Host-Plant Variations Affect the Biotic Potential, Survival, and Population Projection of Myzus persicae (Hemiptera: Aphididae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Host Plants
2.2. Aphids
2.3. Life Table Study
2.4. Population Projection
2.5. Statistical Analysis
3. Results
3.1. Age-Stage Two-Sex Life Tables
3.2. Population Projection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Egan, S.P.; Ott, J.R. Host plant quality and local adaptation determine the distribution of a gall-forming herbivore. Ecology 2007, 88, 2868–2879. [Google Scholar] [CrossRef] [PubMed]
- Drès, M.; Mallet, J. Host races in plant-feeding insects and their importance in sympatric speciation. Phil. Trans. R. Soc. Lond. B 2002, 357, 471–492. [Google Scholar] [CrossRef] [PubMed]
- Blackman, R.L.; Eastop, V.S. Aphids on the World’s Crops: An Identification and Information Guide, 2nd ed.; John Wiley and Sons: Chichester, UK, 2000. [Google Scholar]
- Meng, J.; Zhang, C.; Chen, X.; Cao, Y.; Shang, S. Differential protein expression in the susceptible and resistant Myzus persicae (Sulzer) to imidacloprid. Pest Biochem. Physio. 2014, 115, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Herman, M.A.B.; Nault, B.A.; Smart, C.D. Effects of plant growth-promoting rhizobacteria on bell pepper production and green peach aphid infestations in New York. Crop Prot. 2008, 27, 996–1002. [Google Scholar] [CrossRef]
- Saljoqi, A.U.R.; Khan, K.; Rahman, S.U. Integrated management of potato-peach aphid, Myzus persicae (Sulzer). Sarhad J. Agric. 2009, 25, 573–580. [Google Scholar]
