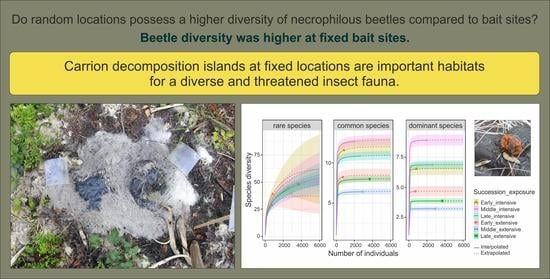
Carcasses at Fixed Locations Host a Higher Diversity of Necrophilous Beetles
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site Description
2.2. Experimental design
2.2.1. Wildlife Carcass Exposure
2.2.2. Pitfall Trap Installation and Beetle Sampling
2.3. Statistics
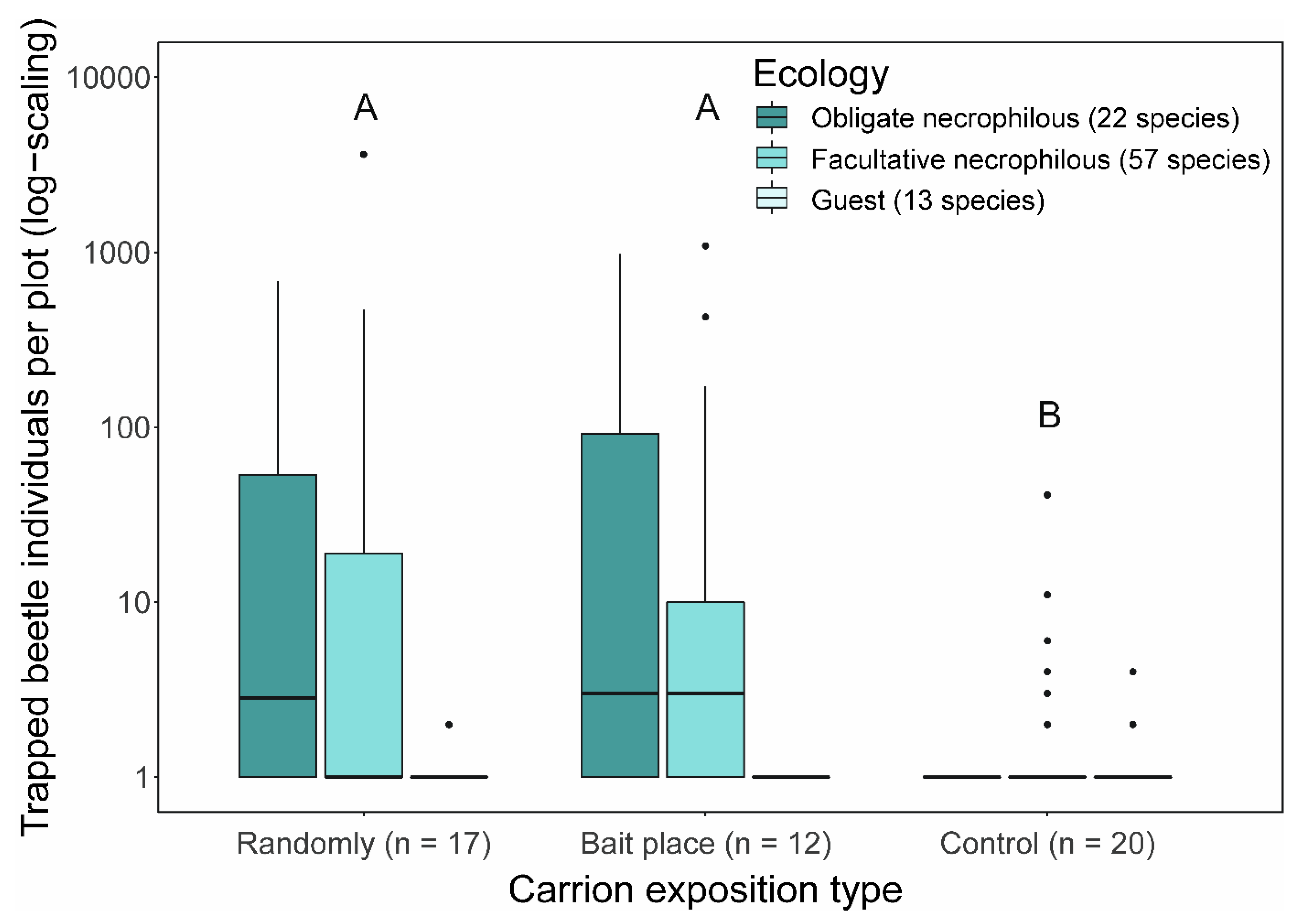
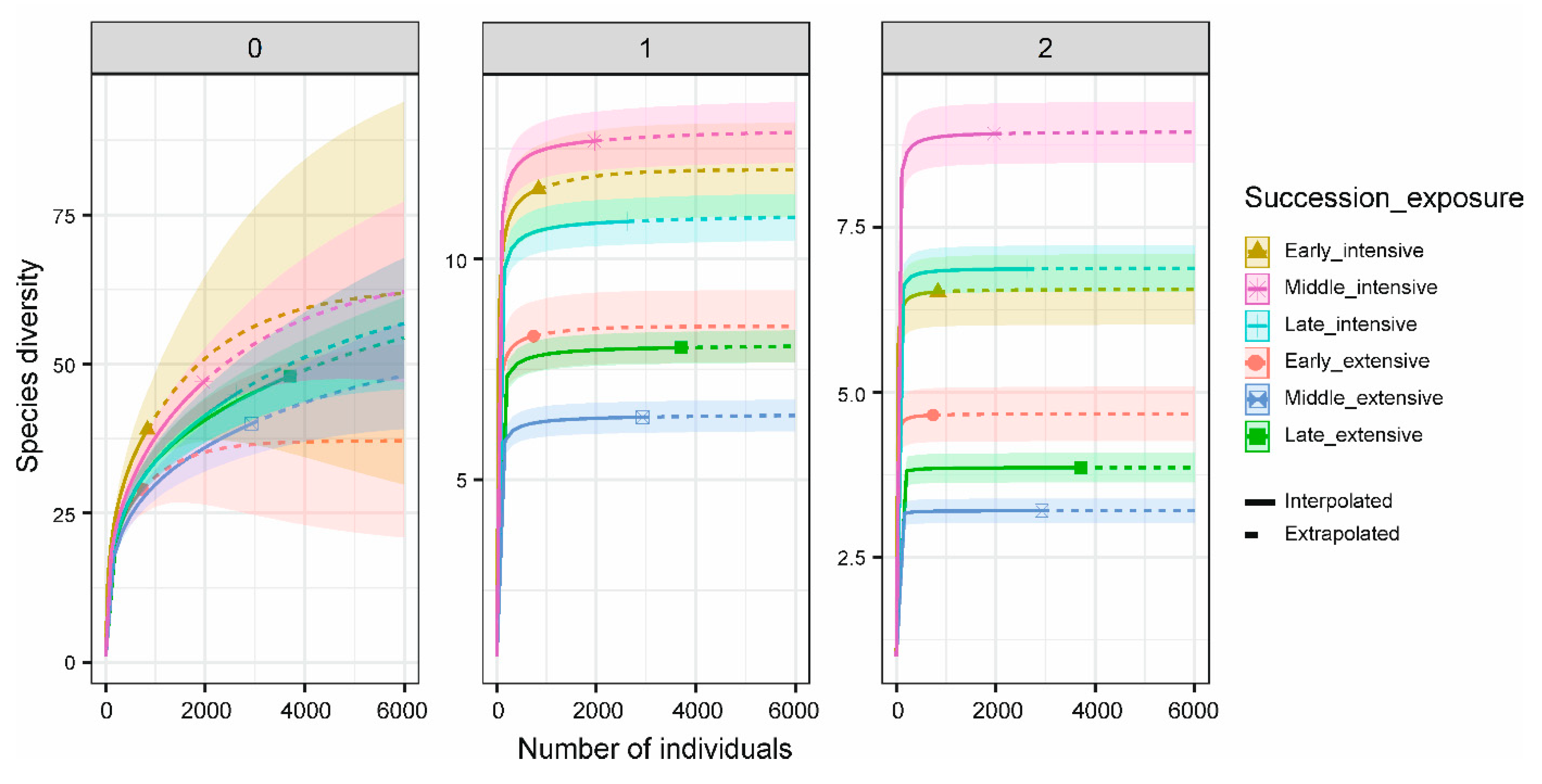
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Swift, M.J.; Heal, O.W.; Anderson, J.M. Decomposition in Terrestrial Ecosystems; Blackwell Scientific Publications: Oxford, UK, 1979. [Google Scholar]
- Moore, J.C.; Berlow, E.L.; Coleman, D.C.; de Ruiter, P.C.; Dong, Q.; Hastings, A.; Johnson, N.C.; McCann, K.S.; Melville, K.; Morin, P.J.; et al. Detritus, trophic dynamics and biodiversity. Ecol. Lett. 2004, 7, 584–600. [Google Scholar] [CrossRef]
- Parmenter, R.R.; MacMahon, J.A. Carrion decomposition and nutrient cycling in a semiarid shrub--steppe ecosystem. Ecol. Monogr. 2009, 79, 637–661. [Google Scholar] [CrossRef]
- Barton, P.S.; Cunningham, S.A.; Lindenmayer, D.B.; Manning, A.D. The role of carrion in maintaining biodiversity and ecological processes in terrestrial ecosystems. Oecologia 2013, 171, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Benbow, M.E.; Barton, P.S.; Ulyshen, M.D.; Beasley, J.C.; DeVault, T.L.; Strickland, M.S.; Tomberlin, J.K.; Jordan, H.R.; Pechal, J.L. Necrobiome framework for bridging decomposition ecology of autotrophically and heterotrophically derived organic matter. Ecol. Monogr. 2019, 89, e01331. [Google Scholar] [CrossRef]
- Gessner, M.O.; Swan, C.M.; Dang, C.K.; McKie, B.G.; Bardgett, R.D.; Wall, D.H.; Hättenschwiler, S. Diversity meets decomposition. Trends Ecol. Evol. 2010, 25, 372–380. [Google Scholar] [CrossRef]
- Benbow, M.E.; Receveur, J.P.; Lamberti, G.A. Death and decomposition in aquatic ecosystems. Front. Ecol. Evol. 2020, 8, 17. [Google Scholar] [CrossRef]
- Lamberti, G.A.; Levesque, N.M.; Brueseke, M.A.; Chaloner, D.T.; Benbow, M.E. Editorial: Animal mass mortalities in aquatic ecosystems: How common and influential? Front. Ecol. Evol. 2020, 8, 343. [Google Scholar] [CrossRef]
- Yang, L.H.; Edwards, K.F.; Byrnes, J.E.; Bastow, J.L.; Wright, A.N.; Spence, K.O. A meta-analysis of resource pulse-consumer interactions. Ecol. Monogr. 2010, 80, 125–151. [Google Scholar] [CrossRef]
- Seibold, S.; Hagge, J.; Müller, J.; Gruppe, A.; Brandl, R.; Bässler, C.; Thorn, S. Experiments with dead wood reveal the importance of dead branches in the canopy for saproxylic beetle conservation. For. Ecol. Manag. 2018, 409, 564–570. [Google Scholar] [CrossRef]
- Müller, J.; Ulyshen, M.; Seibold, S.; Cadotte, M.; Chao, A.; Bässler, C.; Vogel, S.; Hagge, J.; Weiß, I.; Baldrian, P.; et al. Primary determinants of communities in deadwood vary among taxa but are regionally consistent. Oikos 2020, 129, 1579–1588. [Google Scholar] [CrossRef]
- Braack, L.E.O. Community dynamics of carrion-attendant arthropods in tropical African woodland. Oecologia 1987, 72, 402–409. [Google Scholar] [CrossRef]
- Payne, J.A.; King, E.W.; Beinhart, G. Arthropod succession and decomposition of buried pigs. Nature 1968, 219, 1180–1181. [Google Scholar] [CrossRef]
