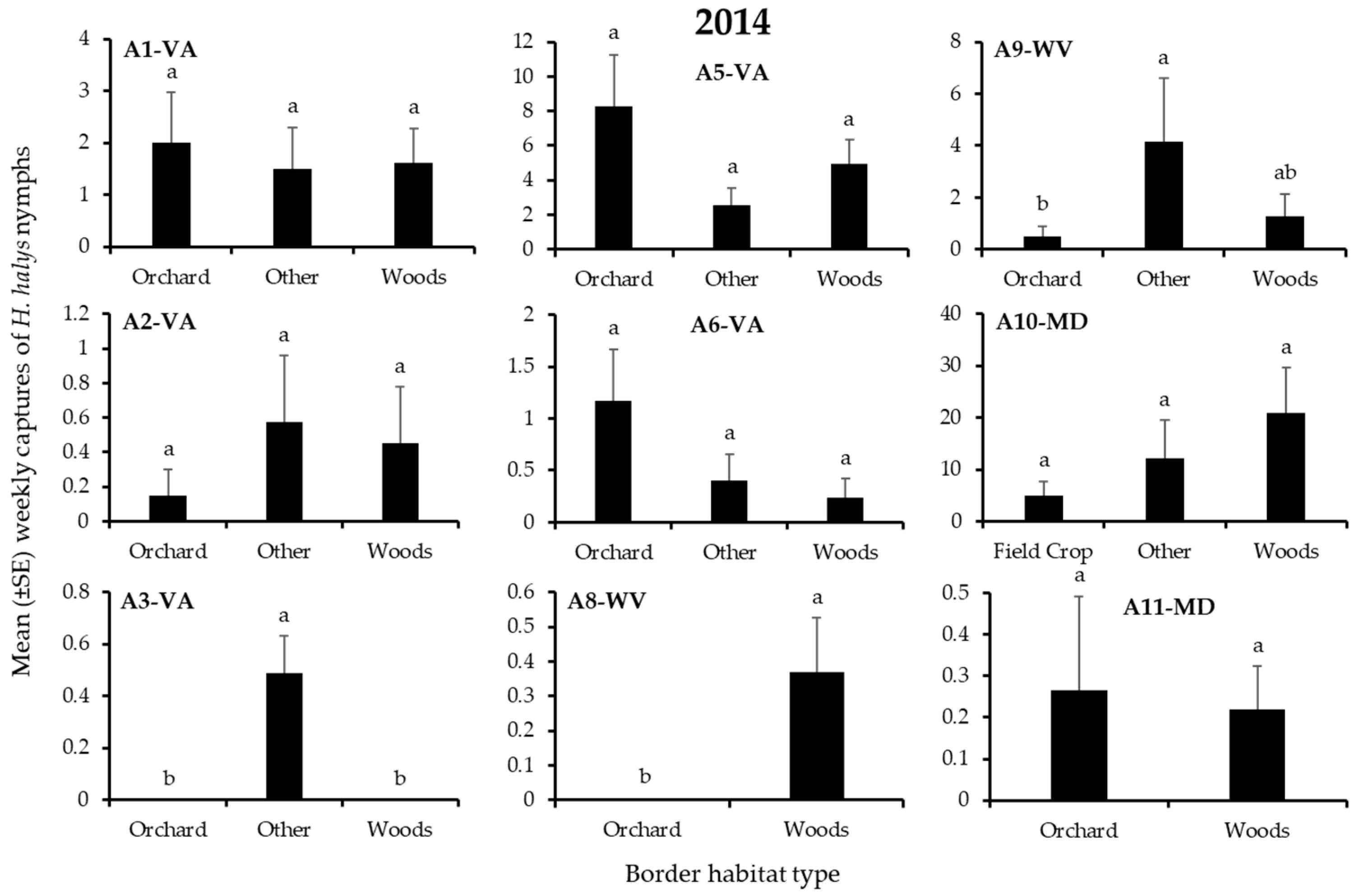
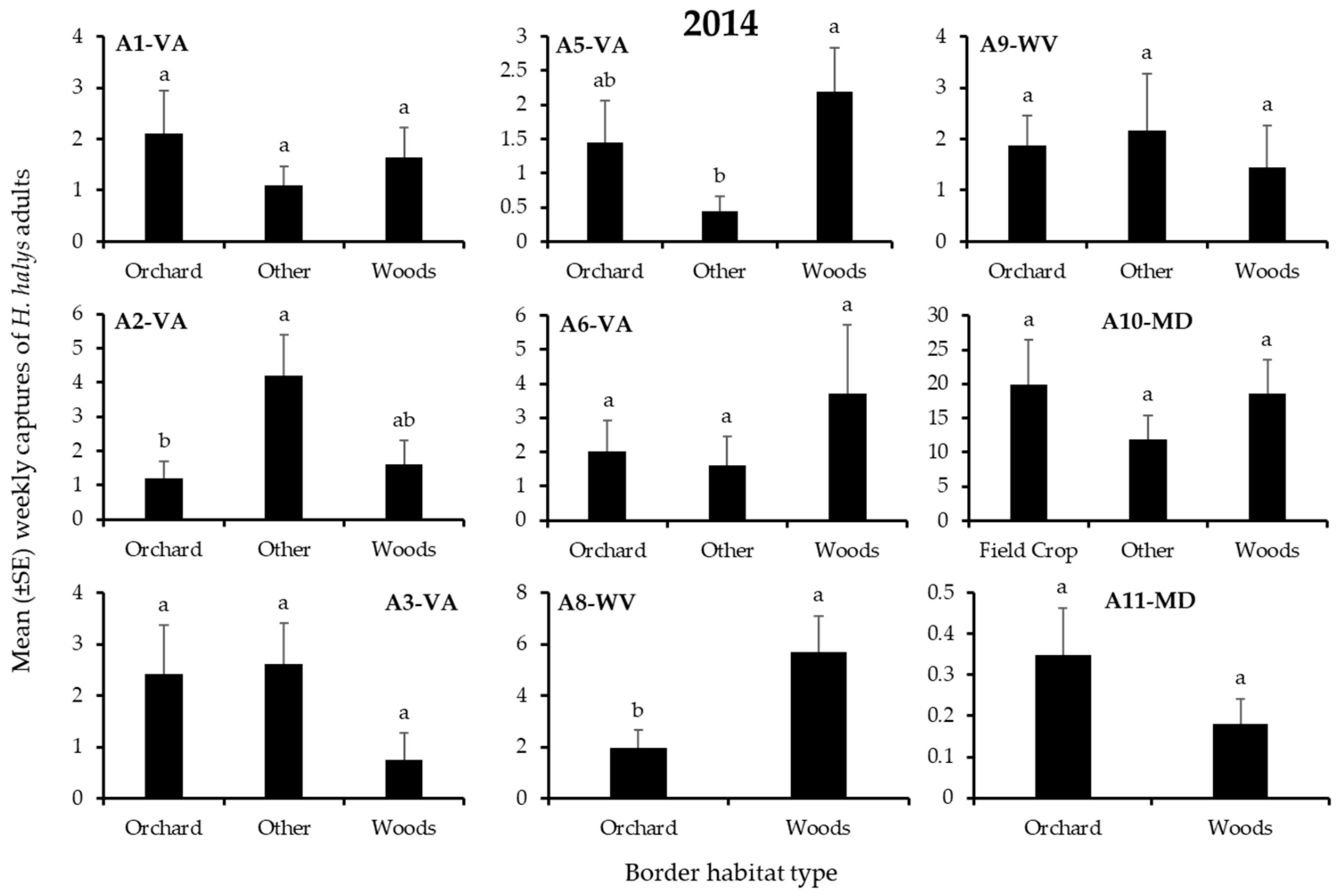
3.1. H. Halys Captures in Apple
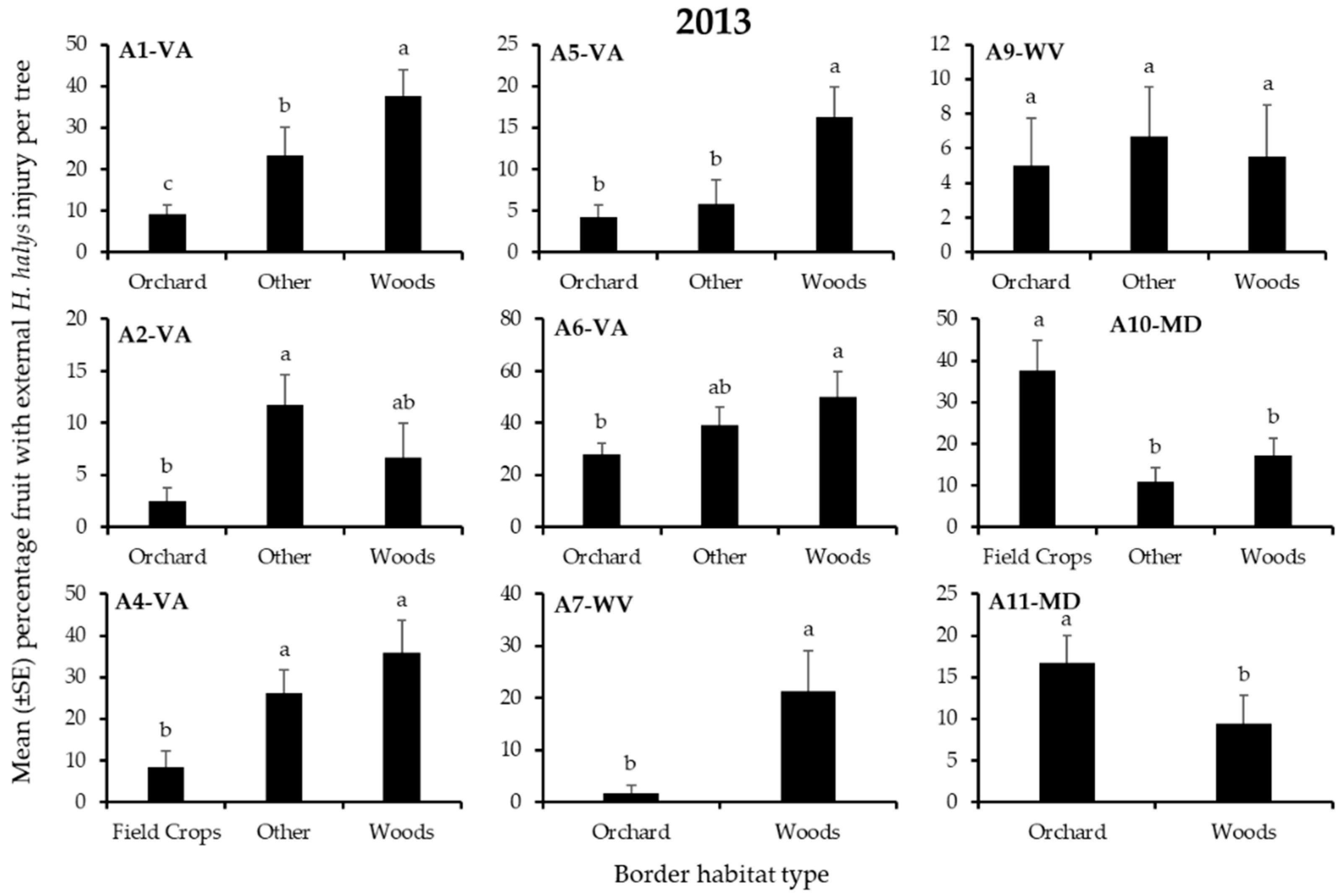
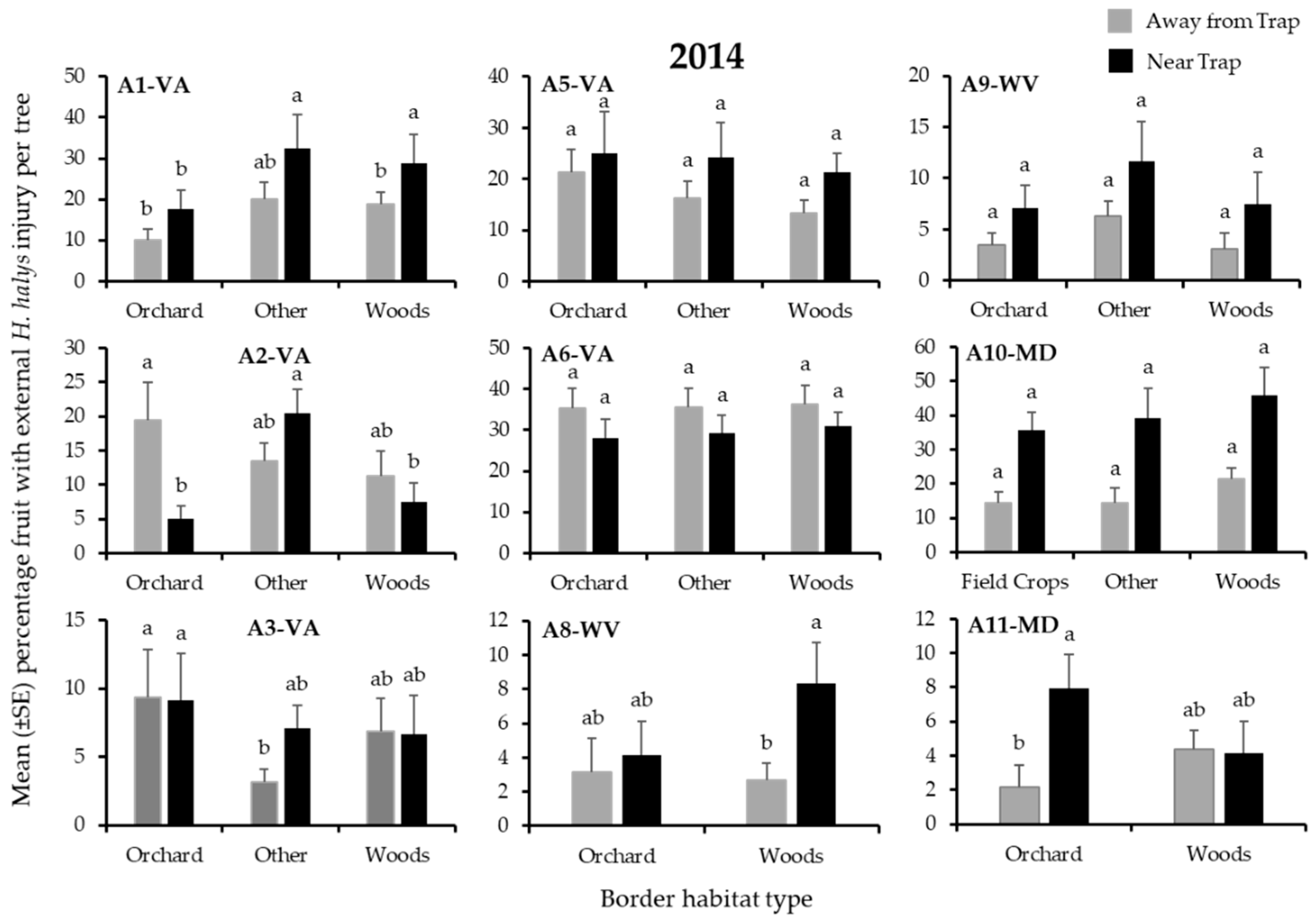
3.2. Apple Injury from H. halys
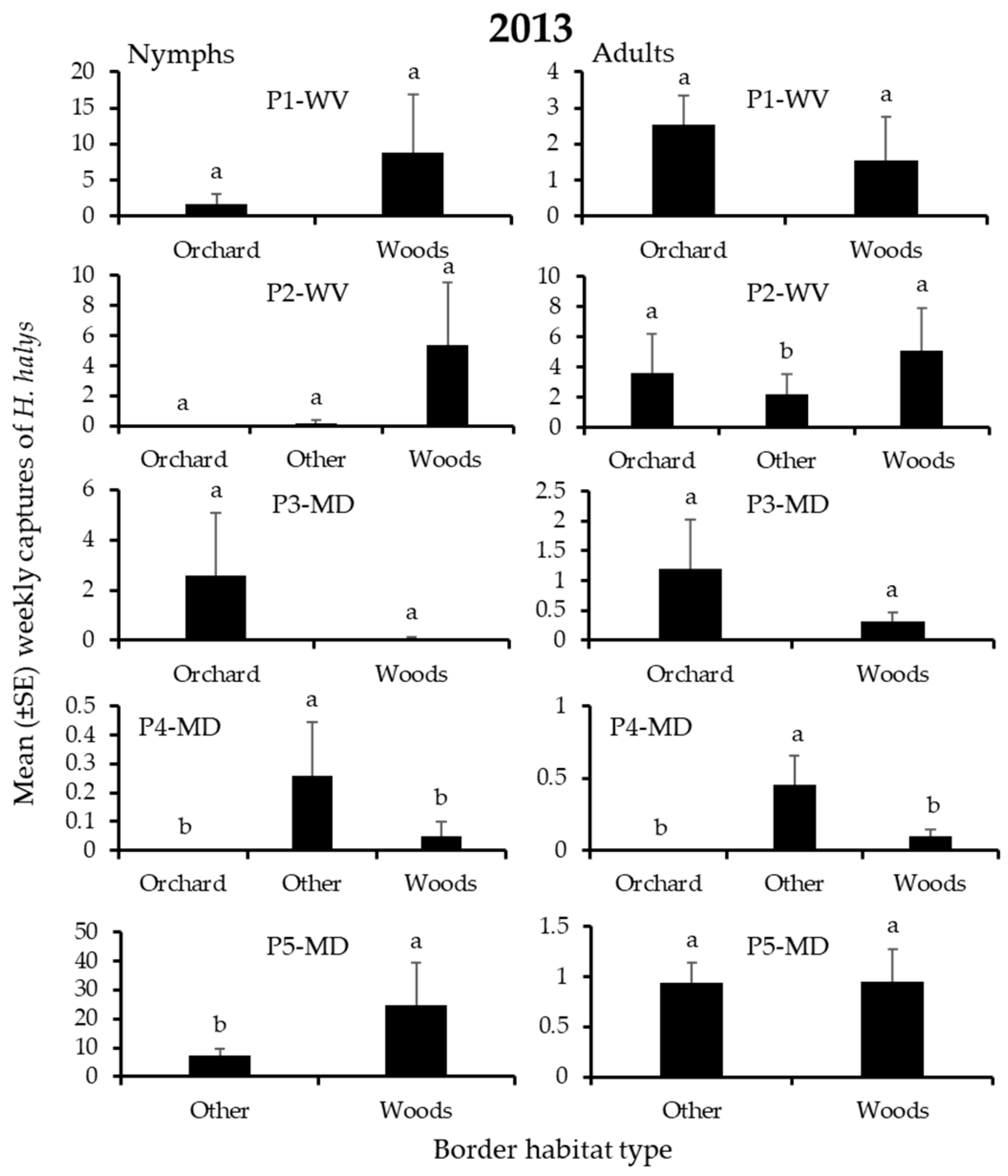
3.3. H. halys Captures in Peach
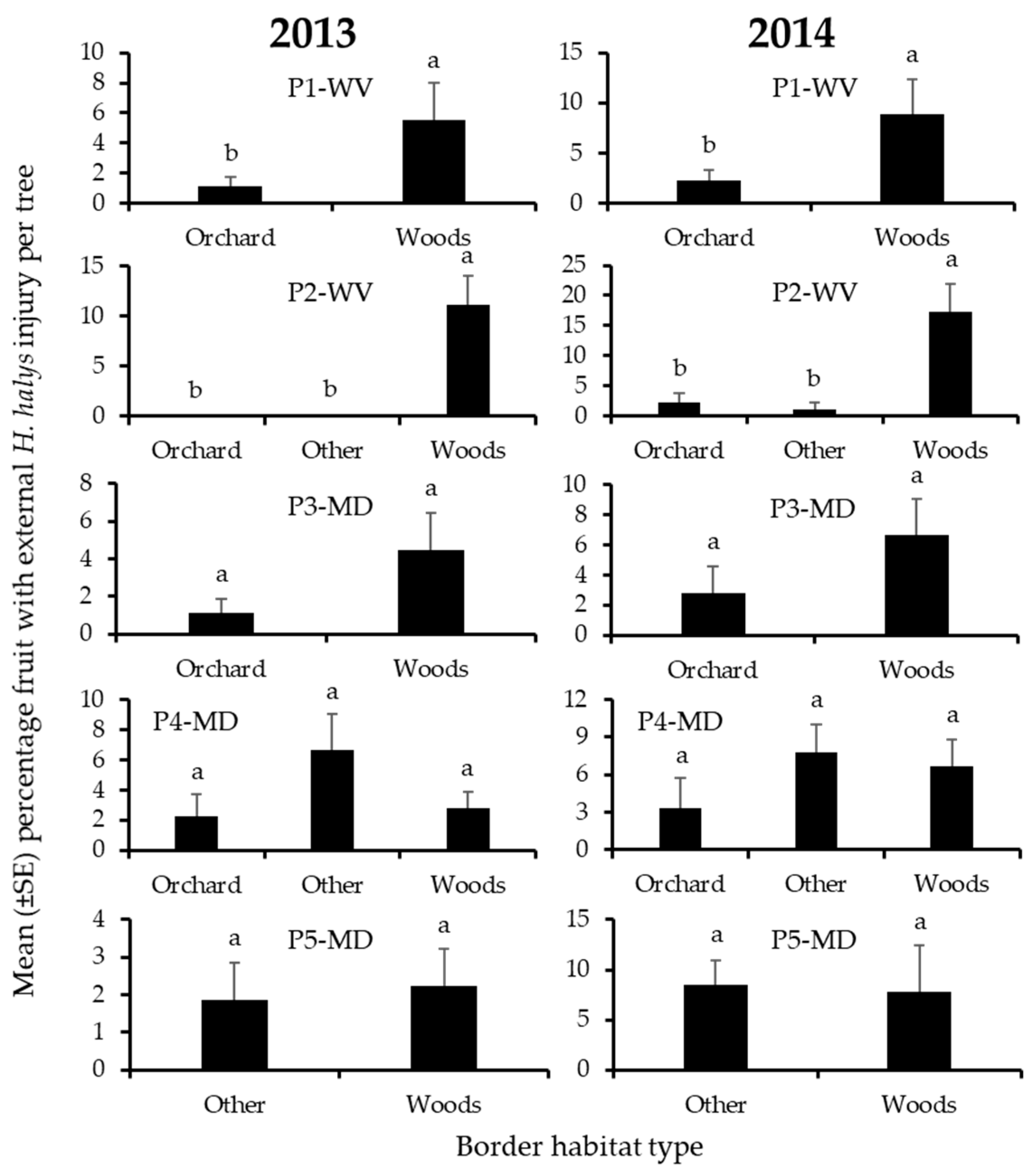
3.4. Peach Injury from H. halys
3.5. Border Risk Assessment Based on Highest Captures and Injury at a Common Border
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leskey, T.C.; Nielsen, A.L. Impact of the invasive brown marmorated stink bug in North America and Europe: History, biology, ecology, and management. Annu. Rev. Entomol. 2018, 63, 599–618. [Google Scholar] [CrossRef] [PubMed]
- Leskey, T.C.; Short, B.D.; Butler, B.R.; Wright, S.E. Impact of the invasive brown marmorated stink bug, Halyomorpha halys (Stål), in mid-Atlantic tree fruit orchards in the United States: Case studies of commercial management. Psyche 2012, 2012, 535062. [Google Scholar] [CrossRef]
- Leskey, T.C.; Lee, D.-H.; Short, B.D.; Wright, S.E. Impact of insecticides on the invasive Halyomorphahalys (Hemiptera: Pentatomidae): Analysis of insecticide lethality. J. Econ. Entomol. 2012, 105, 1726–1735. [Google Scholar] [CrossRef] [PubMed]
- Bosco, L.; Moraglio, S.T.; Tavella, L. Halyomorpha halys, a serious threat for hazelnut in newly invaded areas. J. Pest. Sci. 2017, 91, 661–670. [Google Scholar] [CrossRef]
- Maistrello, L.; Vaccari, G.; Caruso, S.; Costi, E.; Bortolini, S.; Macavei, L.; Foca, G.; Ulrici, A.; Bortolotti, P.P.; Nannini, R.; et al. Monitoring of the invasive Halyomorpha halys, a new key pest of fruit orchards in northern Italy. J. Pest. Sci. 2017, 90, 1231–1244. [Google Scholar] [CrossRef]
- Nielsen, A.L.; Hamilton, G.C. Life history of the invasive species Halyomorpha halys (Hemiptera: Pentatomidae) in northeastern United States. Ann. Entomol. Soc. Am. 2009, 102, 608–616. [Google Scholar] [CrossRef]
- Bakken, A.J.; Schoof, S.C.; Bickerton, M.; Kamminga, K.L.; Jenrette, J.C.; Malone, S.; Abney, M.A.; Herbert, D.A.; Reisig, D.; Kuhar, T.P.; et al. Occurrence of brown marmorated stink bug (Hemiptera: Pentatomidae) on wild hosts in nonmanaged woodlands and soybean fields in North Carolina and Virginia. Environ. Entomol. 2015, 44, 1011–1021. [Google Scholar] [CrossRef]
