Identification of Blackberry (Rubus fruticosus) Volatiles as Drosophila suzukii Attractants
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fly Rearing
2.2. Chemical Analysis of R. fruticosus Volatiles
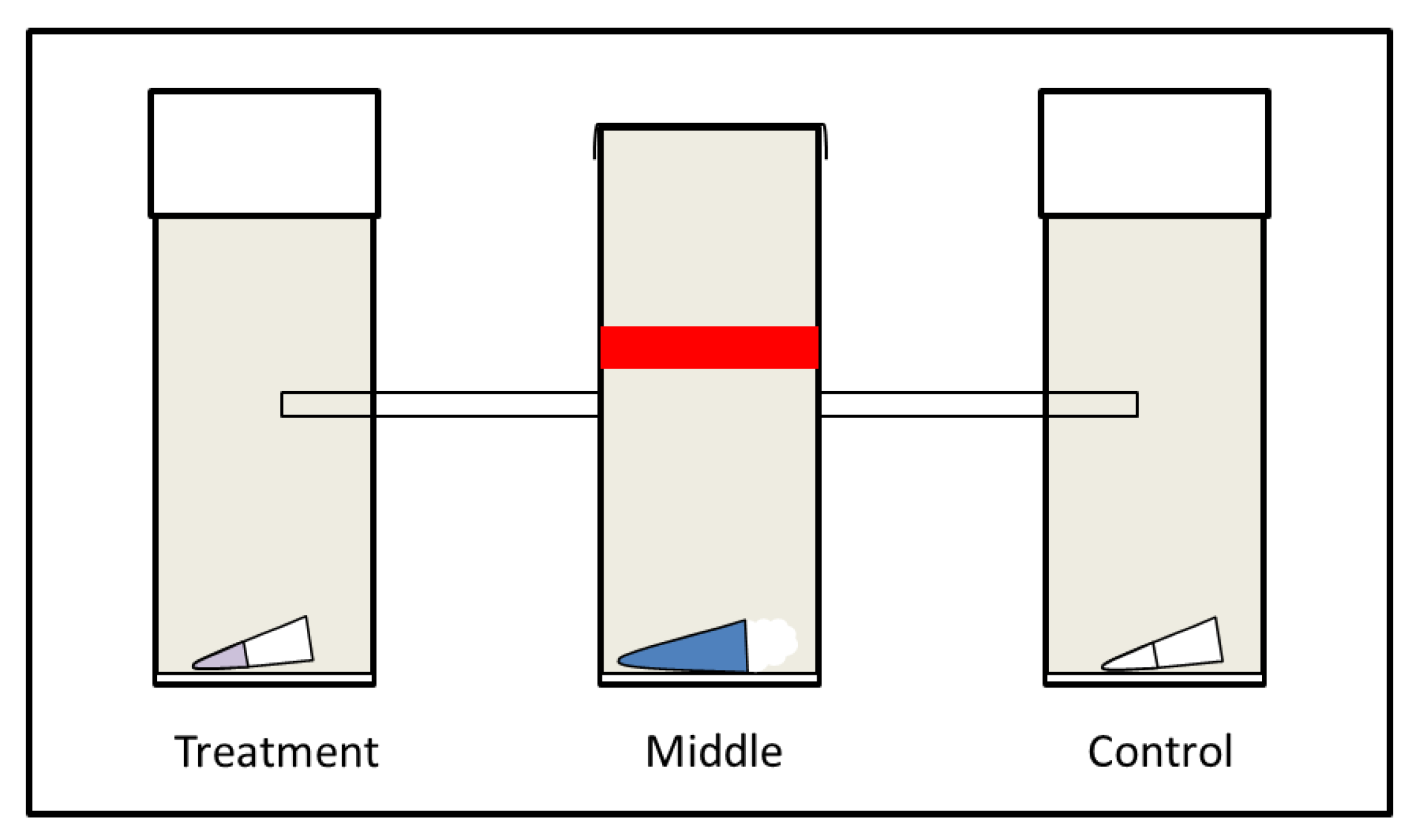
2.3. Experimental Set-Up
2.4. Behavioural Experiments with Rubus fruticosus Berries
2.5. Behavioural Experiments with Individual Compounds
2.6. Statistical Analysis
3. Results
3.1. Identification and Quantification of R. fruticosus Volatiles
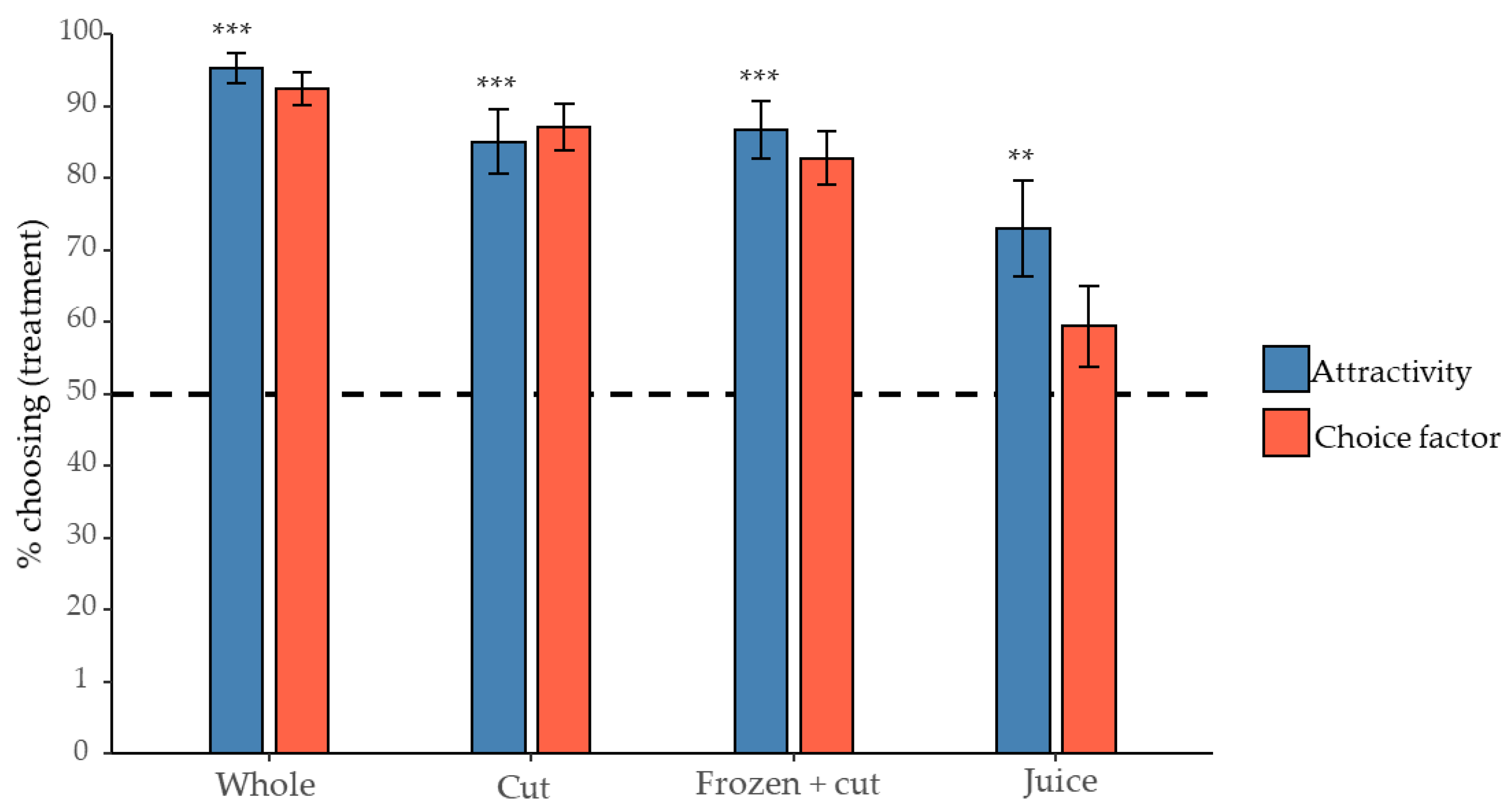
3.2. The Effect of the Condition of R. fruticosus Berry on D. suzukii Attraction
3.3. The Attractivity of Individual R. fruticosus Volatiles on D. suzukii
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calabria, G.; Máca, J.; Bächli, G.; Serra, L.; Pascual, M. First records of the potential pest species Drosophila suzukii (Diptera: Drosophilidae) in Europe. J. Appl. Entomol. 2012, 136, 139–147. [Google Scholar] [CrossRef]
- Lee, J.C.; Bruck, D.J.; Dreves, A.J.; Ioriatti, C.; Vogt, H.; Baufeld, P. In Focus: Spotted wing drosophila, Drosophila suzukii, across perspectives. Pest Manag. Sci. 2011, 67, 1349–1351. [Google Scholar] [CrossRef] [PubMed]
- Asplen, M.K.; Anfora, G.; Biondi, A.; Choi, D.-S.; Chu, D.; Daane, K.M.; Gibert, P.; Gutierrez, A.P.; Hoelmer, K.A.; Hutchison, W.D.; et al. Invasion biology of spotted wing Drosophila (Drosophila suzukii): A global perspective and future priorities. J. Pest Sci. 2015, 88, 469–494. [Google Scholar] [CrossRef]
- Cini, A.; Ioriatti, C.; Anfora, G. A review of the invasion of Drosophila suzukii in Europe and a draft research agenda for integrated pest management. Bull. Insectology 2012, 65, 149–160. [Google Scholar]
- Shaw, B.; Brain, P.; Wijnen, H.; Fountain, M.T. Reducing Drosophila suzukii emergence through inter-species competition. Pest Manag. Sci. 2018, 74, 1466–1471. [Google Scholar] [CrossRef] [PubMed]
- Leach, H.; Van Timmeren, S.; Isaacs, R. Exclusion Netting Delays and Reduces Drosophila suzukii (Diptera: Drosophilidae) Infestation in Raspberries. J. Econ. Entomol. 2016, 109, 2151–2158. [Google Scholar] [CrossRef] [PubMed]
- Reher, T.; Van Kerckvoorde, V.; Verheyden, L.; Wenseleers, T.; Beliën, T.; Bylemans, D.; Martens, J.A. Evaluation of hop (Humulus lupulus) as a repellent for the management of Drosophila suzukii. Crop Prot. 2019, 124. [Google Scholar] [CrossRef]
- Cha, D.H.; Roh, G.H.; Hesler, S.P.; Wallingford, A.; Stockton, D.G.; Park, S.K.; Loeb, G.M. 2-Pentylfuran: A Novel Repellent of Drosophila Suzukii. Pest Manag. Sci. 2021, 77, 1757–1764. [Google Scholar] [CrossRef]