- Pechal, J.L.; Crippen, T.L.; Tarone, A.M.; Lewis, A.J.; Tomberlin, J.K.; Benbow, M.E. Microbial community functional change during vertebrate carrion decomposition. PLoS ONE 2013, 8, e79035. [Google Scholar] [CrossRef]
- Pechal, J.L.; Benbow, M.E.; Crippen, T.L.; Tarone, A.M.; Tomberlin, J.K. Delayed insect access alters carrion decomposition and necrophagous insect community assembly. Ecosphere 2014, 5, art45. [Google Scholar] [CrossRef]
- Bump, J.K.; Peterson, R.O.; Vucetich, J.A. Wolves modulate soil nutrient heterogeneity and foliar nitrogen by configuring the distribution of ungulate carcasses. Ecology 2009, 90, 3159–3167. [Google Scholar] [CrossRef]
- Coe, M. The decomposition of elephant carcases in the Tsavo (East) National Park, Kenya. J. Arid Environ. 1978, 1, 71–86. [Google Scholar] [CrossRef]
- Benbow, M.E.; Tomberlin, J.K.; Tarone, A.M. Carrion Ecology, Evolution, and Their Applications; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Wilmers, C.C.; Stahler, D.R.; Crabtree, R.L.; Smith, D.W.; Getz, W.M. Resource dispersion and consumer dominance: Scavenging at wolf-and hunter-killed carcasses in Greater Yellowstone, USA. Ecol. Lett. 2003, 6, 996–1003. [Google Scholar] [CrossRef]
- Cortés-Avizanda, A.; Jovani, R.; Carrete, M.; Donázar, J.A. Resource unpredictability promotes species diversity and coexistence in an avian scavenger guild: A field experiment. Ecology 2012, 93, 2570–2579. [Google Scholar] [CrossRef] [PubMed]
- Stiegler, J.; Von Hoermann, C.; Müller, J.; Benbow, M.E.; Heurich, M. Carcass provisioning for scavenger conservation in a temperate forest ecosystem. Ecosphere 2020, 11, e03063. [Google Scholar] [CrossRef]
- Lai, Y.C.; Liu, Y.R. Noise promotes species diversity in nature. Phys. Rev. Lett. 2005, 94, 038102. [Google Scholar] [CrossRef]
- Wilmers, C.C.; Crabtree, R.L.; Smith, D.W.; Murphy, K.M.; Getz, W.M. Trophic facilitation by introduced top predators: Grey wolf subsidies to scavengers in Yellowstone National Park. J. Anim. Ecol. 2003, 72, 909–916. [Google Scholar] [CrossRef]
- Schoenly, K.; Reid, W. Dynamics of heterotrophic succession in carrion arthropod assemblages: Discrete seres or a continuum of change? Oecologia 1987, 73, 192–202. [Google Scholar] [CrossRef]
- Boulton, A.J.; Lake, P.S. Dynamics of heterotrophic succession in carrion arthropod assemblages. Oecologia 1988, 76, 477–480. [Google Scholar] [CrossRef]
- Schoenly, K.G.; Reid, W. Dynamics of heterotrophic succession in carrion revisited. Oecologia 1989, 79, 140–142. [Google Scholar] [CrossRef] [PubMed]
- Moura, M.O.; Monteiro-Filho, E.L.d.A.; de Carvalho, C.J.B. Heterotrophic succession in carrion arthropod assemblages. Brazilian Arch. Biol. Technol. 2005, 48, 477–486. [Google Scholar] [CrossRef]
- Matuszewski, S.; Bajerlein, D.; Konwerski, S.; Szpila, K. Insect succession and carrion decomposition in selected forests of Central Europe. Part 3: Succession of carrion fauna. Forensic Sci. Int. 2011, 207, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Mondor, E.B.; Tremblay, M.N.; Tomberlin, J.K.; Benbow, E.M.; Tarone, A.M.; Crippen, T.L. The ecology of carrion decomposition. Nat Educ Knowl. 2012, 3, 21. [Google Scholar]
- Fiedler, A.; Halbach, M.; Sinclair, B.; Benecke, M. What is the edge of a forest? A diversity analysis of adult Diptera found on decomposing piglets inside and on the edge of a Western German woodland inspired by a courtroom question. Entomol. Heute 2008, 20, 173–191. [Google Scholar]