- Bergmann, E.J.; Venugopal, P.D.; Martinson, H.M.; Raupp, M.J.; Shrewsbury, P.M. Host plant use by the invasive Halyomorpha halys (Stål) on woody ornamental trees and shrubs. PLoS ONE 2016, 11, e0149975. [Google Scholar] [CrossRef]
- Lee, D.-H.; Nielsen, A.L.; Leskey, T.C. Dispersal capacity and behavior of nymphal stages of Halyomorpha halys (Hemiptera: Pentatomidae) evaluated under laboratory and field conditions. J. Insect. Behav. 2014, 27, 639–651. [Google Scholar] [CrossRef]
- Wiman, N.G.; Walton, V.M.; Shearer, P.W.; Rondon, S.I.; Lee, J.C. Factors affecting flight capacity of brown marmorated stink bug, Halyomorpha halys (Hemiptera: Pentatomidae). J. Pest. Sci. 2014, 88, 37–47. [Google Scholar] [CrossRef]
- Lee, D.-H.; Leskey, T.C. Flight behavior of foraging and overwintering brown marmorated stink bug, Halyomorpha halys (Hemiptera: Pentatomidae). Bull. Entomol. Res. 2015, 105, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Acebes-Doria, A.L.; Leskey, T.C.; Bergh, J.C. Temporal and directional patterns of nymphal Halyomorpha halys (Hemiptera: Pentatomidae) movement on the trunk of selected wild and tree fruit hosts in the Mid-Atlantic region. Environ. Entomol. 2017, 46, 258–267. [Google Scholar]
- Acebes-Doria, A.L.; Leskey, T.C.; Bergh, J.C. Injury to apples and peaches at harvest from feeding by Halyomorpha halys (Stål) (Hemiptera: Pentatomidae) nymphs early and late in the season. Crop Prot. 2016, 89, 58–65. [Google Scholar] [CrossRef]
- Venugopal, P.D.; Coffey, P.L.; Dively, G.P.; Lamp, W.O. Adjacent habitat influence on stink bug (Hemiptera: Pentatomidae) densities and associated damage at field corn and soybean edges. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, P.D.; Martinson, H.M.; Bergmann, E.J.; Shrewsbury, P.M.; Raupp, M.J. Edge effects influence the abundance of the invasive Halyomorpha halys (Hemiptera: Pentatomidae) in woody plant nurseries. Environ. Entomol. 2015, 44, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.V.; Stallings, J.W.; Leskey, T.C.; Krawczyk, G.; Polk, D.; Butler, B.; Bergh, J.C. Spatial distribution of brown marmorated stink bug injury at harvest mid-Atlantic in apple orchards. J. Econ. Entomol. 2014, 107, 1839–1848. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.V.; Bergh, J.C.; Wright, S.E.; Leskey, T.C. Factors affecting captures of brown marmorated stink bug, Halyomorpha halys (Hemiptera: Pentatomidae), in baited pyramid traps. J. Entomol. Sci. 2013, 48, 43–51. [Google Scholar] [CrossRef]