- de Souza, M.T.; de Souza, M.T.; Bernardi, D.; Krinski, D.; de Melo, D.J.; da Costa Oliveira, D.; Rakes, M.; Zarbin, P.H.G.; de Noronha Sales Maia, B.H.L.; Zawadneak, M.A.C. Chemical composition of essential oils of selected species of Piper and their insecticidal activity against Drosophila suzukii and Trichopria anastrephae. Environ. Sci. Pollut. Res. 2020, 27, 13056–13065. [Google Scholar] [CrossRef]
- Wallingford, A.K.; Cha, D.H.; Loeb, G.M. Evaluating a push–pull strategy for management of Drosophila suzukii Matsumura in red raspberry. Pest Manag. Sci. 2018, 74, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Renkema, J.M.; Devkota, S. Pupation depth of spotted wing drosophila (Drosophila suzukii) and effects of field sanitation in Florida strawberries. In Proceedings of the Acta Horticulturae; Gulf Coast Research and Education Center: Wimauma, FL, USA, 2016; Volume 1156, pp. 849–855. [Google Scholar]
- Hooper, H.; Grieshop, M.J. Postharvest Burial of Drosophila suzukii (Diptera: Drosophilidae) Infested Fruit Waste Reduces Adult Emergence. Environ. Entomol. 2020, 49, 59–65. [Google Scholar] [CrossRef]
- Hampton, E.; Koski, C.; Barsoian, O.; Faubert, H.; Cowles, R.S.; Alm, S.R. Use of Early Ripening Cultivars to Avoid Infestation and Mass Trapping to Manage Drosophila suzukii (Diptera: Drosophilidae) in Vaccinium corymbosum (Ericales: Ericaceae). Hortic. Entomol. 2014, 107, 1849–1857. [Google Scholar] [CrossRef] [PubMed]
- Tonina, L.; Grassi, A.; Caruso, S.; Mori, N.; Gottardello, A.; Anfora, G.; Giomi, F.; Vaccari, G.; Ioriatti, C. Comparison of attractants for monitoring Drosophila suzukii in sweet cherry orchards in Italy. J. Appl. Entomol. 2018, 142, 18–25. [Google Scholar] [CrossRef]
- Van Kerckvoorde, V.; Clymans, R.; Bangels, E.; Alhmedi, A.; De Ketelaere, B.; De Clercq, P.; Bylemans, D.; Belien, T. Tunnel entries and a killing agent uncover the importance of fly retention in Drosophila suzukii traps. Pest Manag. Sci. 2020, 76, 3459–3468. [Google Scholar] [CrossRef]
- Cha, D.H.; Adams, T.; Werle, C.T.; Sampson, B.J.; Adamczyk, J.J.; Rogg, H.; Landolt, P.J. A four-component synthetic attractant for Drosophila suzukii (Diptera: Drosophilidae) isolated from fermented bait headspace. Pest Manag. Sci. 2014, 70, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Larson, N.R.; Strickland, J.; Shields, V.D.C.; Zhang, A. Controlled-Release Dispenser and Dry Trap Developments for Drosophila suzukii Detection. Front. Ecol. Evol. 2020, 8, 45. [Google Scholar] [CrossRef]