- La Rossa, F.R.; Vasicek, A.; López, M.C. Effects of pepper (Capsicum annuum) cultivars on the biology and life table parameters of Myzus persicae (Sulz.) (Hemiptera: Aphididae). Neotrop. Entomol. 2013, 42, 634–641. [Google Scholar] [CrossRef]
- Capinera, J.L. Green Peach Aphid, Myzus persicae (Sulzer) (Insecta: Hemiptera: Aphididae); The Institute of Food and Agricultural Sciences (IFAS) University of Florida: Gainesville, FL, USA, 2011. [Google Scholar]
- Goundoudaki, S.; Tsitsipis, J.A.; Margaritopoulos, J.T.; Zarpas, K.D.; Divanidis, S. Performance of the tobacco aphid Myzus persicae (Hemiptera: Aphididae) on Oriental and Virginia tobacco varieties. Agric. For. Entomol. 2003, 5, 285–291. [Google Scholar] [CrossRef]
- Mdellel, L.; Kamel, M.B.H. Effects of different cultivars of pepper on the biological parameters of the green peach aphid. Eur. J. Environ. Sci. 2014, 4, 102–105. [Google Scholar]
- Polat Akköprü, E.; Atlıhan, R.; Okut, H.; Chi, H. Demographic assessment of plant cultivar resistance to insect pests: A case study of the dusky-veined walnut aphid (Hemiptera: Callaphididae) on five walnut cultivars. J. Econ. Entomol. 2015, 108, 378–387. [Google Scholar] [CrossRef]
- Awmack, C.S.; Leather, S.R. Host plant quality and fecundity in herbivorous insects. Annu. Rev. Entomol. 2002, 47, 817–844. [Google Scholar] [CrossRef]
- Bashir, F.; Azim, N.M.N.; Akhter, N.; Muzaffar, G. Effect of texture/morphology of host plants on the biology of Brevicoryne brassicae (L.) (Homoptera: Aphididae). Int. J. Curr. Res. 2013, 5, 178–180. [Google Scholar]
- Liu, J.; Wu, K.; Hopper, K.R.; Zhao, K. Population dynamics of Aphis glycines (Homoptera: Aphididae) and its natural enemies in soybean in northern China. Ann. Entomol. Soc. Am. 2004, 97, 235–239. [Google Scholar] [CrossRef]