- Morrison, W.R., III; Cullum, J.P.; Leskey, T.C. Evaluation of trap designs and deployment strategies for capturing Halyomorpha halys (Hemiptera: Pentatomidae). J. Econ. Entomol. 2015, 108, 1683–1692. [Google Scholar] [CrossRef]
- Leskey, T.C.; Agnello, A.; Bergh, J.C.; Dively, G.P.; Hamilton, G.C.; Jentsch, P.; Khrimian, A.; Krawczyk, G.; Kuhar, T.P.; Lee, D.-H.; et al. Attraction of the invasive Halyomorpha halys (Hemiptera: Pentatomidae) to traps baited with semiochemical stimuli across the United States. Environ. Entomol. 2015, 44, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Rice, K.B.; Morrison, W.R., III; Short, B.D.; Acebes-Doria, A.L.; Bergh, J.C.; Leskey, T.C. Improved trap designs and retention mechanisms for Halyomorpha halys (Hemiptera: Pentatomidae). J. Econ. Entomol. 2018, 111, 2136–2142. [Google Scholar] [CrossRef] [PubMed]
- Acebes-Doria, A.L.; Morrison, W.R., III; Short, B.D.; Rice, K.B.; Bush, H.G.; Kuhar, T.P.; Duthie, C.; Leskey, T.C. Monitoring and biosurveillance tools for the brown marmorated stink bug, Halyomorpha halys (Stål) (Hemiptera: Pentatomidae). Insects 2018, 9, 82. [Google Scholar] [CrossRef]
- Acebes-Doria, A.L.; Agnello, A.M.; Alston, D.G.; Andrews, H.; Beers, E.H.; Bergh, J.C.; Bessin, R.; Blaauw, B.R.; Buntin, G.D.; Burkness, E.C.; et al. Season-long monitoring of the brown marmorated stink bug (Hemiptera: Pentatomidae) throughout the United States using commercially available traps and lures. J. Econ. Entomol. 2020, 113, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Blaauw, B.R.; Polk, D.; Nielsen, A.L. IPM-CPR for peaches: Incorporating behaviorally-based methods to manage Halyomorpha halys and key pests in peach. Pest Manag. Sci. 2015, 71, 1513–1522. [Google Scholar] [CrossRef] [PubMed]
- Akotsen-Mensah, C.; Blaauw, B.; Short, B.; Leskey, T.C.; Bergh, J.C.; Polk, D.; Nielsen, A.L. Using IPM-CPR as a management program for apple orchards. J. Econ. Entomol. 2020, 113, 1894–1902. [Google Scholar] [CrossRef]
- Leskey, T.C.; Short, B.D.; Ludwick, D. Comparison and refinement of integrated pest management tactics for Halyomorpha halys (Hemiptera: Pentatomidae) management in apple orchards. J. Econ. Entomol. 2020, 113, 1725–1734. [Google Scholar] [CrossRef] [PubMed]
- Morrison, W.R., III; Lee, D.-H.; Short, B.D.; Khrimian, A.; Leskey, T.C. Establishing the behavioral basis for an attract-and-kill strategy to manage the invasive Halyomorpha halys in apple orchards. J. Pest Sci. 2016, 89, 81–96. [Google Scholar] [CrossRef]