- Goff, M.L. Early post-mortem changes and stages of decomposition in exposed cadavers. Exp. Appl. Acarol. 2009, 49, 21–36. [Google Scholar] [CrossRef]
- Introna, F.; Campobasso, C.P. Forensic dipterology. In Contributions to a Manual of Palaearctic Diptera, General and Applied Dipterology; Papp, L., Darvas, B., Eds.; Science Herald: Budapest, Hungary, 2000; Volume 1, pp. 793–846. [Google Scholar]
- von Hoermann, C.; Ruther, J.; Ayasse, M. The attraction of virgin female hide beetles (Dermestes maculatus) to cadavers by a combination of decomposition odour and male sex pheromones. Front. Zool. 2012, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Amendt, J.; Krettek, R.; Zehner, R. Forensic entomology. Naturwissenschaften 2004, 91, 51–65. [Google Scholar] [CrossRef]
- Strümpher, W.P.; Farrell, J.; Scholtz, C.H. Trogidae (Coleoptera: Scarabaeoidea) in forensic entomology: Occurrence of known and new species in Queensland, Australia. Austral Entomol. 2014, 53, 368–372. [Google Scholar] [CrossRef]
- von Hoermann, C.; Weithmann, S.; Deißler, M.; Ayasse, M.; Steiger, S. Forest habitat parameters influence abundance and diversity of cadaver-visiting dung beetles in Central Europe. R. Soc. Open Sci. 2020, 7, 191722. [Google Scholar] [CrossRef]
- Heurich, M.; Beudert, B.; Rall, H.; Křenová, Z. National parks as model regions for interdisciplinary long-term ecological research: The Bavarian Forest and Šumavá National Parks underway to transboundary ecosystem research. In Long-Term Ecological Research; Müller, F., Baessler, C., Schubert, H., Klotz, S., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 327–344. [Google Scholar]
- Cailleret, M.; Heurich, M.; Bugmann, H. Reduction in browsing intensity may not compensate climate change effects on tree species composition in the Bavarian Forest National Park. For. Ecol. Manag. 2014, 328, 179–192. [Google Scholar] [CrossRef]
- Seibold, S.; Büche, B.; Szallies, A.; Müller, J. Neue Käfernachweise im Nationalpark Bayerischer Wald im Rahmen von Totholzexperimenten (Insecta: Coleoptera). Beiträge zur Bayerischen Entomofaunistik 2017, 17, 1–17. [Google Scholar]
- Möst, L.; Hothorn, T.; Müller, J.; Heurich, M. Creating a landscape of management: Unintended effects on the variation of browsing pressure in a national park. For. Ecol. Manag. 2015, 338, 46–56. [Google Scholar] [CrossRef]
- Müller, J.; Brandl, R. Assessing biodiversity by remote sensing in mountainous terrain: The potential of LiDAR to predict forest beetle assemblages. J. Appl. Ecol. 2009, 46, 897–905. [Google Scholar] [CrossRef]
- Farwig, N.; Brandl, R.; Siemann, S.; Wiener, F.; Müller, J. Decomposition rate of carrion is dependent on composition not abundance of the assemblages of insect scavengers. Oecologia 2014, 175, 1291–1300. [Google Scholar] [CrossRef]
- Dekeirsschieter, J.; Verheggen, F.J.; Haubruge, E.; Brostaux, Y. Carrion beetles visiting pig carcasses during early spring in urban, forest and agricultural biotopes of Western Europe. J. Insect. Sci. 2011, 11, 73. [Google Scholar] [CrossRef]