- Maremela, M.; Tiroesele, B.; Obopile, M.; Tshegofatso, A.B. Effects of Brassica cultivar on population growth and life table parameters of the cabbage aphid, Brevicoryne brassicae L. (Hemiptera: Aphididae). J. Entomol. Res. 2013, 37, 95–100. [Google Scholar]
- Atlihan, R.; Kasap, I.; Özgökçe, M.S.; Polat-Akköprü, E.; Chi, H. Population growth of Dysaphis pyri (Hemiptera: Aphididae) on different pear cultivars with discussion on curve fitting in life table studies. J. Econ. Entomol. 2017, 110, 1890–1898. [Google Scholar] [CrossRef]
- Simon, J.C.; Peccoud, J. Rapid evolution of aphid pests in agricultural environments. Curr. Opin. Insect Sci. 2018, 26, 17–24. [Google Scholar] [CrossRef]
- Hosseini-Tabesh, B.; Sahragard, A.; Karimi-Malati, A.A. laboratory and field condition comparison of life table parameters of Aphis gossypii Glover (Hemiptera: Aphididae). J. Plant Prot. Res. 2015, 55, 1–7. [Google Scholar] [CrossRef]
- McDonald, S.A.; Halbert, S.E.; Tolin, S.A.; Nault, B.A. Seasonal abundance and diversity of aphids (Homoptera: Aphididae) in a pepper production region in Jamaica. Environ. Entomol. 2003, 32, 499–509. [Google Scholar] [CrossRef]
- Chi, H.; Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. 1985, 24, 225–240. [Google Scholar]
- Chi, H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Chang, C.; Huang, C.Y.; Dai, S.M.; Atlıhan, R.; Chi, H. Genetically engineered ricin suppresses Bactrocera dorsalis (Diptera: Tephritidae) based on demographic analysis of group-reared life table. J. Econ. Entomol. 2016, 109, 987–992. [Google Scholar] [CrossRef]
- Haas, J.; Lozano, E.R.; Poppy, G.M. A simple, light clip-cage for experiments with aphids. Agric. Forest Entomol. 2018, 20, 589–592. [Google Scholar] [CrossRef]