- Morrison, W.R., III; Blaauw, B.R.; Short, B.D.; Nielsen, A.L.; Bergh, J.C.; Krawczyk, G.; Park, Y.-L.; Butler, B.; Khrimian, A.; Leskey, T.C. Successful management of Halyomorpha halys (Hemiptera: Pentatomidae) in commercial apple orchards with an attract-and-kill strategy. Pest Manag. Sci. 2018, 75, 104–114. [Google Scholar] [CrossRef]
- Short, B.D.; Khrimian, A.; Leskey, T.C. Pheromone-based decision support tools for management of Halyomorpha halys in apple orchards: Development of a treatment threshold. J. Pest Sci. 2016, 90, 1191–1204. [Google Scholar] [CrossRef]
- Leskey, T.C.; Khrimian, A.; Weber, D.C.; Aldrich, J.C.; Short, B.D.; Lee, D.-H.; Morrison, W.R., III. Behavioral responses of the invasive Halyomorpha halys (Stål) to traps baited with stereoisomeric mixtures of 10,11-Epoxy-1-bisabolen-3-OL. J. Chem. Ecol. 2015, 41, 418–429. [Google Scholar] [PubMed]
- Khrimian, A.; Zhang, A.; Weber, D.C.; Ho, H.Y.; Aldrich, J.R.; Vermillion, K.E.; Siegler, M.A.; Shirali, S.; Guzman, F.; Leskey, T.C. Discovery of the aggregation pheromone of the brown marmorated stink bug (Halyomorpha halys) through the creation of stereoisomeric libraries of 1-bisabolen-3-ols. J. Nat. Prod. 2014, 77, 1708–1717. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.C.; Leskey, T.C.; Walsh, G.C.; Khrimian, A. Synergy of aggregation pheromone with methyl (E,E,Z)-2,4,6-decatrienoate in attraction of Halyomorpha halys (Hemiptera: Pentatomidae). J. Econ. Entomol. 2014, 107, 1061–1068. [Google Scholar] [CrossRef]
- Joseph, S.V.; Nita, M.; Leskey, T.C.; Bergh, J.C. Temporal effects on the incidence and severity of brown marmorated stink bug (Hemiptera: Pentatomidae) feeding injury to peaches and apples during the fruiting period in Virginia. J. Econ. Entomol. 2015, 108, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Aho, K.A. Foundational and Applied Statistics for Biologists Using R; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2014. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 3 November 2020).
- Hothorn, T.; Bretz, F.; Westfall, P.; Heiderger, R.M.; Schuetzenmeister, A.; Scheibe, S. Package ‘Multcomp’. 2020. Available online: https://cran.r-project.org/web/packages/multcomp/multcomp.pdf (accessed on 3 November 2020).
