Niche Modeling May Explain the Historical Population Failure of Phytoseiulus persimilis in Taiwan: Implications of Biocontrol Strategies
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Species Occurrence Dataset
2.2. Environmental Variables
2.3. Distribution Modeling
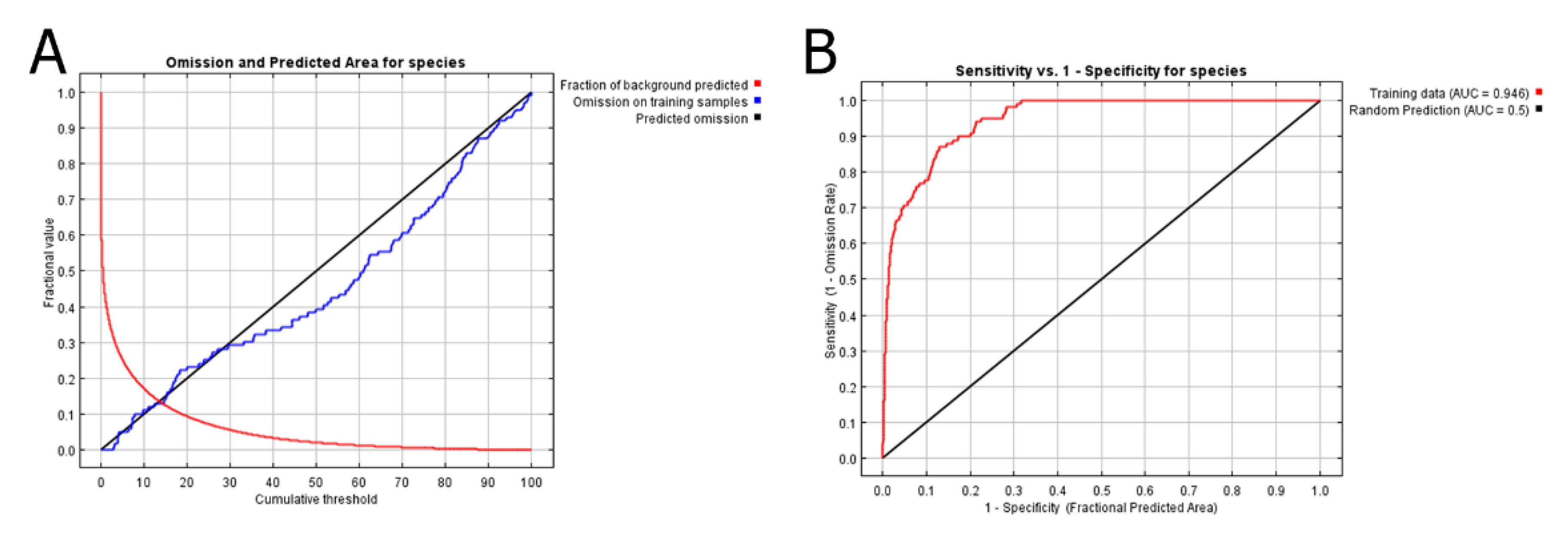
3. Results
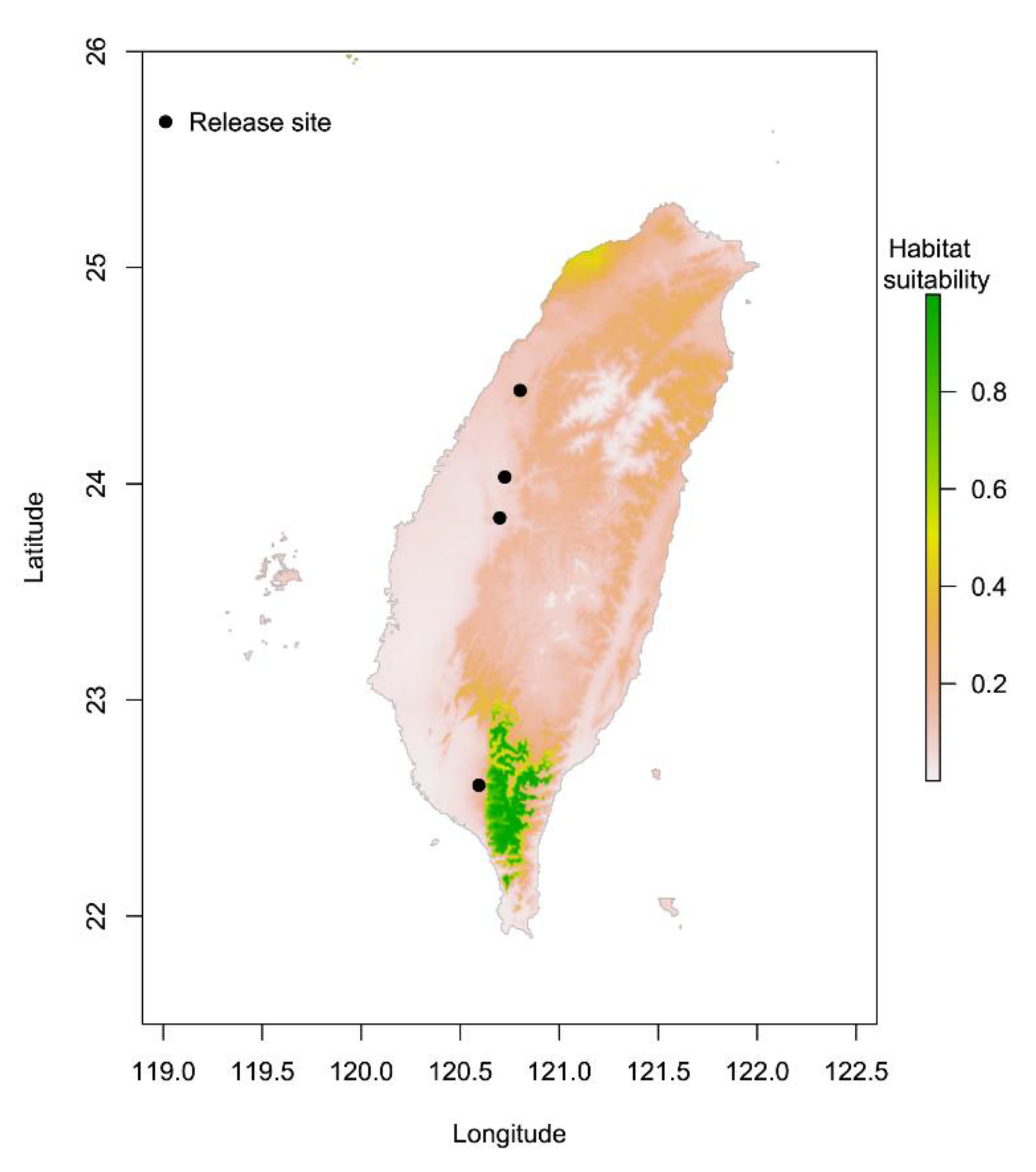
3.1. Comparison of Suitable Distributions of P. persimilis and Four Release Sites in Taiwan
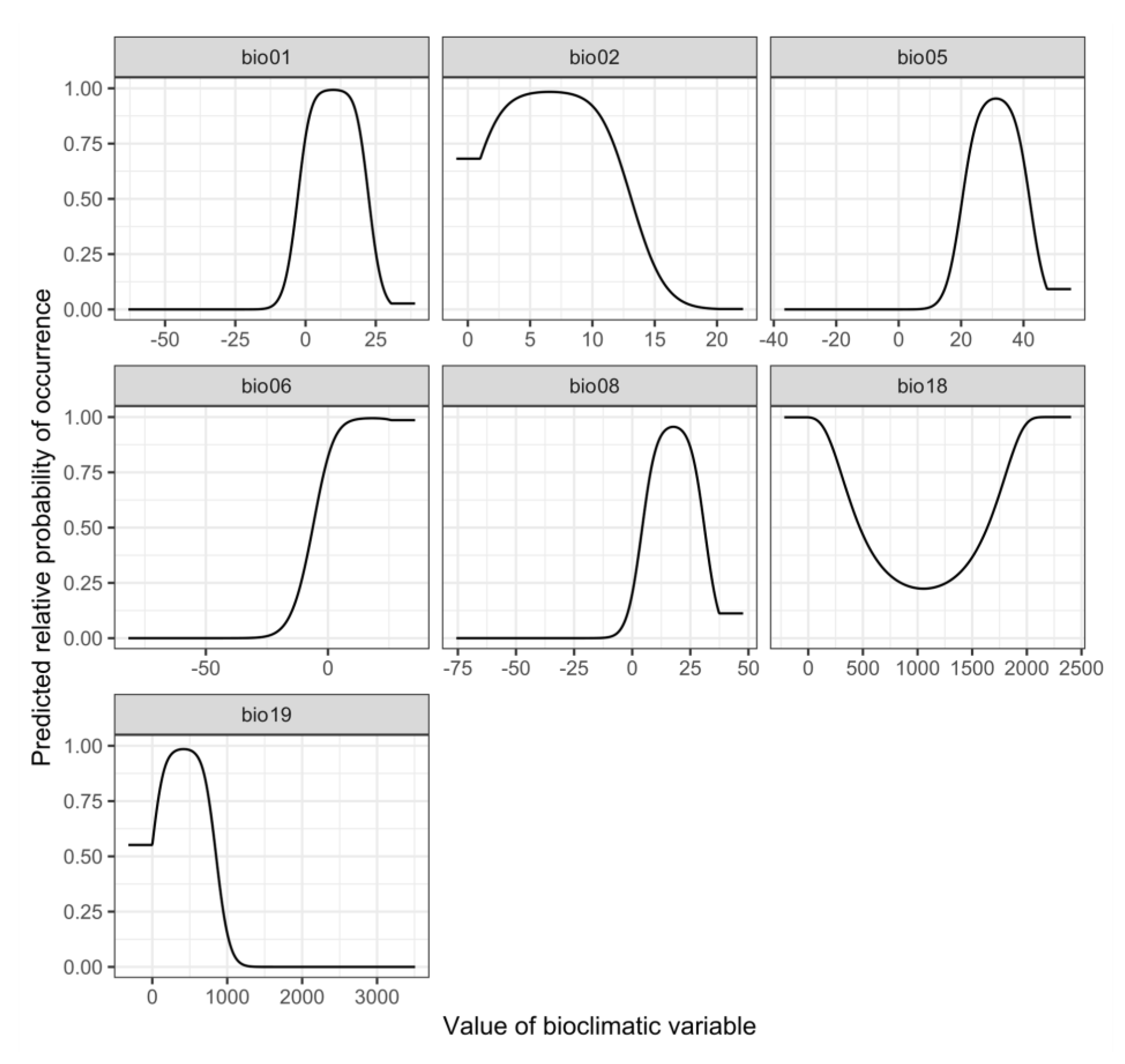
3.2. Environmental Variables Influencing the Distribution of Phytoseiulus persimilis
4. Discussion
4.1. Potential Suitable Areas for P. persimilis and the Four Release Sites
4.2. Environmental Variables Affect the Establishment of P. pesimilis
4.3. Necessity of Predicting the Potential Distributions of Exotic Natural Enemies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Lenteren, J.; Babendreier, D.; Bigler, F.; Burgio, G.; Hokkanen, H.M.T.; Kuske, S.; Loomans, A.J.M.; Menzler-Hokkanen, I.; van Rijn, P.C.J.; Thomas, M.B.; et al. Environmental risk assessment of exotic natural enemies used in inundative biological control. BioControl 2003, 48, 3–38. [Google Scholar] [CrossRef]
- van Lenteren, J.C.; Bale, J.; Bigler, F.; Hokkanen, H.M.T.; Loomans, A.J.M. Assessing risks of releasing exotic biological control agents of arthropod pests. Annu. Rev. Entomol. 2006, 51, 609–634. [Google Scholar] [CrossRef]