- von Hoermann, C.; Jauch, D.; Kubotsch, C.; Reichel-Jung, K.; Steiger, S.; Ayasse, M. Effects of abiotic environmental factors and land use on the diversity of carrion-visiting silphid beetles (Coleoptera: Silphidae): A large scale carrion study. PLoS ONE 2018, 13, e0196839. [Google Scholar] [CrossRef]
- Matuszewski, S.; Bajerlein, D.; Konwerski, S.; Szpila, K. Insect succession and carrion decomposition in selected forests of Central Europe. Part 2: Composition and residency patterns of carrion fauna. Forensic Sci. Int. 2010, 195, 42–51. [Google Scholar] [CrossRef]
- R: A Language and Environment for Statistical Computing (Wien: R Foundation for Statistical Computing). Available online: https://www.R-project.org/ (accessed on 10 October 2020).
- Hill, M.O. Diversity and evenness: A unifying notation and its consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: An R package for interpolation and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- De Cáceres, M.; Legendre, P. Associations between species and groups of sites: Indices and statistical inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef]
- Esser, J. Rote Liste und Gesamtartenliste der Kapuzinerkäferartigen (Bostrichoidea), Buntkäferartigen (Cleroidea), Plattkäferartigen (Cucujoidea), Schnellkäferartigen (Elateroidea), Werftkäferartigen (Lymexyloidea) und Schwarzkäferartigen (Tenebrioidea); Universitätsverlag der TU Berlin: Berlin, Germany, 2017. [Google Scholar]
- VanLaerhoven, S.L.; Benbow, M.E.; Tomberlin, J.K.; Tarone, A.M. Modeling species interactions within carrion food webs. In Carrion Ecology, Evolution, and Their Applications; Benbow, M.E., Tomberlin, J.K., Tarone, A.M., Eds.; CRC: Boca Raton, FL, USA, 2015; pp. 231–245. [Google Scholar]
- Pukowski, E. Ökologische Untersuchungen an Necrophorus F. Z. Morphol. Okol. Tiere 1933, 27, 518–586. [Google Scholar] [CrossRef]
- Kentner, E.; Streit, B. Temporal distribution and habitat preference of congeneric insect species found at rat carrion. Pedobiologia 1990, 34, 347–359. [Google Scholar]
- Scott, M.P. The ecology and behavior of burying beetles. Annu. Rev. Entomol. 1998, 43, 595–618. [Google Scholar] [CrossRef]
- Matuszewski, S.; Bajerlein, D.; Konwerski, S.; Szpila, K. An initial study of insect succession and carrion decomposition in various forest habitats of Central Europe. Forensic Sci. Int. 2008, 180, 61–69. [Google Scholar] [CrossRef]
- Kadlec, J.; Mikatova, S.; Maslo, P.; Sipkova, H.; Sipek, P.; Sladecek, F.X.J. Delaying insect access alters community composition on small carrion: A quantitative approach. Entomol. Exp. Appl. 2019, 167, 729–740. [Google Scholar] [CrossRef]
- Anderson, G.S. Factors that influence insect succession on carrion. In Forensic Entomology: The Utility of Arthropods in Legal Investigations; Byrd, J., Castner, E., Eds.; CRC Press: Boca Raton, FL, USA, 2009; pp. 201–250. [Google Scholar]
- Anderson, G.S. Forensic entomology. In Forensic Science, an Introduction to Scientific and Investigative Techniques; James, S.H., Nordby, J., Eds.; CRC Press: Boca Raton, FL, USA, 2009; pp. 137–165. [Google Scholar]
- Lindgren, N.K.; Bucheli, S.R.; Archambeault, A.D.; Bytheway, J.A. Exclusion of forensically important flies due to burying behavior by the red imported fire ant (Solenopsis invicta) in southeast Texas. Forensic Sci. Int. 2011, 204, e1–e3. [Google Scholar] [CrossRef]