- Horsfall, J.L. Life History Studies of Myzus persicae Sulzer; Pennsylvania Agricultural Experiment Station: Rock Springs, FL, USA, 1924; Volume 185, p. 16. [Google Scholar]
- Chi, H. TIMING-MSChart: A Computer Program for the Population Projection Based on Age-Stage, two-Sex Life Table; National Chung Hsing University: Taichung, Taiwan, 2020. [Google Scholar]
- Chi, H. TWOSEX-MSChart: A Computer Program for the Age-Stage, Two-Sex Life Table Analysis; National Chung Hsing University: Taichung, Taiwan, 2020. [Google Scholar]
- Akca, I.; Ayvaz, T.; Yazici, E.; Smith, C.L.; Chi, H. Demography and population projection of Aphis fabae (Hemiptera: Aphididae): With additional comments on life table research criteria. J. Econ. Entomol. 2015, 108, 1466–1478. [Google Scholar] [CrossRef]
- Tuan, S.J.; Yeh, C.C.; Atlihan, R.; Chi, H. Linking life table and predation rate for biological control: A comparative study of Eocanthecona furcellata (Hemiptera: Pentatomidae) fed on Spodoptera litura (Lepidoptera: Noctuidae) and Plutella xylostella (Lepidoptera: Plutellidae). J. Econ. Entomol. 2016, 109, 13–24. [Google Scholar] [CrossRef]
- Davis, J.A.; Radcliffe, E.B. Reproduction and feeding behavior of Myzus persicae on four cereals. J. Econ. Entomol. 2008, 101, 9–16. [Google Scholar] [CrossRef]
- Razmjou, J.; Golizadeh, A. Performance of corn leaf aphid, Rhopalosiphum maidis (Fitch) (Homoptera: Aphididae) on selected maize hybrids under laboratory conditions. Appl. Entomol. Zool. 2010, 45, 267–274. [Google Scholar] [CrossRef]