- Cock, M.J.W.; van Lenteren, J.C.; Brodeur, J.; Barrat, B.I.P.; Bigler, F.; Bolckmans, K.; Cônsoli, F.L.; Haas, F.; Mason, P.G.; Parra, J.R.P. Do new access and benefit sharing procedures under the convention on biological diversity threaten the future of biological control? BioControl 2010, 55, 199–218. [Google Scholar] [CrossRef]
- Hajek, A.E.; Hurley, B.P.; Kenis, M.; Garnas, J.R.; Bush, S.J.; Wingfield, M.J.; van Lenteren, J.C.; Cock, M.J.W. Exotic biological control agents: A solution or contribution to arthropod invasions? Biol. Invasions 2016, 18, 953–969. [Google Scholar] [CrossRef]
- Wyckhuys, K.A.G.; Hughes, A.C.; Buamas, C.; Johnson, A.C.; Vasseur, L.; Reymondin, L.; Deguine, J.-P.; Sheil, D. Biological control of an agricultural pest protects tropical forests. Commun. Biol. 2019, 2, 10. [Google Scholar] [CrossRef]
- Kaufman, L.V.; Yalemar, J.; Wright, W.G. Classical biological control of the erythrina gall wasp, Quadrastichus erythrinae, in Hawaii: Conserving an endangered habitat. Biol. Control 2020, 142, 104161. [Google Scholar] [CrossRef]
- Messelink, G.J.; Bennison, J.; Alomar, O.; Ingegno, B.L.; Tavella, L.; Shipp, L.; Palevsky, E.; Wäckers, F.L. Approaches to conserving natural enemy populations in greenhouse crops: Current methods and future prospects. BioControl 2014, 59, 377–393. [Google Scholar] [CrossRef]
- van Lenteren, J.C.; Loomans, A.J.M.; Babendreier, D.; Bigler, F. Harmonia axyridis: An environmental risk assessment for Northwest Europe. In From Biological Control to Invasion: The Ladybird Harmonia Axyridis as a Model Species; Roy, H.E., Wajnberg, E., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 37–54. [Google Scholar] [CrossRef]