- Acebes-Doria, A.L.; Leskey, T.C.; Bergh, J.C. Host plant effects on Halyomorpha halys (Hemiptera: Pentatomidae) nymphal development and survivorship. Environ. Entomol. 2016, 45, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Funayama, K. Comparison of the susceptibility to injury of apple cultivars by stink bugs. Jpn. J. Appl. Entomol. Zool. 2002, 46, 37–40. [Google Scholar] [CrossRef][Green Version]
- Inkley, D.B. Characteristics of home invasion by the brown marmorated stink bug (Hemiptera: Pentatomidae). J. Entomol. Sci. 2012, 47, 125–130. [Google Scholar] [CrossRef]
- Venugopal, P.D.; Dively, G.P.; Lamp, W.O. Spatiotemporal dynamics of the invasive Halyomorpha halys (Hemiptera: Pentatomidae) in and between adjacent corn and soybean fields. J. Econ. Entomol. 2015, 108, 2231–2241. [Google Scholar] [CrossRef] [PubMed]
- Ludwick, D.; Morrison, W.R.; Acebes-Doria, A.L.; Agnello, A.M.; Bergh, J.C.; Buffington, M.L.; Hamilton, G.C.; Harper, J.K.; Hoelmer, K.A.; Krawczyk, G.; et al. Invasion of the brown marmorated stink bug (Hemiptera: Pentatomidae) into the USA: Developing a national response to an invasive species crisis through collaborative research and outreach efforts. J. IPM 2020, 11, 1–16. [Google Scholar] [CrossRef]
- Kilpatrick, D.M.; Acebes-Doria, A.L.; Rice, K.B.; Short, B.D.; Adams, C.G.; Gut, L.J.; Leskey, T.C. Estimating monitoring trap plume reach and trapping area for nymphal and adult Halyomorpha halys (Hemiptera: Pentatomidae) in crop and non-crop habitats. Environ. Entomol. 2019, 48, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.L.; Shearer, P.W.; Hamilton, G.C. Toxicity of insecticides to Halyomorpha halys (Hemiptera: Pentatomidae) using glass-vial bioassays. J. Econ. Entomol. 2008, 101, 1439–1442. [Google Scholar] [CrossRef]
- Bergmann, E.J.; Raupp, M.J. Efficacy of common ready to use insecticides against Halyomorpha halys (Hemiptera: Pentatomidae). Fla. Entomol. 2014, 97, 791–800. [Google Scholar] [CrossRef]

| Crop | State | Year(s) | Block Code | Area (ha) | Frequency of Border Habitats | |||
|---|---|---|---|---|---|---|---|---|
| Woods | Another Orchard | Field Crop | Other Habitats 1 | |||||
| apple | Virginia | 2013–2014 | A1-VA | 7.31 | 2 | 1 | 0 | 1 |
| apple | Virginia | 2013–2014 | A2-VA | 3.28 | 1 | 1 | 0 | 2 |
| apple | Virginia | 2014 | A3-VA | 4.27 | 1 | 2 | 0 | 1 |
| apple | Virginia | 2013 | A4-VA | 5.27 | 2 | 1 | 1 | 0 |
| apple | Virginia | 2013–2014 | A5-VA | 4.70 | 2 | 1 | 0 | 1 |
| apple | Virginia | 2013–2014 | A6-VA | 4.43 | 1 | 2 | 0 | 1 |