- Obopile, M.; Ositile, B. Life table and population parameters of cowpea aphid, Aphis craccivora Koch (Homoptera: Aphididae) on five cowpea Vigna unguiculata (L. Walp.) cultivars. J. Pest Sci. 2010, 83, 9–14. [Google Scholar] [CrossRef]
- Madahi, K.; Sahragard, A. Comparative life table of Aphis pomi (Hemiptera: Aphididae) on two host plants Malus pumila L. and Chaenomeles japonica under laboratory conditions. J. Crop Prot. 2012, 1, 321–330. [Google Scholar]
- Bussaman, P.; Sauth, C.; Chandrapatya, A.; Atlihan, R.; Gökçe, A.; Saska, P.; Chi, H. Fast population growth in physogastry reproduction of Luciaphorus perniciosus (Acari: Pygmephoridae) at different temperatures. J. Econ. Entomol. 2017, 109, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.Z.; Chen, B.H.; Güncan, A.; Atlihan, R.; Gökçe, A.; Smith, C.L.; Chi, H. Demography and mass-rearing Harmonia dimidiata (Coleoptera: Coccinellidae) using Aphis gossypii (Hemiptera: Aphididae) and eggs of Bactrocera dorsalis (Diptera: Tephritidae). J. Econ. Entomol. 2018, 2, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Güncan, A.; Gümüs, E. Influence of different hazelnut cultivars on some demographic characteristics of the Filbert aphid (Hemiptera: Aphididae). J. Econ. Entomol. 2017, 110, 1856–1862. [Google Scholar] [CrossRef] [PubMed]
- Jahan, F.; Abbasipour, H.; Askarianzadeh, A.; Hassanshahi, G.; Saeedizadeh, A. Biology and life table parameters of Brevicoryne brassicae (Hemiptera: Aphididae) on cauliflower cultivars. J. Insect Sci. 2014, 14, 284. [Google Scholar] [CrossRef]
- Ulusoy, M.R.; Ölmez-Bayhan, S. Effect of certain Brassica plants on biology of the cabbage aphid Brevicoryne brassicae under laboratory conditions. Phytoparasitica 2006, 34, 133–138. [Google Scholar] [CrossRef]