- DeVault, T.L.; Rhodes, O.E., Jr.; Shivik, J.A. Scavenging by vertebrates: Behavioral, ecological, and evolutionary perspectives on an important energy transfer pathway in terrestrial ecosystems. Oikos 2003, 102, 225–234. [Google Scholar] [CrossRef]
- van den Heever, L.; Thompson, L.J.; Bowerman, W.W.; Smit-Robinson, H.; Shaffer, L.J.; Harrell, R.M.; Ottinger, M.A. Reviewing the role of vultures at the human–wildlife–livestock disease interface: An African perspective. J. Raptor Res. 2021, 55. [Google Scholar] [CrossRef]
- Connell, J.H.; Slatyer, R.O. Mechanisms of succession in natural communities and their role in community stability and organization. Am. Nat. 1977, 111, 1119–1144. [Google Scholar] [CrossRef]
- Jones, C.G.; Lawton, J.H.; Shachak, M. Organisms as ecosystem engineers. In Ecosystem Management; Samson, F.B., Knopf, F.L., Eds.; Springer: New York, NY, USA, 1994; pp. 130–147. [Google Scholar]
- Moleón, M.; Martínez-Carrasco, C.; Muellerklein, O.C.; Getz, W.M.; Muñoz-Lozano, C.; Sánchez-Zapata, J.A. Carnivore carcasses are avoided by carnivores. J. Anim. Ecol. 2017, 86, 1179–1191. [Google Scholar] [CrossRef]
- Muñoz-Lozano, C.; Martín-Vega, D.; Martínez-Carrasco, C.; Sánchez-Zapata, J.A.; Morales-Reyes, Z.; Gonzálvez, M.; Moleón, M. Avoidance of carnivore carcasses by vertebrate scavengers enables colonization by a diverse community of carrion insects. PLoS ONE 2019, 14, e0221890. [Google Scholar] [CrossRef]
- Opitz, W.; Arnett, J.R.H. Cleridae Latreille 1804. In American Beetles, Polyphaga: Scarabaeoidea through Curculionoidea; Arnett, R.H., Thomas, M.C., Skelley, P.E., Frank, J.H., Eds.; CRC Press: Boca Raton, FL, USA, 2002; Volume 2, pp. 267–280. [Google Scholar]
- Knull, J.N. The checkered beetles of Ohio (Coleoptera: Cleridae). Ohio Biol. Surv. Bull. 1951, 8, 268–350. [Google Scholar]
- Kočárek, P. Decomposition and Coleoptera succession on exposed carrion of small mammal in Opava, the Czech Republic. Eur. J. Soil Biol. 2003, 39, 31–45. [Google Scholar] [CrossRef]
- Anton, E.; Niederegger, S.; Beutel, R.G. Beetles and flies collected on pig carrion in an experimental setting in Thuringia and their forensic implications. Med. Vet. Entomol. 2011, 25, 353–364. [Google Scholar] [CrossRef]
- Haelewaters, D.; Vanpoucke, S.; Raes, D.; Krawczynski, R. On carrion-associated beetles in the Sonian Forest (Belgium): Observations on five deer carcasses. Bull. Société R. Belge d’Entomologie 2015, 151, 25–33. [Google Scholar]
- Carter, D.O.; Yellowlees, D.; Tibbett, M. Cadaver decomposition in terrestrial ecosystems. Naturwissenschaften 2007, 94, 12–24. [Google Scholar] [CrossRef]
- Benbow, M.E.; Lewis, A.J.; Tomberlin, J.K.; Pechal, J.L. Seasonal necrophagous insect community assembly during vertebrate carrion decomposition. J. Med. Entomol. 2013, 50, 440–450. [Google Scholar] [CrossRef]
- Saloña, M.I.; Moraza, M.L.; Carles-Tolrá, M.; Iraola, V.; Bahillo, P.; Yélamos, T.; Outerelo, R.; Alcaraz, R. Searching the soil: Forensic importance of edaphic fauna after the removal of a corpse. J. Forensic Sci. 2010, 55, 1652–1655. [Google Scholar] [CrossRef]
- von Hoermann, C.; (Bavarian Forest National Park Administration, Grafenau, Bavaria, Germany). Personal communication, 2018.