- Loomans, A.J.M. Every generalist biological control agent requires a special risk assessment. BioControl 2021, 66, 23–35. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Qiao, H.J.; Hu, J.H.; Huang, J.H. Theoretical basis, future directions, and challenges for ecological niche models. Sci. Sin. Vitae 2013, 43, 915–927. (In Chinese) [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Rome, Q.; Villemant, C.; Courchamp, F. Can species distribution models really predict the expansion of invasive species? PLoS ONE 2018, 13, e0193085. [Google Scholar] [CrossRef]
- Xu, D.; Li, X.; Jin, Y.; Zhuo, Z.; Yang, H.; Hu, J.; Wang, R. Influence of climatic factors on the potential distribution of pest Heortia vitessoides Moore in China. Glob. Ecol. Conserv. 2020, 23, e01107. [Google Scholar] [CrossRef]
- Jarnevich, C.S.; Young, N. Using the MAXENT program for species distribution modelling to assess invasion risk. In Pest Risk Modelling and Mapping for Invasive Alien Species; Venette, R.C., Ed.; CABI: Wallingford, UK, 2015; pp. 65–81. [Google Scholar]
- Tang, J.; Li, J.; Lu, H.; Lu, F.; Lu, B. Potential distribution of an invasive pest, Euplatypus parallelus, in China as predicted by Maxent. Pest. Manag. Sci. 2019, 75, 1630–1637. [Google Scholar] [CrossRef]