- Zarghami, S.; Allahyari, H.; Bagheri, M.R.; Saboori, A. Effect of nitrogen fertilization on life table parameters and population growth of Brevicoryne brassicae. Bull. Insectol. 2010, 63, 39–43. [Google Scholar]
- Musa, P.D.; Ren, S.X. Development and reproduction of Bemisia tabaci (Homoptera: Aleyrodidae) on three bean species. Insect Sci. 2005, 12, 25–30. [Google Scholar] [CrossRef]
- Southwood, T.R.E.; Henderson, P.A. Ecological Methods; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Atlihan, R.; Chi, H. Temperature-dependent development and demography of Scymnus subvillosus (Coleoptera: Coccinellidae) reared on Hyalopterus pruni (Homoptera: Aphididae). J. Econ. Entomol. 2008, 101, 325–333. [Google Scholar] [CrossRef]
- Bailey, R.; Chang, N.T.; Lai, P.Y. Two-sex life table and predation rate of Cybocephalus flavocapitis Smith (Coleoptera: Cybocephalidae) reared on Aulacaspis yasumatsui Takagi (Hemiptera: Diaspididae). Taiwan J. Asia-Pac. Entomol. 2011, 14, 433–439. [Google Scholar] [CrossRef]
- Huang, Y.B.; Chi, H. Age-stage, two-sex life table of Bactrocera cucurbitae (Coquillett) (Diptera: Tephritidae) with a discussion on the problem of applying female age-specific life table to insect populations. Insect Sci. 2012, 19, 263–273. [Google Scholar] [CrossRef]
- Mitra, S.; Mobarak, S.H.; Barik, A. Age-stage, two-sex life table of the biocontrol agent, Altica cyanea on three Ludwigia species. Biologia 2001, 76, 101–112. [Google Scholar] [CrossRef]
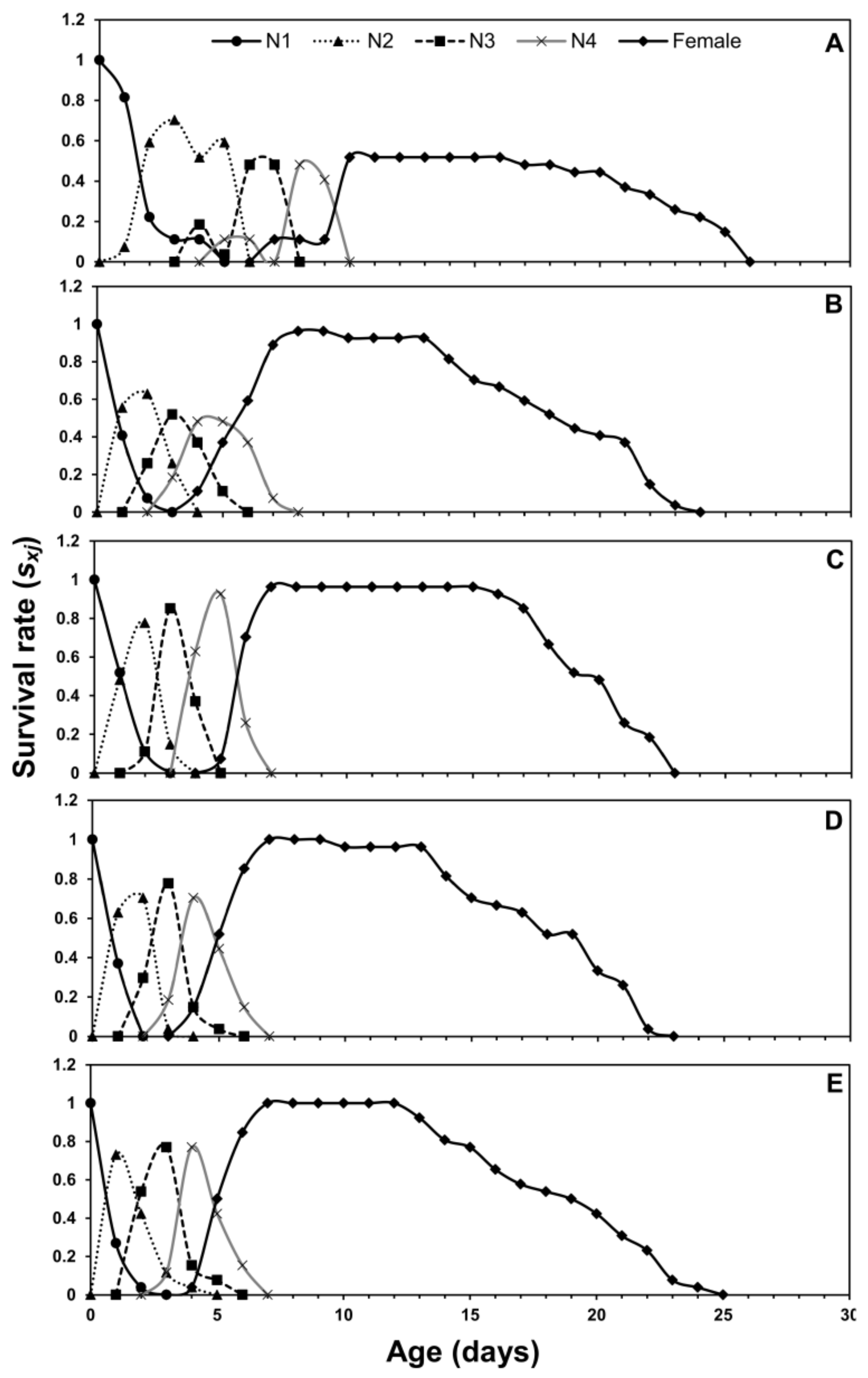
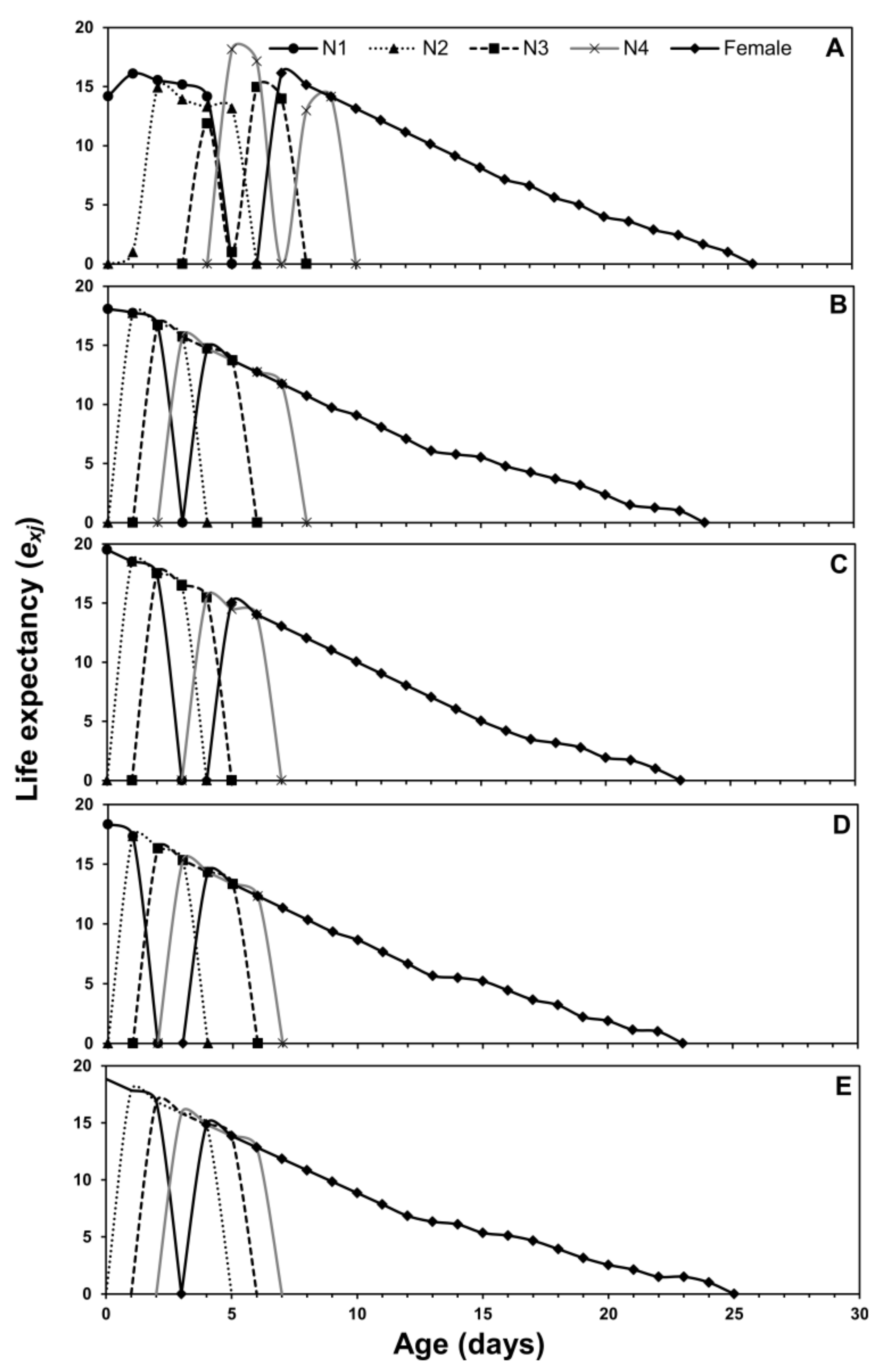
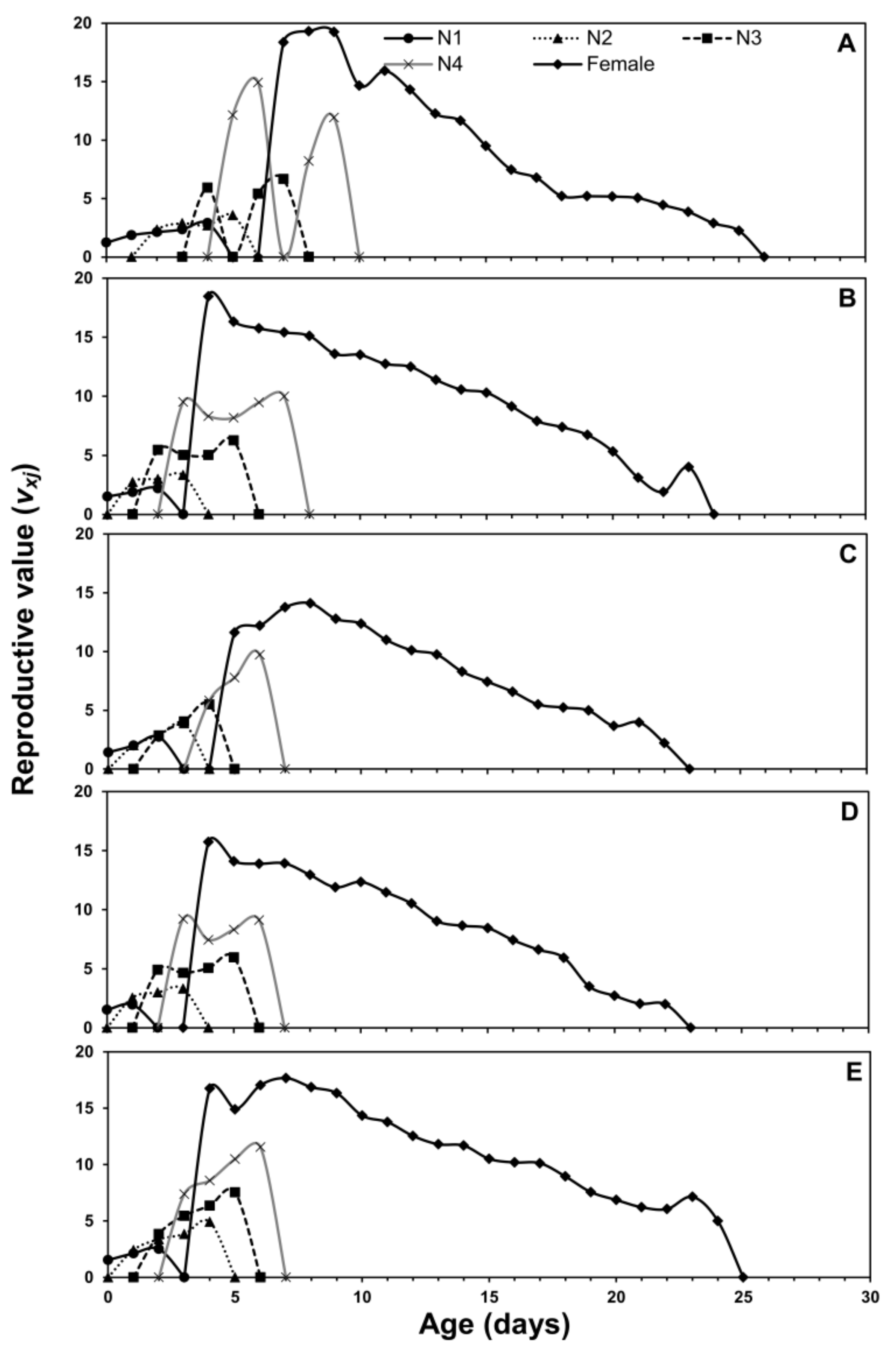
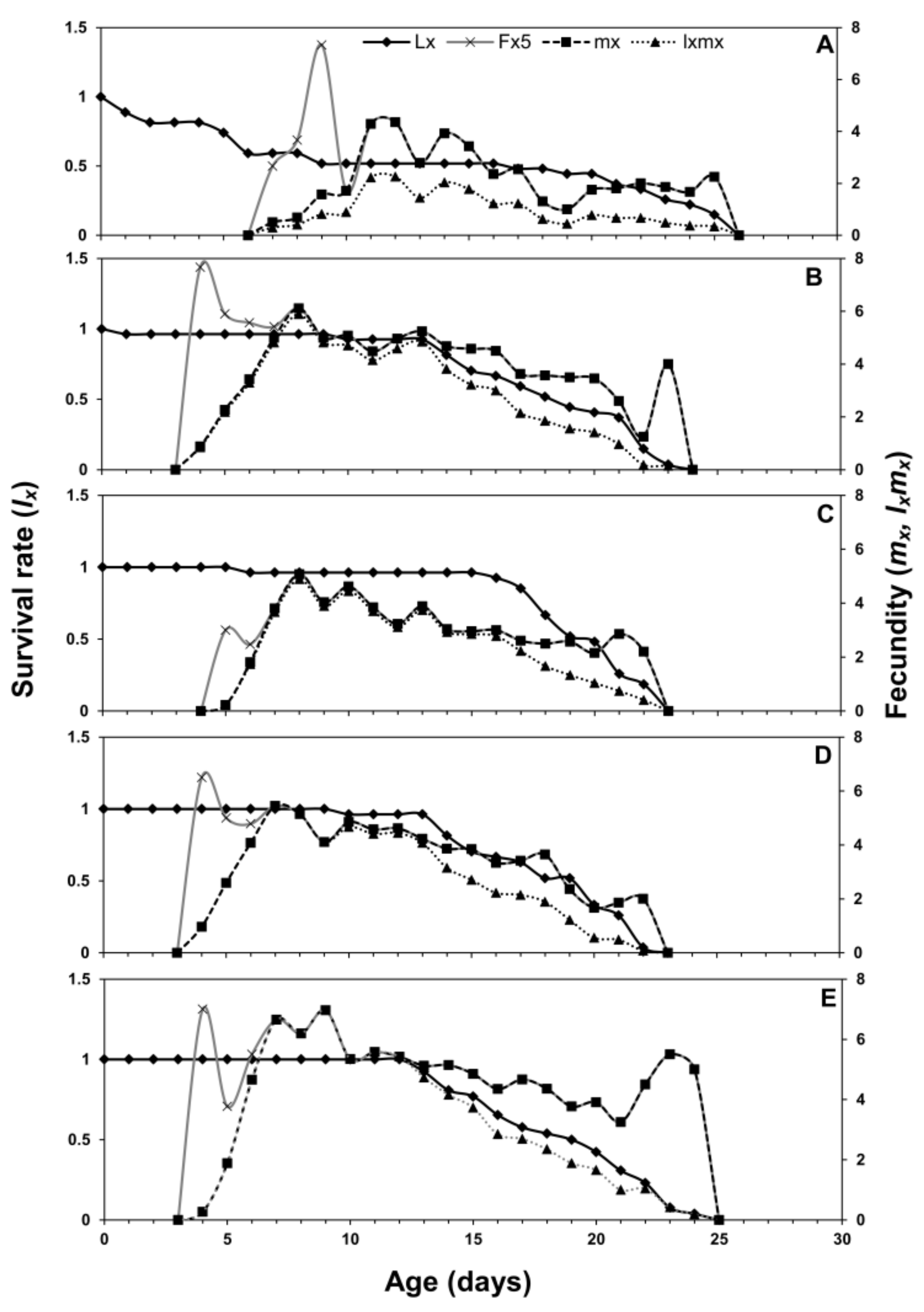

| Life Stage | n | Cabbage | n | Chinese Cabbage | n | Crown Daisy | n | Eggplant | n | Pepper |