- Landolt, P.J.; Adams, T.; Rogg, H. Trapping spotted wing drosophila, Drosophila suzukii (Matsumura) (Diptera: Drosophilidae), with combinations of vinegar and wine, and acetic acid and ethanol. J. Appl. Entomol. 2011, 136, 148–154. [Google Scholar] [CrossRef]
- Snellings, Y.; Herrera, B.; Wildemann, B.; Beelen, M.; Zwarts, L.; Wenseleers, T.; Callaerts, P. The role of cuticular hydrocarbons in mate recognition in Drosophila suzukii. Sci. Rep. 2018, 8, 4996. [Google Scholar] [CrossRef]
- Lee, J.C.; Burrack, H.J.; Barrantes, L.D.; Beers, E.H.; Dreves, A.J.; Hamby, K.A.; Haviland, D.R.; Isaacs, R.; Richardson, T.A.; Shearer, P.W.; et al. Evaluation of monitoring traps for Drosophila suzukii (diptera: Drosophilidae) in North America. J. Econ. Entomol. 2012, 105, 1350–1357. [Google Scholar] [CrossRef]
- Cha, D.H.; Adams, T.; Rogg, H.; Landolt, P.J. Identification and Field Evaluation of Fermentation Volatiles from Wine and Vinegar that Mediate Attraction of Spotted Wing Drosophila, Drosophila suzukii. J. Chem. Ecol. 2012, 38, 1419–1431. [Google Scholar] [CrossRef]
- Feng, Y.; Bruton, R.; Park, A.; Zhang, A. Identification of attractive blend for spotted wing drosophila, Drosophila suzukii, from apple juice. J. Pest Sci. 2018, 91, 1251–1267. [Google Scholar] [CrossRef]
- Burrack, H.J.; Asplen, M.; Bahder, L.; Collins, J.; Drummond, F.A.; Guédot, C.; Isaacs, R.; Johnson, D.; Blanton, A.; Lee, J.C.; et al. Multistate comparison of attractants for monitoring Drosophila suzukii (Diptera: Drosophilidae) in blueberries and caneberries. Environ. Entomol. 2015, 44, 704–712. [Google Scholar] [CrossRef]
- Cha, D.H.; Hesler, S.P.; Wallingford, A.K.; Zaman, F.; Jentsch, P.; Nyrop, J.; Loeb, G.M. Comparison of Commercial Lures and Food Baits for Early Detection of Fruit Infestation Risk by Drosophila suzukii (Diptera: Drosophilidae). J. Econ. Entomol. 2018, 111, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Revadi, S.; Vitagliano, S.; Rossi Stacconi, M.V.; Ramasamy, S.; Mansourian, S.; Carlin, S.; Vrhovsek, U.; Becher, P.G.; Mazzoni, V.; Rota-Stabelli, O.; et al. Olfactory responses of Drosophila suzukii females to host plant volatiles. Physiol. Entomol. 2015, 40, 54–64. [Google Scholar] [CrossRef]
- Lee, J.C.; Bruck, D.J.; Curry, H.; Edwards, D.; Haviland, D.R.; Van Steenwyk, R.A.; Yorgey, B.M. The susceptibility of small fruits and cherries to the spotted-wing drosophila, Drosophila suzukii. Pest Manag. Sci. 2011, 67, 1358–1367. [Google Scholar] [CrossRef]