| apple | West Virginia | 2013 | A7-WV | 6.0 | 3 | 1 | 0 | 0 |
| apple | West Virginia | 2014 | A8-WV | 3.7 | 2 | 1 | 0 | 0 |
| apple | West Virginia | 2013–2014 | A9-WV | 4.4 | 1 | 2 | 0 | 1 |
| apple | Maryland | 2013–2014 | A10-MD | 1.6 | 2 | 0 | 1 | 1 |
| apple | Maryland | 2013–2014 | A11-MD | 1.0 | 1 | 2 | 0 | 1 |
| peach | West Virginia | 2013–2014 | P1-WV | 4.0 | 1 | 3 | 0 | 0 |
| peach | West Virginia | 2013–2014 | P2-WV | 5.2 | 2 | 1 | 0 | 1 |
| peach | Maryland | 2013–2014 | P3-MD | 1.5 | 2 | 1 | 0 | 1 |
| peach | Maryland | 2013–2014 | P4-MD | 2.2 | 2 | 1 | 0 | 1 |
| peach | Maryland | 2013–2014 | P5-MD | 1.3 | 1 | 1 | 0 | 2 |
| Block | Factor | 2013 | 2014 | ||||
|---|---|---|---|---|---|---|---|
| df | χ2 | p | df | χ2 | p | ||
| Nymphs | |||||||
| A1-VA | Border | 2 | 1.22 | 0.54 | 2 | 0.17 | 0.92 |
| A2-VA | Border | 2 | 9.53 | 0.01 | 2 | 0.93 | 0.63 |
| A3-VA | Border | - | - | - | 2 | 30.3 | 0.0001 |
| A4-VA | Border | 2 | 6.30 | 0.04 | - | - | - |
| A5-VA | Border | 2 | 6.49 | 0.04 | 2 | 4.27 | 0.12 |
| A6-VA | Border | 2 | 6.78 | 0.03 | 2 | 3.54 | 0.17 |
| A7-WV | Border | 1 | 5.76 | 0.02 | 1 | - | - |
| A8-WV | Border | 1 | - | - | 1 | 4.22 | 0.04 |
| A9-WV | Border | 2 | 26.9 | 0.0001 | 2 | 6.01 | 0.05 |
| A10-MD | Border | 2 | 0.45 | 0.80 | 2 | 2.67 | 0.26 |
| A11-MD | Border | 1 | 5.67 | 0.02 | 1 | 0.04 | 0.85 |
| Adults | |||||||
| A1-VA | Border | 2 | 1.80 | 0.41 | 2 | 1.00 | 0.61 |
| A2-VA | Border | 2 | 19.7 | 0.0001 | 2 | 6.34 | 0.04 |
| A3-VA | Border | - | - | - | 2 | 3.45 | 0.18 |
| A4-VA | Border | 2 | 4.63 | 0.10 | - | - | - |
| A5-VA | Border | 2 | 7.99 | 0.02 | 2 | 5.74 | 0.05 |
| A6-VA | Border | 2 | 4.91 | 0.09 | 2 | 1.27 | 0.53 |
| A7-WV | Border | 1 | 0.34 | 0.56 | - | - | - |
| A8-WV | Border | 1 | - | - | 1 | 3.12 | 0.05 |
| A9-WV | Border | 2 | 1.20 | 0.55 | 2 | 0.34 | 0.84 |
| A10-MD | Border | 2 | 3.47 | 0.18 | 2 | 1.18 | 0.55 |
| A11-MD | Border | 1 | 0.04 | 0.85 | 1 | 1.82 | 0.18 |
| Block | Factor | df | χ2 | p |
|---|---|---|---|---|
| 2013 | ||||
| A1-VA | Border | 2 | 38.6 | 0.0001 |
| A2-VA | Border | 2 | 21.2 | 0.0001 |
| A4-VA | Border | 2 | 30.1 | 0.0001 |
| A5-VA | Border | 2 | 17.1 | 0.001 |
| A6-VA | Border | 2 | 16.9 | 0.001 |
| A7-WV | Border | 1 | 18.2 | 0.0001 |
| A9-WV | Border | 2 | 0.31 | 0.86 |
| A10-MD | Border | 2 | 27.1 | 0.0001 |
| A11-MD | Border | 1 | 4.17 | 0.04 |
| 2014 | ||||