- Koch, K. Die Käfer Mitteleuropas. Ökologie Band 2; Goecke und Evers Verlag: Krefeld, Germany, 1989. [Google Scholar]
- Philp, B.; Hamlin, I.; Lavery, A. Insects and arachnids of Ardeer, North Ayrshire, Scotland. Glas. Nat. 2020, 27. [Google Scholar] [CrossRef]
- Dekeirsschieter, J.; Frederick, C.; Verheggen, F.J.; Drugmand, D.; Haubruge, E. Diversity of forensic rove beetles (Coleoptera, Staphylinidae) associated with decaying pig carcass in a forest biotope. J. Forensic Sci. 2013, 58, 1032–1040. [Google Scholar] [CrossRef]
- Vindstad, O.P.L.; Schultze, S.; Jepsen, J.U.; Biuw, M.; Kapari, L.; Sverdrup-Thygeson, A.; Ims, R.A. Numerical responses of saproxylic beetles to rapid increases in dead wood availability following geometrid moth outbreaks in sub-arctic mountain birch forest. PLoS ONE 2014, 9, e99624. [Google Scholar] [CrossRef] [PubMed]
- Buckland, P.I.; Buckland, P.C. BugsCEP: Coleopteran Ecology Package (Software); NOAA/NCDC Paleoclimatology Program: Boulder, CO, USA, 2006. [Google Scholar]
- Koch, K. Die Käfer Mitteleuropas. Ökologie Band 1; Goecke und Evers Verlag: Krefeld, Germany, 1989. [Google Scholar]
- Peschke, K.; Friedrich, P.; Kaiser, U.; Franke, S.; Francke, W. Isopropyl (Z9)-hexadecenoate as a male attractant pheromone from the sternal gland of the rove beetle Aleochara curtala (Coleoptera: Staphylinidae). Chemoecology 1999, 9, 47–54. [Google Scholar] [CrossRef]
- Lipkow, E. Habits of Philonthus species and other Staphylinidae (Coleoptera) in dung. Drosera 1982, 1, 47–54. [Google Scholar]
- Weithmann, S.; Kuppler, J.; Degasperi, G.; Steiger, S.; Ayasse, M.; von Hoermann, C. Local and landscape effects on carrion-associated rove beetle (Coleoptera: Staphylinidae) communities in German forests. Insects 2020, 11, 828. [Google Scholar] [CrossRef]
- von Lengerken, H. Die Brutfürsorge- und Brutpflegeinstinkte der Käfer; Akademische Verlagsgesellschaft Geest und Portig K.G.: Leipzig, Germany, 1954. [Google Scholar]
- Teichert, M. Nahrungsspeicherung von Geotrupes vernalis L. und Geotrupes stercorosus Scriba (Coleopt. Scarab.). Wiss. Z. Martin-Luther-Univ. Halle-Wittenb. 1956, 5, 669–672. [Google Scholar]
- Byk, A.; Semkiw, P. Habitat preferences of the forest dung beetle Anoplotrupes stercorosus (Scriba, 1791) (Coleoptera: Geotrupidae) in the Białowieża Forest. Acta Sci. Pol. Silvarum Colendarum Ratio Ind. Lignaria 2010, 9, 17–28. [Google Scholar]
- Jarmusz, M.; Bajerlein, D. Anoplotrupes stercorosus (Scr.) and Trypocopris vernalis (L.) (Coleoptera: Geotrupidae) visiting exposed pig carrion in forests of Central Europe: Seasonality, habitat preferences and influence of smell of decay on their abundances. Entomol. Gen. 2015, 35, 213–228. [Google Scholar] [CrossRef]
- Růžička, J.; Jakubec, P. Icones Insectorum Europae Centralis. Coleoptera: Agyrtidae, Silphidae. Folia Heyrovskiana, Series B 2016, 26, 1–17. [Google Scholar]
- Růžička, J.; (Czech University of Life Sciences Prague, Prague, Czech Republic). Personal communication, 2021.
- Newton, A.F. Review of Agyrtidae (Coleoptera), with a new genus and species from New Zealand. Annal. Zool. 1997, 47, 111–156. [Google Scholar]
- Rade, E. Necrophilus subterraneus Dej. und andere Käfer des Göttinger Gebietes 1893. Entomol. Nachr. 1893, 19, 357–363. [Google Scholar]
- Zwick, P. Die Jugendstadien des Käfers Necrophilus subterraneus (Coleoptera: Silphidae: Agyrtinae). Beiträge Naturkd. Osthessen 1981, 17, 133–140. [Google Scholar]
- Schawaller, W. Die Gattung Necrophilus Latreille 1829 im Himalaya (Insecta: Coleoptera: Agyrtidae). Senckenb. Biol. 1986, 66, 311–319. [Google Scholar]
- Růžička, J. Agyrtidae. In Catalogue of Palaearctic Coleoptera. Hydrophiloidea – Staphylinoidea, Revised and Updated ed.; Löbl, I., Löbl, D., Eds.; Brill: Leiden, The Nederlands; Boston, MA, USA, 2015; Volume 2/1, pp. 5, 177–180. [Google Scholar]
- Newton, A.F. StaphBase: Staphyliniformia world catalog database (version January 2021). In Species 2000 & ITIS Catalogue of Life, 20th February 2019; Roskov, Y., Ower, G., Orrell, T., Nicolson, D., Bailly, N., Kirk, P.M., Bourgoin, T., DeWalt, R.E., Decock, W., Nieukerken, E., et al., Eds.; Naturalis: Leiden, The Nederlands, 2021; Available online: www.catalogueoflife.org/col (accessed on 18 March 2021).