- Abraham, J.; Zhang, A.; Angeli, S.; Abubeker, S.; Michel, C.; Feng, Y.; Rodriguez-Saona, C. Behavioral and Antennal Responses of Drosophila suzukii (Diptera: Drosophilidae) to Volatiles From Fruit Extracts. Environ. Entomol. 2015, 44, 356–367. [Google Scholar] [CrossRef]
- Diepenbrock, L.M.; Burrack, H.J. Variation of within-crop microhabitat use by Drosophila suzukii (Diptera: Drosophilidae) in blackberry. J. Appl. Entomol. 2017, 141, 1–7. [Google Scholar] [CrossRef]
- Burrack, H.J.; Fernandez, G.E.; Spivey, T.; Kraus, D.A. Variation in selection and utilization of host crops in the field and laboratory by Drosophila suzukii Matsumara (Diptera: Drosophilidae), an invasive frugivore. Pest Manag. Sci. 2013, 69, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Serrano, E.; Beltran, J.; Hernandez, F. Application of multiple headspace-solid-phase microextraction followed by gas chromatography-mass spectrometry to quantitative analysis of tomato aroma components. J. Chromatogr. A 2009, 1216, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Guggenberger, M.; Potthast, A.; Rosenau, T.; Böhmdorfer, S. Quantification of Volatiles from Technical Lignins by Multiple Headspace Sampling-Solid-Phase Microextraction-Gas Chromatography-Mass Spectrometry. ACS Sustain. Chem. Eng. 2019, 7, 9896–9903. [Google Scholar] [CrossRef]
- Costa, R.; Tedone, L.; De Grazia, S.; Dugo, P.; Mondello, L. Multiple headspace-solid-phase microextraction: An application to quantification of mushroom volatiles. Anal. Chim. Acta 2013, 770, 1–6. [Google Scholar] [CrossRef]
- D’Agostino, M.F.; Sanz, J.; Sanz, M.L.; Giuffre, A.M.; Sicari, V.; Soria, A.C. Optimization of a Solid-Phase Microextraction method for the Gas Chromatography-Mass Spectrometry analysis of blackberry (Rubus ulmifolius Schott) fruit volatiles. Food Chem. 2015, 178, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Goelen, T.; Sobhy, I.S.; Vanderaa, C.; de Boer, J.G.; Delvigne, F.; Francis, F.; Wäckers, F.; Rediers, H.; Verstrepen, K.J.; Wenseleers, T.; et al. Volatiles of bacteria associated with parasitoid habitats elicit distinct olfactory responses in an aphid parasitoid and its hyperparasitoid. Funct. Ecol. 2020, 34, 507–520. [Google Scholar] [CrossRef]
- Goelen, T.; Sobhy, I.S.; Vanderaa, C.; Wäckers, F.; Rediers, H.; Wenseleers, T.; Jacquemyn, H.; Lievens, B. Bacterial phylogeny predicts volatile organic compound composition and olfactory response of an aphid parasitoid. Oikos 2020, 129, 1415–1428. [Google Scholar] [CrossRef]