| A1-VA | Border | 2 | 8.54 | 0.01 |
| A1-VA | Adjacent Trap | 1 | 3.63 | 0.06 |
| A1-VA | Interaction | 2 | 0.17 | 0.92 |
| A2-VA | Border | 2 | 4.49 | 0.11 |
| A2-VA | Adjacent Trap | 1 | 13.9 | 0.0002 |
| A2-VA | Interaction | 2 | 19.5 | 0.0001 |
| A3-VA | Border | 2 | 8.49 | 0.01 |
| A3-VA | Adjacent Trap | 1 | 0.00 | 0.95 |
| A3-VA | Interaction | 2 | 3.11 | 0.21 |
| A5-VA | Border | 2 | 4.78 | 0.09 |
| A5-VA | Adjacent Trap | 1 | 0.51 | 0.47 |
| A5-VA | Interaction | 2 | 1.06 | 0.59 |
| A6-VA | Border | 2 | 0.04 | 0.98 |
| A6-VA | Adjacent Trap | 1 | 3.50 | 0.06 |
| A6-VA | Interaction | 2 | 0.12 | 0.94 |
| A8-WV | Border | 2 | 0.73 | 0.39 |
| A8-WV | Adjacent Trap | 1 | 10.2 | 0.001 |
| A8-WV | Interaction | 2 | 1.39 | 0.24 |
| A9-WV | Border | 2 | 2.45 | 0.29 |
| A9-WV | Adjacent Trap | 1 | 3.80 | 0.05 |
| A9-WV | Interaction | 2 | 0.14 | 0.93 |
| A10-MD | Border | 2 | 6.41 | 0.04 |
| A10-MD | Adjacent Trap | 1 | 18.4 | 0.0001 |
| A10-MD | Interaction | 2 | 1.12 | 0.57 |
| A11-MD | Border | 2 | 3.46 | 0.06 |
| A11-MD | Adjacent Trap | 1 | 8.86 | 0.003 |
| A11-MD | Interaction | 2 | 4.97 | 0.03 |
| Block | Factor | 2013 | 2014 | ||||
|---|---|---|---|---|---|---|---|
| df | χ2 | p | df | χ2 | p | ||
| Nymphs | |||||||
| P1-WV | Border | 1 | 2.14 | 0.14 | 1 | 0.30 | 0.58 |
| P2-WV | Border | 2 | 3.98 | 0.08 | 2 | 3.17 | 0.20 |
| P3-MD | Border | 1 | 2.33 | 0.13 | 1 | 1.33 | 0.25 |
| P4-MD | Border | 2 | 3.03 | 0.22 | 2 | 0.83 | 0.66 |
| P5-MD | Border | 1 | 4.31 | 0.04 | 1 | 4.31 | 0.04 |
| Adults | |||||||
| P1-WV | Border | 1 | 0.45 | 0.50 | 1 | 0.76 | 0.38 |
| P2-WV | Border | 2 | 0.69 | 0.71 | 2 | 4.27 | 0.12 |
| P3-MD | Border | 1 | 1.68 | 0.19 | 1 | 0.72 | 0.39 |
| P4-MD | Border | 2 | 8.40 | 0.02 | 2 | 6.01 | 0.05 |
| P5-MD | Border | 1 | 0.00 | 0.97 | 1 | 0.002 | 0.97 |
| Block | Factor | 2013 | 2014 | ||||
|---|---|---|---|---|---|---|---|
| df | χ2 | p | df | χ2 | p | ||
| P1-WV | Border | 1 | 5.38 | 0.02 | 1 | 4.52 | 0.03 |
| P2-WV | Border | 2 | 39.4 | 0.0001 | 2 | 16.9 | 0.0002 |
| P3-MD | Border | 1 | 2.98 | 0.08 | 1 | 1.64 | 0.20 |
| P4-MD | Border | 2 | 3.54 | 0.17 | 2 | 1.61 | 0.45 |
| P5-MD | Border | 1 | 0.02 | 0.88 | 1 | 0.02 | 0.88 |
| Block | 2013 | 2014 | ||||
|---|---|---|---|---|---|---|
| Adults 1 | Nymphs | Injury | Adults | Nymphs | Injury | |
| A1-VA | Other | Other | Woods | Orchard | Orchard | Other |
| A2-VA | Woods | Other | Other | Other | Other | Other |