- Slatculescu, A.M.; Clow, K.M.; McKay, R.; Talbot, B.; Logan, J.J.; Thickstun, C.R.; Jardine, C.M.; Ogden, N.H.; Knudby, A.J.; Kulkarni, M.A. Species distribution models for the eastern blacklegged tick, Ixodes scapularis, and the Lyme disease pathogen, Borrelia burgdorferi, in Ontario, Canada. PLoS ONE 2020, 15, e0238126. [Google Scholar] [CrossRef]
- Negrini, M.; Fidelis, E.G.; Picanço, M.C.; Ramos, R.S. Mapping of the Steneotarsonemus spinki invasion risk in suitable areas for rice (Oryza sativa) cultivation using MaxEnt. Exp. Appl. Acarol. 2020, 80, 445–461. [Google Scholar] [CrossRef]
- Chant, D.A.; McMurtry, J.A. Illustrated Keys and Diagnoses for the Genera and Subgenera of the Phytoseiidae of the World (Acari: Mesostigmata); Indira Publication House: West Bloomfield, MI, USA, 2007. [Google Scholar]
- McMurtry, J.A.; De Moraes, G.J.; Sourassou, N.F. Revision of the lifestyles of phytoseiid mites (Acari: Phytoseiidae) and implications for biological control strategies. Syst. Appl. Acarol. 2013, 18, 297–320. [Google Scholar] [CrossRef]
- Liao, J.R.; Ho, C.C.; Lee, H.C.; Ko, C.C. Phytoseiidae of Taiwan (Acari: Mesostigmata); National Taiwan University Press: Taipei, Taiwan, 2020. [Google Scholar]
- EPPO. List of Biological Control Agents Widely Used in the EPPO Region: PM6/3(2) 2020 Version. Available online: https://www.eppo.int/RESOURCES/eppo_standards/pm6_biocontrol (accessed on 30 August 2020).
- Athias-Henriot, C. Phytoseiidae et Aceosejidae (Acarina, Gamasina) d’Algerie. I. Genres Blattisocius Keegan, Iphiseius Berlese, Amblyseius Berlese, Phytoseius Ribaga, Phytoseiulus Evans. Bull. Soc. Hist. Nat. Afr. N.L. 1957, 48, 319–352. [Google Scholar]