- Messadi, D.; Helaimia, F.; Ali-Mokhnache, S.; Boumahraz, M. Accurate determination of retention indices in programmed temperature gas chromatography. Chromatographia 1990, 29, 429–434. [Google Scholar] [CrossRef]
- Lawson, C.; Hanson, R. Solving Least Squares Problems; Society for Industrial and Applied Mathematics: Philadelphia, PA, USA, 1995. [Google Scholar]
- Cloonan, K.R.; Abraham, J.; Angeli, S.; Syed, Z.; Rodriguez-Saona, C. Advances in the Chemical Ecology of the Spotted Wing Drosophila (Drosophila suzukii) and its Applications. J. Chem. Ecol. 2018, 44, 922–939. [Google Scholar] [CrossRef] [PubMed]
- Jacques, A.C.; Chaves, F.C.; Zambiazi, R.C.; Brasil, M.C.; Caramão, E.B. Bioactive and volatile organic compounds in Southern Brazilian blackberry (Rubus Fruticosus) fruit cv. Tupy. Food Sci. Technol. 2014, 34, 636–643. [Google Scholar] [CrossRef]
- Kirkpatrick, D.M.; Leach, H.L.; Xu, P.; Dong, K.; Isaacs, R.; Gut, L.J. Comparative Antennal and Behavioral Responses of Summer and Winter Morph Drosophila suzukii (Diptera: Drosophilidae) to Ecologically Relevant Volatiles. Environ. Entomol. 2018, 47, 700–706. [Google Scholar] [CrossRef]
- Bolton, L.G.; Piñero, J.C.; Barrett, B.A.; Cha, D.H. Electrophysiological and Behavioral Responses of Drosophila suzukii (Diptera: Drosophilidae) Towards the Leaf Volatile β-cyclocitral and Selected Fruit-Ripening Volatiles. Environ. Entomol. 2019, 48, 1049–1055. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, Z.; Shi, M.; Kenis, M.; Dong, W.; Zhang, F.; Zhang, J.; Xiao, C.; Chen, L. Antennal and behavioral responses of Drosophila suzukii to volatiles from a non-crop host, Osyris wightiana. Insects 2021, 12, 166. [Google Scholar] [CrossRef] [PubMed]
- Keesey, I.W.; Knaden, M.; Hansson, B. Olfactory specialization in Drosophila suzukii supports an ecological shift in host preference from rotten to fresh fruit. J. Chem. Ecol. 2015, 41, 121–128. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, W.X.; Zhang, F.; Kenis, M.; Griepink, F.; Zhang, J.P.; Chen, L.; Xiao, C. Identification of active components from volatiles of Chinese bayberry, Myrica rubra attractive to Drosophila suzukii. Arthropod. Plant. Interact. 2018, 12, 435–442. [Google Scholar] [CrossRef]
- Cha, D.H.; Yee, W.L.; Goughnour, R.B.; Sim, S.B.; Powell, T.H.Q.; Feder, J.L.; Linn, C.E. Identification of Host Fruit Volatiles from Domestic Apple (Malus domestica), Native Black Hawthorn (Crataegus douglasii) and Introduced Ornamental Hawthorn (C. monogyna) Attractive to Rhagoletis pomonella Flies from the Western United States. J. Chem. Ecol. 2012, 38, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.H.; Zhai, Y.F.; Chen, H.; Wang, Y.M.; Liu, Q.; Hu, Q.L.; Ren, F.S.; Yu, Y. Ecological niche difference associated with varied ethanol tolerance between Drosophila suzukii and Drosophila melanogaster (Diptera: Drosophilidae). Florida Entomol. 2018, 101, 498–504. [Google Scholar] [CrossRef]