|---|---|---|---|---|---|---|---|---|---|---|
| N1 | 54 | 2.42 ± 0.16 a | 54 | 1.50 ± 0.09 b | 54 | 1.63 ± 0.09 b | 54 | 1.37 ± 0.07 c | 52 | 1.31 ± 0.08 c |
| N2 | 38 | 3.26 ± 0.14 a | 52 | 1.50 ± 0.07 b | 54 | 1.41 ± 0.07 b | 54 | 1.37 ± 0.07 b | 52 | 1.31 ± 0.06 c |
| N3 | 32 | 1.81 ± 0.07 a | 52 | 1.31 ± 0.06 b | 54 | 1.33 ± 0.06 b | 54 | 1.26 ± 0.07 b | 52 | 1.54 ± 0.08 a |
| N4 | 28 | 2.00 ± 0.00 a | 52 | 1.65 ± 0.09 b | 52 | 1.85 ± 0.05 a | 54 | 1.48 ± 0.07 c | 52 | 1.46 ± 0.08 c |
| Preadult | 9.36 ± 0.23 a | 5.96 ± 0.16 bc | 6.19 ± 0.07 b | 5.48 ± 0.12 c | 5.62 ± 0.11 c | |||||
| Adult longevity | 28 | 13.7 ± 0.41 a | 52 | 12.7 ± 0.50 a | 52 | 13.8 ± 0.28 a | 54 | 12.8 ± 0.48 a | 52 | 13.2 ± 0.52 a |
| Total Longevity | 14.1 ± 1.33 b | 18.1 ± 0.67 a | 19.5 ± 0.46 a | 18.3 ± 0.47 a | 18.8 ± 0.518 a | |||||
| Fecundity | 36.5 ± 1.26 c | 60.6 ± 2.68 a | 47.1 ± 0.91 bc | 54.3 ± 2.16 b | 69.6 ± 2.42 a | |||||
| TPRP | 9.71 ± 0.29 a | 6.00 ± 0.16 b | 6.35 ± 0.09 b | 5.52 ± 0.13 c | 5.62 ± 0.11 c | |||||
| APRP | 0.36 ± 0.24 a | 0.04 ± 0.03 b | 0.15 ± 0.05 b | 0.04 ± 0.03 b | 0.00 ± 0.00 c | |||||
| Ovi. days | 12.1 ± 0.55 a | 12.3 ± 0.52 a | 13.6 ± 0.30 a | 12.4 ± 0.05 a | 12.9 ± 0.50 a | |||||
| Preadult survival rate (sa) | 0.51 ± 0.06 b | 0.96 ± 0.02 a | 0.96 ± 0.02 a | 1.00 ± 0.00 a | 1.00 ± 0.00 a |
| Parameters | Host Plants | ||||
|---|---|---|---|---|---|
| Cabbage | Chinese Cabbage | Crown Daisy | Eggplant | Pepper | |
| Net reproductive rate (R0) (offspring) | 18.9 ± 2.57 d | 58.4 ± 2.99 b | 45.3 ± 1.48 c | 54.3 ± 2.14 b,c | 69.6 ± 2.38 a |
| Intrinsic rate of increase (r−1) (d−1) | 0.21 ± 0.01 c | 0.41 ± 0.01 a | 0.34 ± 0.01 b | 0.42 ± 0.01 a | 0.42 ± 0.01 a |