- Růžička, J.; Puetz, A. New species and new records of Agyrtidae (Coleoptera) from China, India, Myanmar, Thailand and Vietnam. Acta Entomol. Musei Natl. Pragae 2009, 49, 631–650. [Google Scholar]
- Nikitsky, N.B. Morfologia lichinki Sphaerites glabratus F. I filogenia Histeroidea. Zool. Zhurnal. 1976, 55, 531–537. [Google Scholar]
- Newton, A.F. 13.1 Sphaeritidae Shuckard, 1839. In Handbook of Zoology, Arthropoda: Insecta. Coleoptera, Beetles. Morphology and Systematics (Archostemata, Adephaga, Myxophaga, Polyphaga Partim); Beutel, R.G., Leschen, R.A.B., Eds.; Walter de Gruyter: Berlin, Germany, 2016; Volume 1, pp. 274–277. [Google Scholar]
- Lackner, T. Coleoptera: Sphaeritidae, Histeridae. Folia Heyrovskyana Ser. B 2015, 23, 1–33. [Google Scholar]
- Löbl, I. Sphaeritidae. In Catalogue of Palaearctic Coleoptera. Hydrophiloidea-Staphylinoidea, Revised and Updated ed.; Löbl, I., Löbl, D., Eds.; Brill: Leiden, The Nederlands; Boston, MA, USA, 2015; Volume 2/1, p. 76. [Google Scholar]
- Thorn, S.; Bässler, C.; Svoboda, M.; Müller, J. Effects of natural disturbances and salvage logging on biodiversity-lessons from the Bohemian Forest. For. Ecol. Manag. 2017, 388, 113–119. [Google Scholar] [CrossRef]


| Species | Family | Ecology | Indiv (Random) | Indiv (Fixed) | Indiv (Controls) |
|---|---|---|---|---|---|
| Saprinus semistriatus (L. G. Scriba, 1790) | Histeridae | fac | 165 | 427 | 3 |
| Margarinotus striola (C. Sahlb., 1819) | Histeridae | fac | 145 | 157 | 6 |
| Margarinotus brunneus (F., 1775) | Histeridae | fac | 0 | 1 | 0 |
| Hister unicolor L., 1758 | Histeridae | fac | 1 | 0 | 0 |
| Sphaerites glabratus (F., 1792) | Sphaeritidae | fac | 6 | 3 | 0 |
| Nicrophorus humator (Gled., 1767) | Silphidae | obl | 68 | 58 | 0 |
| Nicrophorus investigator Zett., 1824 | Silphidae | obl | 100 | 70 | 0 |
| Nicrophorus interruptus Steph., 1830 | Silphidae | obl | 6 | 1 | 0 |
| Nicrophorus vespilloides Herbst, 1783 | Silphidae | obl | 686 | 614 | 0 |
| Nicrophorus vespillo (L., 1758) | Silphidae | obl | 4 | 3 | 0 |
| Necrodes littoralis (L., 1758) | Silphidae | obl | 328 | 268 | 0 |
| Thanatophilus rugosus (L., 1758) | Silphidae | obl | 26 | 101 | 0 |
| Thanatophilus sinuatus (F., 1775) | Silphidae | obl | 439 | 976 | 0 |
| Oiceoptoma thoracicum (L., 1758) | Silphidae | obl | 290 | 528 | 0 |
| Necrophilus subterraneus (Dahl, 1807) | Agyrtidae | fac | 1 | 7 | 0 |
| Agyrtes castaneus (F., 1792) | Agyrtidae | obl | 0 | 0 | 1 |
| Sciodrepoides watsoni (Spence, 1813) | Leiodidae | obl | 1 | 0 | 0 |
| Sciodrepoides fumatus (Spence, 1813) | Leiodidae | obl | 4 | 0 | 0 |
| Catops kirbyi (Spence, 1813) | Leiodidae | obl | 1 | 1 | 0 |
| Apocatops nigrita (Er., 1837) | Leiodidae | obl | 1 | 0 | 0 |
| Megarthrus depressus sensu FHL Bd 4–15, | Staphylinidae | fac | 0 | 1 | 0 |
| Proteinus brachypterus (F., 1792) | Staphylinidae | fac | 0 | 3 | 0 |
| Omalium rivulare (Payk., 1789) | Staphylinidae | fac | 42 | 24 | 2 |
| Omalium septentrionis C. Thoms., 1857 | Staphylinidae | fac | 71 | 87 | 1 |
| Omalium rugatum Muls. et Rey, 1880 | Staphylinidae | fac | 0 | 1 | 0 |
| Anthobium melanocephalum (Ill., 1794) | Staphylinidae | fac | 0 | 7 | 0 |
| Arpedium quadrum (Grav., 1806) | Staphylinidae | fac | 14 | 5 | 4 |
| Oxytelus laqueatus (Marsh., 1802) | Staphylinidae | fac | 25 | 4 | 0 |
| Anotylus rugosus (F., 1775) | Staphylinidae | fac | 0 | 1 | 0 |
| Anotylus sculpturatus (Grav., 1806) | Staphylinidae | fac | 1 | 4 | 0 |
| Stenus clavicornis (Scop., 1763) | Staphylinidae | guest | 0 | 1 | 0 |
| Rugilus rufipes (Germar, 1836) | Staphylinidae | fac | 6 | 10 | 1 |
| Rugilus mixtus Lohse, 1956 | Staphylinidae | fac | 0 | 1 | 0 |
| Xantholinus tricolor (F., 1787) | Staphylinidae | fac | 0 | 1 | 0 |
| Xantholinus longiventris Heer, 1839 | Staphylinidae | fac | 3 | 2 | 1 |
| Atrecus affinis (Payk., 1789) | Staphylinidae | fac | 0 | 1 | 0 |
| Philonthus laevicollis (Lacord., 1835) | Staphylinidae | fac | 12 | 7 | 0 |
| Philonthus rufipes (Steph., 1832) | Staphylinidae | fac | 13 | 11 | 0 |
| Philonthus carbonarius Grav., 1802 | Staphylinidae | fac | 266 | 102 | 0 |
| Philonthus addendus Sharp, 1867 | Staphylinidae | fac | 1 | 0 | 0 |
| Philonthus pseudovarians A. Strand, 1941 | Staphylinidae | fac | 0 | 4 | 0 |
| Philonthus splendens (F., 1792) | Staphylinidae | fac | 3 | 0 | 1 |
| Philonthus fimetarius (Grav., 1802) | Staphylinidae | fac | 143 | 79 | 1 |