- Dweck, H.K.M.; Ebrahim, S.A.M.; Kromann, S.; Bown, D.; Hillbur, Y.; Sachse, S.; Hansson, B.S.; Stensmyr, M.C. Olfactory preference for egg laying on citrus substrates in Drosophila. Curr. Biol. 2013, 23, 2472–2480. [Google Scholar] [CrossRef]
- Elamrani, A.; David, J.R.; Idaomar, M. Parallel changes in enzyme activity and oviposition behavior in adults of Drosophila melanogaster submitted to alcohols, acetaldehyde or acetone. Invertebr. Reprod. Dev. 2001, 40, 17–25. [Google Scholar] [CrossRef]
- Rasgado, M.A.; Malo, E.A.; Cruz-Lopez, L.; Rojas, J.C.; Toledo, J. Olfactory response of the Mexican fruit fly (Diptera: Tephritidae) to Citrus aurantium volatiles. J. Econ. Entomol. 2009, 102, 585–594. [Google Scholar] [CrossRef]
- Erland, L.A.E.; Rheault, M.R.; Mahmoud, S.S. Insecticidal and oviposition deterrent effects of essential oils and their constituents against the invasive pest Drosophila suzukii (Matsumura) (Diptera: Drosophilidae). Crop Prot. 2015, 78, 20–26. [Google Scholar] [CrossRef]
- Niogret, J.; Epsky, N.D. Attraction of Ceratitis capitata (Diptera: Tephritidae) sterile males to essential oils: The importance of linalool. Environ. Entomol. 2018, 47, 1287–1292. [Google Scholar] [CrossRef] [PubMed]
- Zito, P.; Guarino, S.; Peri, E.; Sajeva, M.; Colazza, S. Electrophysiological and behavioural responses of the housefly to “sweet” volatiles of the flowers of Caralluma europaea (Guss.) N.E. Br. Arthropod. Plant. Interact. 2013, 7, 485–489. [Google Scholar] [CrossRef]
- Diaz-Santiz, E.; Rojas, J.C.; Cruz-López, L.; Hernández, E.; Malo, E.A. Olfactory response of Anastrepha striata (Diptera: Tephritidae) to guava and sweet orange volatiles. Insect Sci. 2016, 23, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cui, Z.; Si, P.; Liu, Y.; Zhou, Q.; Wang, G. Characterization of a specific odorant receptor for linalool in the Chinese citrus fly Bactrocera minax (Diptera: Tephritidae). Insect Biochem. Mol. Biol. 2020, 122, 103389. [Google Scholar] [CrossRef] [PubMed]
- Bruce, T.J.A.; Wadhams, L.J.; Woodcock, C.M. Insect host location: A volatile situation. Trends Plant Sci. 2005, 10, 269–274. [Google Scholar] [CrossRef]