- Migeon, A.; Tixier, M.-S.; Navajas, M.; Litskas, V.D.; Stavrinides, M.C. A predator-prey system: Phytoseiulus persimilis (Acari: Phytoseiidae) and Tetranychus urticae (Acari: Tetranychidae): Worldwide occurrence datasets. Acarologia 2019, 59, 301–307. [Google Scholar] [CrossRef]
- Takahashi, F.; Chant, D.A. Phylogenetic relationships in the genus Phytoseiulus Evans (Acari: Phytoseiidae). II. Taxonomic review. Int. J. Acarol. 1993, 19, 23–37. [Google Scholar] [CrossRef]
- Demite, P.R.; De Moraes, G.J.; McMurtry, J.A.; Denmark, H.A.; Castilho, R.C. Phytoseiidae Database. Available online: http://www.lea.esalq.usp.br/phytoseiidae/ (accessed on 24 January 2021).
- Stenseth, C. Effect of temperature and humidity on the development of Phytoseiulus Persimilis and its ability to regulate populations of Tetranychus urticae [Acarina: Phytoseiidae. Tetranychidae]. Entomophaga 1979, 24, 311–317. [Google Scholar] [CrossRef]
- Laing, J.E. Life history and life table of Phytoseiulus persimilis. Acarologia 1968, 10, 578–588. [Google Scholar]
- Skirvin, D.J.; Fenlon, J.S. The effect of temperature on the functional response of Phytoseiulus persimilis (Acari: Phytoseiidae). Exp. Appl. Acarol. 2003, 31, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, S.; Kishimoto, H.; Amano, H. Phytoseiid Mite Portal. Available online: https://phytoseiidae.acarology-japan.org/ (accessed on 10 January 2021).