| Finite rate of increase (λ) (d−1) | 1.23 ± 0.01 c | 1.51 ± 0.01 a | 1.41 ± 0.06 b | 1.52 ± 0.12 a | 1.53 ± 0.01 a |
| Mean generation time (T) (d) | 14.2 ± 0.40 a | 9.83 ± 0.21 b | 10.9 ± 0.12 b | 9.43 ± 0.16 b | 9.96 ± 0.17 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, M.Y.; Naseem, T.; Arshad, M.; Ashraf, I.; Rizwan, M.; Tahir, M.; Rizwan, M.; Sayed, S.; Ullah, M.I.; Khan, R.R.; et al. Host-Plant Variations Affect the Biotic Potential, Survival, and Population Projection of Myzus persicae (Hemiptera: Aphididae). Insects 2021, 12, 375. https://doi.org/10.3390/insects12050375
Ali MY, Naseem T, Arshad M, Ashraf I, Rizwan M, Tahir M, Rizwan M, Sayed S, Ullah MI, Khan RR, et al. Host-Plant Variations Affect the Biotic Potential, Survival, and Population Projection of Myzus persicae (Hemiptera: Aphididae). Insects. 2021; 12(5):375. https://doi.org/10.3390/insects12050375
Chicago/Turabian StyleAli, Muhammad Yasir, Tayyaba Naseem, Muhammad Arshad, Ijaz Ashraf, Muhammad Rizwan, Muhammad Tahir, Misbah Rizwan, Samy Sayed, Muhammad Irfan Ullah, Rashad Rasool Khan, and et al. 2021. "Host-Plant Variations Affect the Biotic Potential, Survival, and Population Projection of Myzus persicae (Hemiptera: Aphididae)" Insects 12, no. 5: 375. https://doi.org/10.3390/insects12050375
APA StyleAli, M. Y., Naseem, T., Arshad, M., Ashraf, I., Rizwan, M., Tahir, M., Rizwan, M., Sayed, S., Ullah, M. I., Khan, R. R., Amir, M. B., Pan, M., & Liu, T.-X. (2021). Host-Plant Variations Affect the Biotic Potential, Survival, and Population Projection of Myzus persicae (Hemiptera: Aphididae). Insects, 12(5), 375. https://doi.org/10.3390/insects12050375