- Bruce, T.J.A.; Pickett, J.A. Perception of plant volatile blends by herbivorous insects—Finding the right mix. Phytochemistry 2011, 72, 1605–1611. [Google Scholar] [CrossRef] [PubMed]
- Szendrei, Z.; Rodriguez-Saona, C. A meta-analysis of insect pest behavioral manipulation with plant volatiles. Entomol. Exp. Appl. 2010, 134, 201–210. [Google Scholar] [CrossRef]
- Graham, K.V.; Choi, M.Y.; Lee, J.C. Attracting chrysopidae with plant volatiles for lace bug (hemiptera: Tingidae) control in rhododendrons and azaleas. J. Insect Sci. 2020, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Guarino, S.; Basile, S.; Arif, M.A.; Manachini, B. Odorants of Capsicum spp. Dried Fruits as Candidate Attractants for Lasioderma serricorne F. (Coleoptera: Anobiidae). Insects 2021, 12, 61. [Google Scholar] [CrossRef]
- Guarino, S.; Arif, M.A.; Millar, J.G.; Colazza, S.; Peri, E. Volatile unsaturated hydrocarbons emitted by seedlings of Brassica species provide host location cues to Bagrada hilaris. PLoS ONE 2018, 13, e0209870. [Google Scholar] [CrossRef]
- Røstelien, T. Recognition of Plant Odor Information in Moths. In Olfactory Concepts of Insect Control-Alternative to Insecticides; Picimbon, J.-F., Ed.; Springer International Publishing: Cham, Switzerland, 2019; Volume 2, pp. 49–91. ISBN 978-3-030-05165-5. [Google Scholar]
- Jaffe, B.D.; Avanesyan, A.; Bal, H.K.; Feng, Y.; Grant, J.; Grieshop, M.J.; Lee, J.C.; Liburd, O.E.; Rhodes, E.; Rodriguez-Saona, C.; et al. Multistate Comparison of Attractants and the Impact of Fruit Development Stage on Trapping Drosophila suzukii (Diptera: Drosophilidae) in Raspberry and Blueberry. Environ. Entomol. 2018, 47, 935–945. [Google Scholar] [CrossRef]
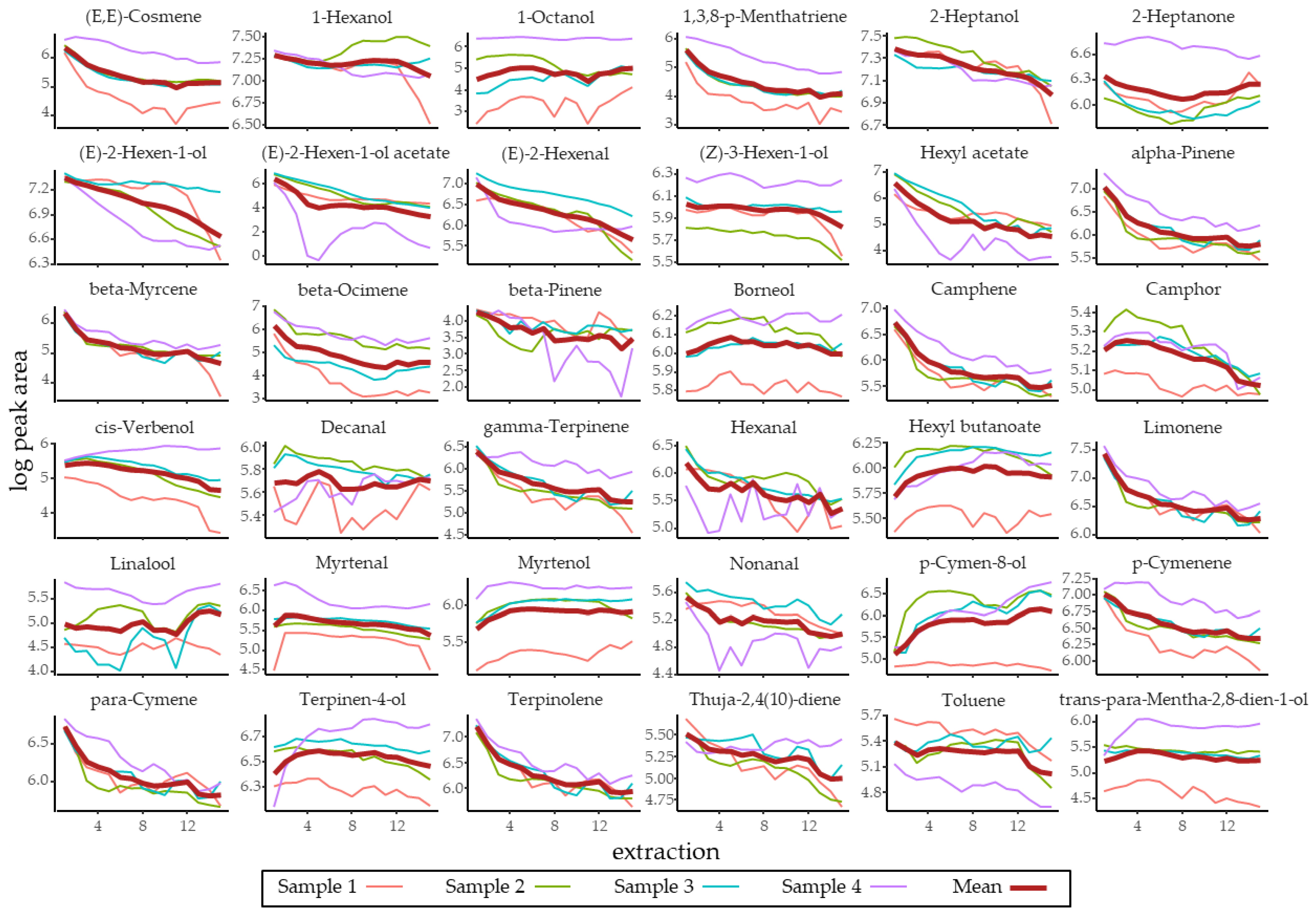

| Compound | RT | RI | Log(Peak Area) | Volume Present in 1 Berry (µL) | Volume Used in Bioassay (µL) |
|---|---|---|---|---|---|
| Acetaldehyde | 0.93 | 408 | - | - | 0.2 |
| Ethanol | 1.02 | 446 | - | - | 0.2 |
| Toluene | 4.95 | 759 | 6.41 | 8.59 × 10−6 | 0.00009 |
| Hexanal | 5.90 | 793 | 6.85 | 7.94 × 10−5 | 0.0008 |
| (E)-2-Hexenal | 7.46 | 849 | 7.58 | 2.35 × 10−4 | 0.002 |
| (Z)-3-Hexen-1-ol | 7.65 | 856 | 7.13 | 7.93 × 10−5 | 0.0008 |
| (E)-2-Hexen-1-ol | 7.99 | 868 | 8.22 | 1.11 × 10−3 | 0.01 |
| 1-Hexanol | 8.09 | 872 | 8.36 | 1.15 × 10−3 | 0.01 |
| 2-Heptanone * | 8.55 | 888 | 7.32 | 1.21 × 10−4 | 0.001 |
| 2-Heptanol | 8.93 | 902 | 8.39 | 1.87 × 10−3 | 0.02 |
| beta-Pinene | 9.50 | 919 | 4.9 | - | - |
| alpha-Pinene | 9.69 | 932 | 7.41 | 4.35 × 10−5 | 0.0004 |
| Camphene | 10.09 | 943 | 7.12 | 2.63 × 10−5 | 0.0003 |