- Rasmy, A.H.; Ellaithy, A.Y.M. Introduction of Phytoseiulus persimilis for two spotted spider mite control in greenhouses in Egypt [Acari: Phytoseiidae, tetranychidae]. Entomophaga 1988, 33, 435–438. [Google Scholar] [CrossRef]
- Lee, W.T.; Lo, K.C. Integrated control of two-spotted spider mite on strawberry in Taiwan. Chinese J. Entomol. Special Publ. 1989, 3, 125–137. (In Chinese) [Google Scholar]
- Ho, C.C. A preliminary study on the biological control of Tetranychus kanzawai in tea field by Amblyseius fallacis and Phytoseiulus persimilis (Acarina: Tetranychidae, Phytoseiidae). J. Agric. Res. China 1990, 39, 133–140. (In Chinese) [Google Scholar]
- Hao, H.H.; Wang, H.L.; Lee, W.T.; Lo, K.C. Studies on biological control of spider mites on papaya. J. Agric. Res. China 1996, 45, 411–421. (In Chinese) [Google Scholar]
- Peterson, A.T.; Papes, M.; Eaton, M. Transferability and model evaluation in ecological niche modeling: A comparison of GARP and Maxent. Ecography 2007, 30, 550–560. [Google Scholar] [CrossRef]
- Fourcade, Y.; Engler, J.O.; Rödder, D.; Secondi, J. Mapping species distributions with MAXENT using a geographically biased sample of presence data: A performance assessment of methods for correcting sampling bias. PLoS ONE 2014, 9, e97122. [Google Scholar] [CrossRef]
- QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation Project. Available online: http://qgis.osgeo.org (accessed on 16 January 2021).
- Phillips, S.J. A Brief Tutorial on MAXENT. Available online: http://biodiversityinformatics.amnh.org/open_source/maxent/ (accessed on 20 December 2020).
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1 km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- De Meyer, M.; Robertson, M.P.; Mansell, M.W.; Ekesi, S.; Tsuruta, K.; Mwaiko, W.; Vayssières, J.-F.; Peterson, A.T. Ecological niche and potential geographic distribution of the invasive fruit fly Bactrocera invadens (Diptera, Tephritidae). Bull. Entomol. Res. 2010, 100, 35–48. [Google Scholar] [CrossRef]
- Hijmans, R. Package “Raster”. Available online: http://raster.r-forge.r-project.org/ (accessed on 22 December 2020).
- Bivand, R.; Keitt, T.; Rowlingson, B. Package “Rgdal”. Available online: https://CRAN.R-project.org/package=rgdal (accessed on 22 December 2020).
- Phillips, S.J.; Dudik, M.; Schapire, R.E. Maxent Software for Modeling Species Niches and Distribution (Version 3.4.1). Available online: https://biodiversityinformatics.amnh.org/open_source/maxent/ (accessed on 20 December 2020).
- Hijmans, R.J.; Phillips, S.; Leathwick, J.; Elith, J. Package ‘Dismo’. Available online: http://cran.r-project.org/web/packages/dismo/index.html (accessed on 20 December 2020).