| A3-VA | - | - | - | Other | Other | Orchard |
| A4-VA | Woods | Other | Woods | - | - | - |
| A5-VA | Woods * | Woods | Woods * | Woods | Orchard | Orchard |
| A6-VA | Woods | Woods | Woods | Woods | Orchard | Woods |
| A7-WV | Woods | Woods * | Woods * | - | - | - |
| A8-WV | - | - | - | Woods | Woods | Woods |
| A9-WV | Other | Other | Other | Other | Other | Other |
| A10-MD | Woods | Woods | Field Crop | Field Crop | Woods | Woods |
| A11-MD | Orchard | Woods | Orchard | Orchard | Orchard | Orchard |
| Block | 2013 | 2014 | ||||
|---|---|---|---|---|---|---|
| Adults 1 | Nymphs | Injury | Adults | Nymphs | Injury | |
| P1-WV | Orchard | Woods | Woods | Orchard | Woods | Woods |
| P2-WV | Woods | Woods | Woods | Woods | Woods | Woods |
| P3-MD | Orchard | Orchard | Woods | Orchard | Orchard | Woods |
| P4-MD | Other | Other | Other | Other | Orchard | Other |
| P5-MD | Woods | Woods | Woods | Woods | Other | Other |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bergh, J.C.; Morrison, W.R., III; Stallrich, J.W.; Short, B.D.; Cullum, J.P.; Leskey, T.C. Border Habitat Effects on Captures of Halyomorpha halys (Hemiptera: Pentatomidae) in Pheromone Traps and Fruit Injury at Harvest in Apple and Peach Orchards in the Mid-Atlantic, USA. Insects 2021, 12, 419. https://doi.org/10.3390/insects12050419
Bergh JC, Morrison WR III, Stallrich JW, Short BD, Cullum JP, Leskey TC. Border Habitat Effects on Captures of Halyomorpha halys (Hemiptera: Pentatomidae) in Pheromone Traps and Fruit Injury at Harvest in Apple and Peach Orchards in the Mid-Atlantic, USA. Insects. 2021; 12(5):419. https://doi.org/10.3390/insects12050419
Chicago/Turabian StyleBergh, James Christopher, William R. Morrison, III, Jon W. Stallrich, Brent D. Short, John P. Cullum, and Tracy C. Leskey. 2021. "Border Habitat Effects on Captures of Halyomorpha halys (Hemiptera: Pentatomidae) in Pheromone Traps and Fruit Injury at Harvest in Apple and Peach Orchards in the Mid-Atlantic, USA" Insects 12, no. 5: 419. https://doi.org/10.3390/insects12050419
APA StyleBergh, J. C., Morrison, W. R., III, Stallrich, J. W., Short, B. D., Cullum, J. P., & Leskey, T. C. (2021). Border Habitat Effects on Captures of Halyomorpha halys (Hemiptera: Pentatomidae) in Pheromone Traps and Fruit Injury at Harvest in Apple and Peach Orchards in the Mid-Atlantic, USA. Insects, 12(5), 419. https://doi.org/10.3390/insects12050419