| Philonthus marginatus (O. F. Müller, 1764) | Staphylinidae | fac | 18 | 10 | 0 |
| Creophilus maxillosus (L., 1758) | Staphylinidae | fac | 137 | 172 | 0 |
| Ontholestes tessellatus (Geoffr., 1785) | Staphylinidae | fac | 19 | 17 | 0 |
| Ontholestes murinus (L., 1758) | Staphylinidae | obl | 0 | 3 | 0 |
| Dinothenarus fossor (Scop., 1771) | Staphylinidae | guest | 0 | 0 | 4 |
| Ocypus olens (O. F. Müller, 1764) | Staphylinidae | fac | 0 | 0 | 1 |
| Quedius cinctus (Payk., 1790) | Staphylinidae | fac | 55 | 18 | 0 |
| Lordithon trinotatus (Er., 1839) | Staphylinidae | fac | 32 | 3 | 11 |
| Tachyporus pusillus Grav., 1806 | Staphylinidae | fac | 1 | 0 | 0 |
| Tachinus pallipes Grav., 1806 | Staphylinidae | fac | 471 | 81 | 2 |
| Atheta excellens (Kr., 1856) | Staphylinidae | fac | 1 | 0 | 0 |
| Atheta longicornis (Grav., 1802) | Staphylinidae | fac | 0 | 1 | 0 |
| Acrotona parvula (Mannerh., 1830) | Staphylinidae | fac | 1 | 0 | 0 |
| Oxypoda opaca (Grav., 1802) | Staphylinidae | fac | 0 | 1 | 0 |
| Oxypoda formosa Kr., 1856 | Staphylinidae | fac | 1 | 0 | 0 |
| Aleochara curtula (Goeze, 1777) | Staphylinidae | obl | 1 | 2 | 0 |
| Lampyris noctiluca (L., 1767) | Lampyridae | fac | 1 | 0 | 0 |
| Necrobia violacea (L., 1758) | Cleridae | obl | 1 | 44 | 0 |
| Necrobia rufipes (DeGeer, 1775) | Cleridae | obl | 2 | 3 | 0 |
| Dermestes murinus L., 1758 | Dermestidae | obl | 2 | 1 | 0 |
| Dermestes laniarius Ill., 1801 | Dermestidae | obl | 0 | 1 | 0 |
| Byrrhus pilula (L., 1758) | Byrrhidae | guest | 0 | 0 | 4 |
| Byrrhus glabratus Heer, 1841 | Byrrhidae | guest | 0 | 0 | 1 |
| Omosita depressa (L., 1758) | Nitidulidae | obl | 14 | 314 | 0 |
| Cychramus variegatus (Herbst, 1792) | Nitidulidae | guest | 2 | 0 | 0 |
| Mycetina cruciata (Schaller, 1783) | Endomychidae | guest | 1 | 0 | 0 |
| Anaspis rufilabris (Gyll., 1827) | Scraptiidae | fac | 1 | 0 | 0 |
| Corticeus unicolor Pill. et Mitt., 1783 | Tenebrionidae | guest | 0 | 0 | 1 |
| Trox scaber (L., 1767) | Trogidae | obl | 0 | 1 | 0 |
| Geotrupes stercorarius (L., 1758) | Geotrupidae | fac | 0 | 1 | 0 |
| Anoplotrupes stercorosus (Hartmann in L. G. Scriba, 1791) | Geotrupidae | fac | 3625 | 1086 | 41 |
| Teuchestes fossor (L., 1758) | Scarabaeidae | fac | 0 | 1 | 0 |
| Ammoecius brevis (Er., 1848) | Scarabaeidae | fac | 4 | 8 | 0 |
| Acrossus rufipes (L., 1758) | Scarabaeidae | fac | 45 | 54 | 0 |
| Acrossus luridus (F., 1775) | Scarabaeidae | fac | 1 | 0 | 0 |
| Acrossus depressus (Kugel., 1792) | Scarabaeidae | fac | 42 | 10 | 0 |
| Limarus maculatus (Sturm, 1800) | Scarabaeidae | fac | 12 | 3 | 0 |
| Volinus sticticus (Panzer, 1798) | Scarabaeidae | fac | 0 | 1 | 0 |
| Nimbus contaminatus (Herbst, 1783) | Scarabaeidae | fac | 3 | 0 | 0 |
| Melinopterus sphacelatus (Panzer, 1798) | Scarabaeidae | fac | 0 | 2 | 0 |
| Melinopterus prodromus (Brahm, 1790) | Scarabaeidae | fac | 1 | 8 | 0 |
| Planolinus fasciatus (Olivier, 1789) | Scarabaeidae | fac | 1 | 0 | 0 |
| Calamosternus granarius (L., 1767) | Scarabaeidae | fac | 0 | 1 | 0 |
| Serica brunnea (L., 1758) | Scarabaeidae | guest | 0 | 0 | 1 |
| Ips typographus (L., 1758) | Scolytidae | guest | 0 | 0 | 1 |
| Otiorhynchus scaber (L., 1758) | Curculionidae | guest | 1 | 0 | 0 |
| Phyllobius arborator (Herbst, 1797) | Curculionidae | guest | 0 | 1 | 1 |
| Hylobius abietis (L., 1758) | Curculionidae | guest | 0 | 0 | 2 |
| Plinthus tischeri Germar, 1824 | Curculionidae | guest | 0 | 0 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
von Hoermann, C.; Lackner, T.; Sommer, D.; Heurich, M.; Benbow, M.E.; Müller, J. Carcasses at Fixed Locations Host a Higher Diversity of Necrophilous Beetles. Insects 2021, 12, 412. https://doi.org/10.3390/insects12050412
von Hoermann C, Lackner T, Sommer D, Heurich M, Benbow ME, Müller J. Carcasses at Fixed Locations Host a Higher Diversity of Necrophilous Beetles. Insects. 2021; 12(5):412. https://doi.org/10.3390/insects12050412
Chicago/Turabian Stylevon Hoermann, Christian, Tomáš Lackner, David Sommer, Marco Heurich, M. Eric Benbow, and Jörg Müller. 2021. "Carcasses at Fixed Locations Host a Higher Diversity of Necrophilous Beetles" Insects 12, no. 5: 412. https://doi.org/10.3390/insects12050412
APA Stylevon Hoermann, C., Lackner, T., Sommer, D., Heurich, M., Benbow, M. E., & Müller, J. (2021). Carcasses at Fixed Locations Host a Higher Diversity of Necrophilous Beetles. Insects, 12(5), 412. https://doi.org/10.3390/insects12050412