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENMeval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Hernandez, P.A.; Graham, C.H.; Master, L.L.; Albert, D.L. The effect of sample size and species characteristics on performance of different species distribution modelling methods. Ecography 2006, 29, 773–785. [Google Scholar] [CrossRef]
- Peterson, A.T.; Papeş, M.; Soberón, J. Rethinking receiver operating characteristic analysis applications in ecological niche modeling. Ecol. Model. 2008, 213, 63–72. [Google Scholar] [CrossRef]
- Liu, C.; White, M.; Newell, G. Selecting thresholds for the prediction of species occurrence with presence-only data. J. Biogeogr. 2013, 40, 778–789. [Google Scholar] [CrossRef]
- Kumar, S.; Neven, L.G.; Zhu, H.; Zhang, R. Assessing the global risk of establishment of Cydia pomonella (Lepidoptera: Tortricidae) using CLIMEX and MaxEnt niche models. J. Econ. Entomol. 2015, 108, 1708–1719. [Google Scholar] [CrossRef]
- Peterson, A.T.; Soberón, J.; Anderson, R.P.; Pearson, R.G.; Martínez-Meyer, E.; Nakamura, M.; Araújo, M.B. Ecological Niches and Geographic Distributions: A Modeling Perspective; Princeton University Press: Princeton, NJ, USA, 2011. [Google Scholar]
- Boria, R.A.; Olson, L.E.; Goodman, S.M.; Anderson, R.P. Spatial filtering to reduce sampling bias can improve the performance of ecological niche models. Ecol. Model. 2014, 275, 73–77. [Google Scholar] [CrossRef]
- Velasco, J.A.; Gonzalez-Salazar, C. Akaike information criterion should not be a “test” of geographical prediction accuracy in ecological niche modelling. Ecol. Inform. 2019, 51, 25–32. [Google Scholar] [CrossRef]
- Jayasinghe, S.L.; Kumar, L. Modeling the climate suitability of tea [Camellia sinensis (L.) O. Kuntze] in Sri Lanka in response to current and future climate change scenarios. Agric. For. Meteorol. 2019, 272, 102–117. [Google Scholar] [CrossRef]
- Feeley, K.J.; Silman, M.R. Keep collecting: Accurate species distribution modelling requires more collections than previously thought. Divers. Distrib. 2011, 17, 1132–1140. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Kondo, A. Colonizing characteristics of two phytoseiid mites, Phytoseiulus persimilis Athias-Henriot and Neoseiulus womersleyi (Schicha) (Acari: Phytoseiidae) on greenhouse grapevine and effects of their release on the kanzawa spider mite, Tetranychus kanzawai Kishida (Acari: Tetranychidae). Appl. Entomol. Zool. 2004, 39, 643–649. [Google Scholar] [CrossRef][Green Version]