| Thuja-2,4(10)-diene | 10.28 | 950 | 6.4 | - | - |
| beta-Myrcene | 11.38 | 987 | 6.52 | 1.11 × 10−5 | 0.0001 |
| Hexyl acetate | 12.05 | 1009 | 6.74 | 1.78 × 10−5 | 0.0002 |
| (E)-2-Hexen-1-ol, acetate | 12.13 | 1016 | 6.39 | 1.74 × 10−5 | 0.0002 |
| para-Cymene | 12.40 | 1025 | 7.26 | 3.60 × 10−5 | 0.0004 |
| L-Limonene | 12.53 | 1028 | 7.83 | 1.69 × 10−4 | 0.002 |
| beta-Ocimene | 13.19 | 1044 | 6.33 | 1.05 × 10−5 | 0.0001 |
| gamma-Terpinene | 13.56 | 1055 | 6.91 | 2.24 × 10−5 | 0.0002 |
| 1-Octanol * | 14.13 | 1071 | 6.02 | 5.06 × 10−6 | 0.00005 |
| Terpinolene | 14.63 | 1084 | 7.55 | 7.35 × 10−5 | 0.0007 |
| p-Cymenene | 14.74 | 1088 | 7.75 | 7.55 × 10−5 | 0.0008 |
| Linalool * | 15.10 | 1101 | 6.12 | 6.29 × 10−6 | 0.00006 |
| 1,3,8-p-Menthatriene | 15.61 | 1110 | 5.84 | - | - |
| Nonanal | 15.70 | 1115 | 6.35 | 6.72 × 10−6 | 0.00007 |
| trans-para-Mentha-2,8-dien-1-ol * | 16.03 | 1120 | 6.49 | - | - |
| (E,E)-Cosmene | 16.63 | 1134 | 6.68 | - | - |
| Camphor | 16.92 | 1141 | 6.32 | 2.42 × 10−6 | 0.00002 |
| cis-Verbenol | 17.10 | 1145 | 6.35 | 5.83 × 10−5 | 0.0006 |
| Borneol * | 17.98 | 1164 | 7.19 | 4.07 × 10−5 | 0.0004 |
| Terpinen-4-ol | 18.54 | 1176 | 7.69 | 1.84 × 10−2 | 0.2 |
| p-Cymen-8-ol * | 18.89 | 1183 | 7.05 | 5.84 × 10−5 | 0.0006 |
| Hexyl butanoate * | 18.99 | 1191 | 7.1 | 2.37 × 10−3 | 0.02 |
| Myrtenal | 19.36 | 1192 | 6.86 | 8.07 × 10−6 | 0.00008 |
| Myrtenol * | 19.53 | 1196 | 7.06 | 2.03 × 10−2 | 0.2 |
| Decanal * | 19.73 | 1205 | 6.83 | 1.57 × 10−3 | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dewitte, P.; Van Kerckvoorde, V.; Beliën, T.; Bylemans, D.; Wenseleers, T. Identification of Blackberry (Rubus fruticosus) Volatiles as Drosophila suzukii Attractants. Insects 2021, 12, 417. https://doi.org/10.3390/insects12050417
Dewitte P, Van Kerckvoorde V, Beliën T, Bylemans D, Wenseleers T. Identification of Blackberry (Rubus fruticosus) Volatiles as Drosophila suzukii Attractants. Insects. 2021; 12(5):417. https://doi.org/10.3390/insects12050417
Chicago/Turabian StyleDewitte, Peter, Vincent Van Kerckvoorde, Tim Beliën, Dany Bylemans, and Tom Wenseleers. 2021. "Identification of Blackberry (Rubus fruticosus) Volatiles as Drosophila suzukii Attractants" Insects 12, no. 5: 417. https://doi.org/10.3390/insects12050417
APA StyleDewitte, P., Van Kerckvoorde, V., Beliën, T., Bylemans, D., & Wenseleers, T. (2021). Identification of Blackberry (Rubus fruticosus) Volatiles as Drosophila suzukii Attractants. Insects, 12(5), 417. https://doi.org/10.3390/insects12050417