- Abad-Moyano, R.; Urbaneja, A.; Schausberger, P. Intraguild interactions between Euseius stipulatus and the candidate biocontrol agents of Tetranychus urticae in Spanish clementine orchards: Phytoseiulus persimilis and Neoseiulus californicus. Exp. Appl. Acarol. 2010, 50, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Morewood, W.D. Diapause and cold hardiness of phytoseiid mites (Acarina: Phytoseiidae). Eur. J. Entomol. 1993, 90, 3–10. [Google Scholar]

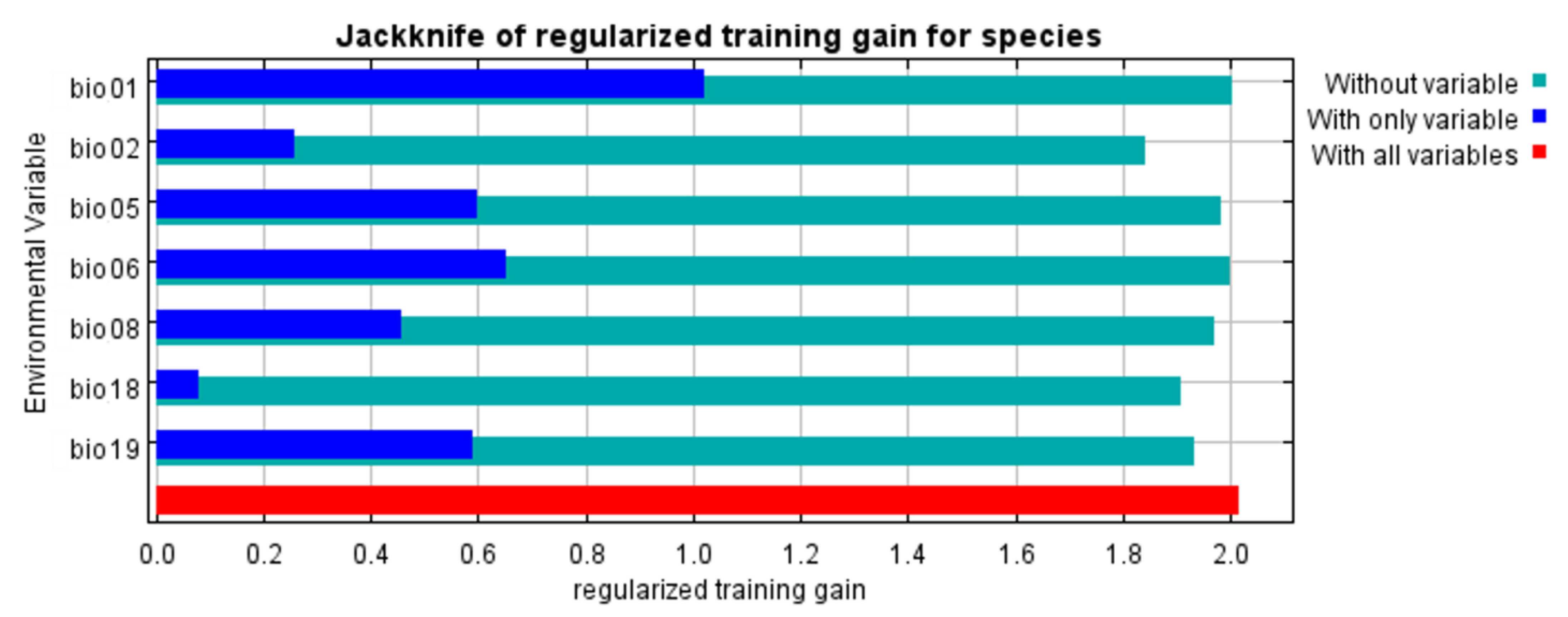
| Variable Codes | Environment Variables | Contribution Rate % | Permutation Importance % |
|---|---|---|---|
| bio06 | Min temperature of coldest month (°C) | 44.7 | 25.2 |
| bio02 | Mean diurnal range (°C) | 24.8 | 8.1 |
| bio18 | Precipitation of warmest quarter (mm) | 10.6 | 5 |
| bio19 | Precipitation of coldest quarter (mm) | 6.4 | 1.7 |
| bio05 | Max temperature of warmest month (°C) | 5.7 | 19.5 |
| bio08 | Mean temperature of wettest quarter (°C) | 4.4 | 18.8 |
| bio01 | Annual mean temperature (°C) | 3.5 | 21.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, J.-R.; Ho, C.-C.; Chiu, M.-C.; Ko, C.-C. Niche Modeling May Explain the Historical Population Failure of Phytoseiulus persimilis in Taiwan: Implications of Biocontrol Strategies. Insects 2021, 12, 418. https://doi.org/10.3390/insects12050418
Liao J-R, Ho C-C, Chiu M-C, Ko C-C. Niche Modeling May Explain the Historical Population Failure of Phytoseiulus persimilis in Taiwan: Implications of Biocontrol Strategies. Insects. 2021; 12(5):418. https://doi.org/10.3390/insects12050418
Chicago/Turabian StyleLiao, Jhih-Rong, Chyi-Chen Ho, Ming-Chih Chiu, and Chiung-Cheng Ko. 2021. "Niche Modeling May Explain the Historical Population Failure of Phytoseiulus persimilis in Taiwan: Implications of Biocontrol Strategies" Insects 12, no. 5: 418. https://doi.org/10.3390/insects12050418
APA StyleLiao, J.-R., Ho, C.-C., Chiu, M.-C., & Ko, C.-C. (2021). Niche Modeling May Explain the Historical Population Failure of Phytoseiulus persimilis in Taiwan: Implications of Biocontrol Strategies. Insects, 12(5), 418. https://doi.org/10.3390/insects12050418






