Investigations on Arthropods Associated with Decay Stages of Buried Animals in Italy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
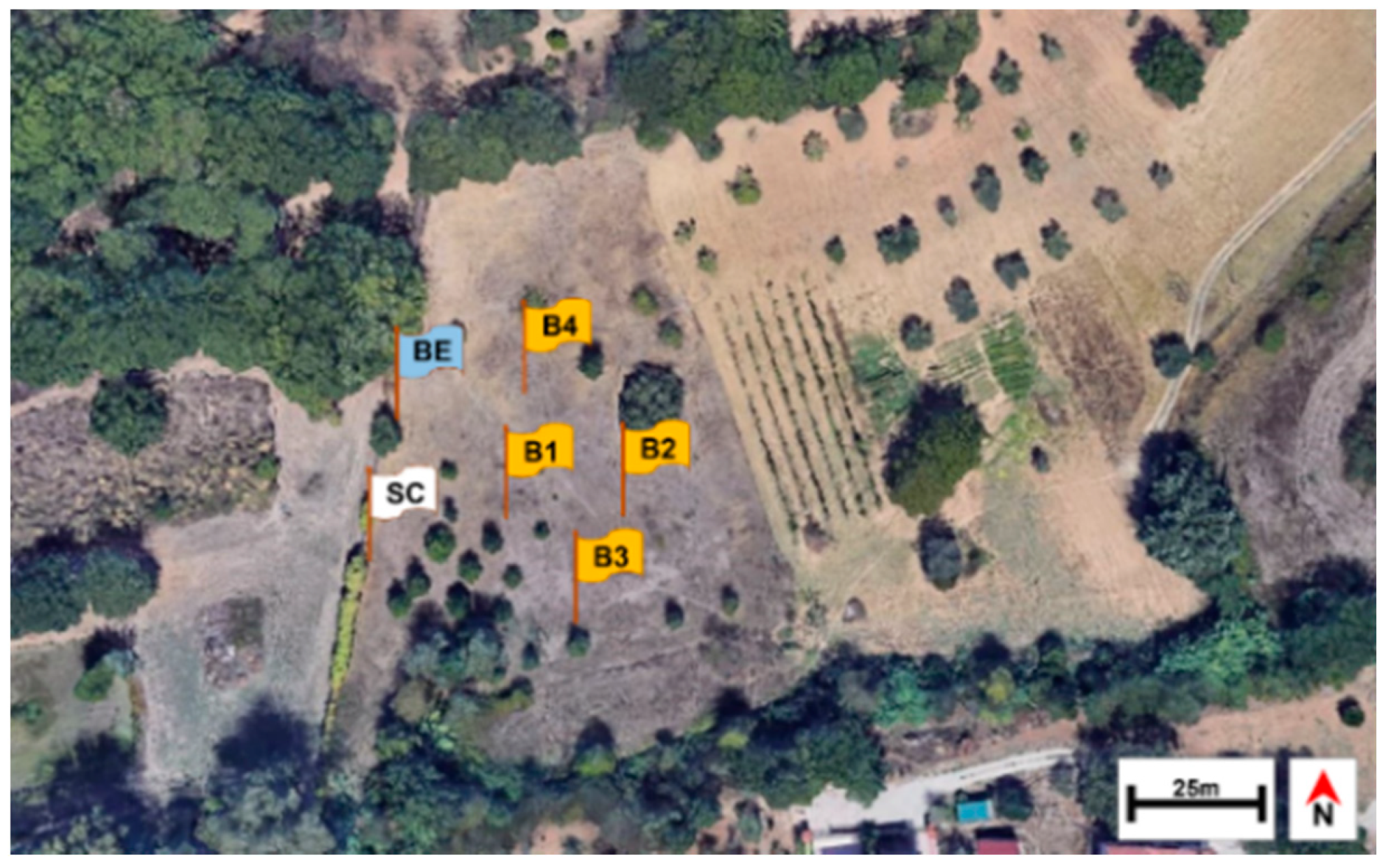
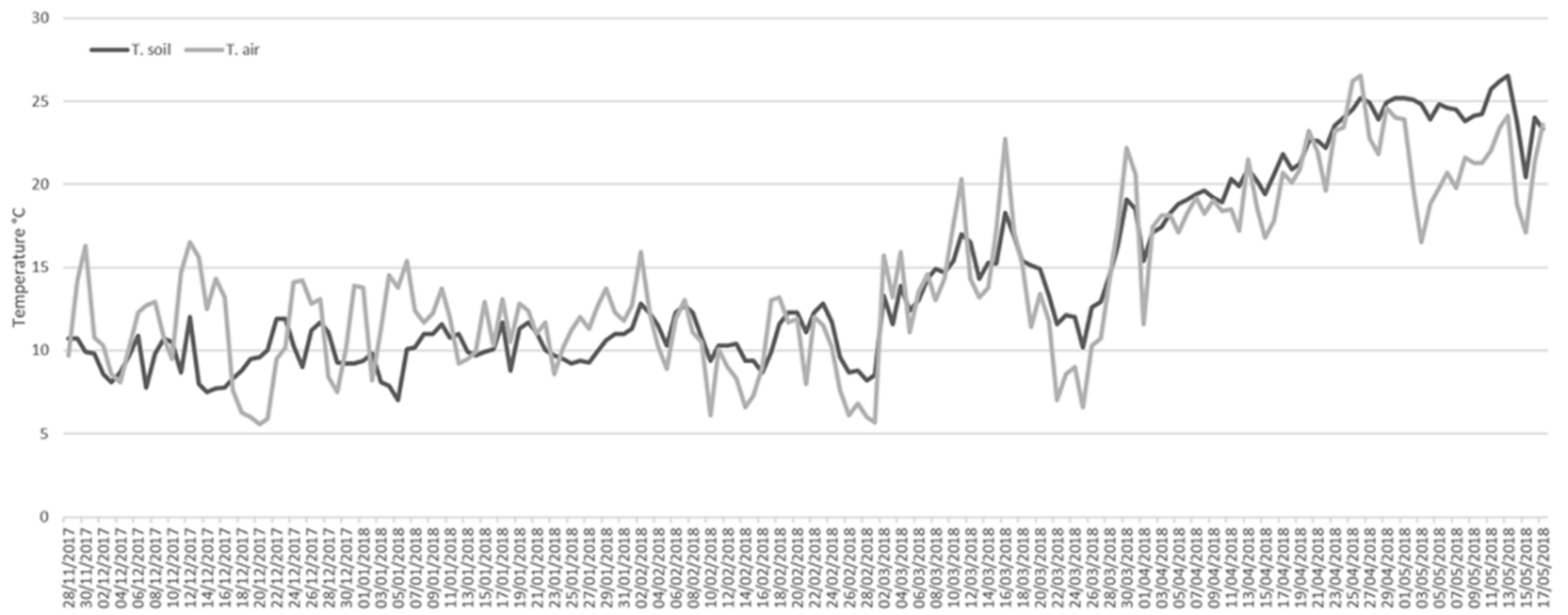
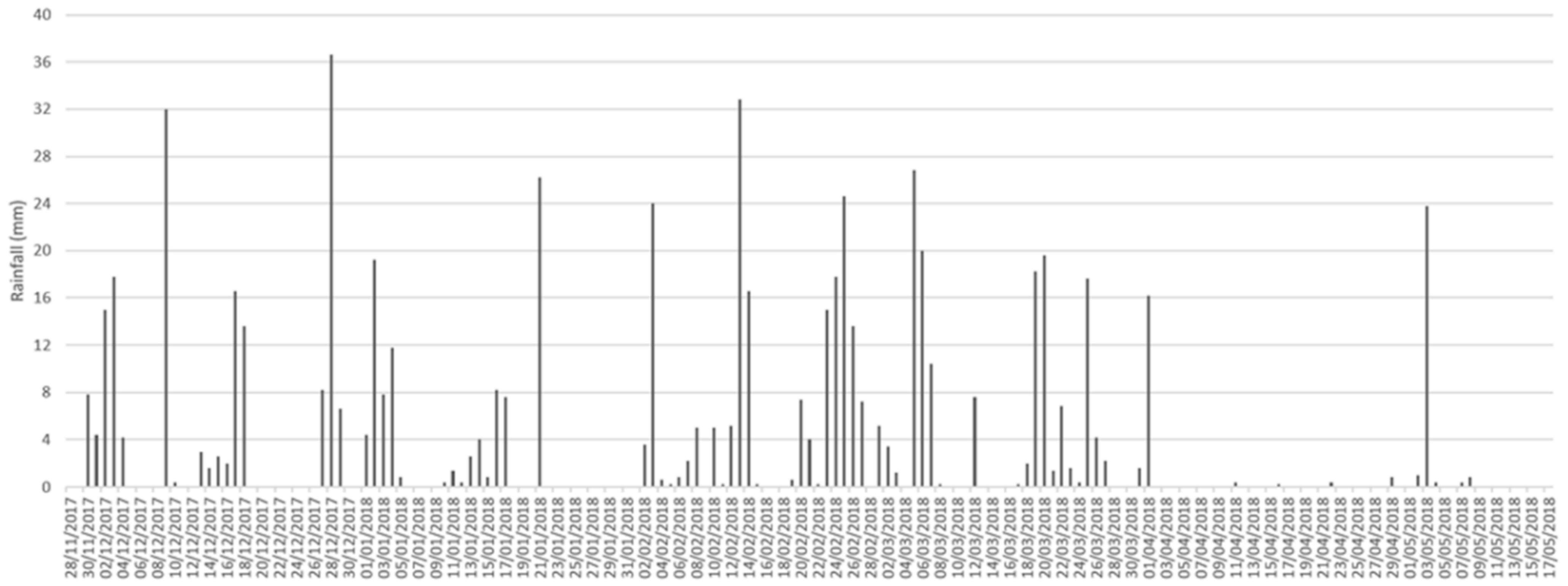
2.1. Study Site
2.2. Arthropod Sampling and Carcass Decay
3. Results
3.1. Decay Rate
3.2. Insect Succession
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, K.G.V. A Manual of Forensic Entomology, 1st ed.; Trustees of the British Museum (Natural History): London, UK, 1986; p. 205. [Google Scholar]
- Byrd, J.H.; Castner, J.L. Forensic Entomology: The Utility of Arthropods in Legal Investigations, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2010; p. 681. [Google Scholar]
- Amendt, J.; Richards, C.S.; Campobasso, C.P.; Zehner, R.; Hall, M.J.R. Forensic entomology: Applications and limitations. Forensic Sci. Med. Pathol. 2011, 7, 379–392. [Google Scholar] [CrossRef]
- Catts, E.P.; Goff, M.L. Forensic entomology in criminal investigations. Ann. Rev. Entomol. 1992, 37, 253–272. [Google Scholar] [CrossRef]
- George, K.A.; Archer, M.S.; Toop, T. Abiotic environmental factors influencing blowfly colonization patterns in the field. Forensic Sci. Int. 2013, 229, 100–107. [Google Scholar] [CrossRef]
- Gilbert, B.M.; Bass, W.M. Seasonal dating of burials from the presence of fly pupae. Am. Antiq. 1967, 32, 534–535. [Google Scholar] [CrossRef]
- Shean, B.S.; Messinger, L.; Papworth, M. Observations of differential decomposition on sun exposed v. shaded pig carrion in coastal Washington state. J. Forensic Sci. 1993, 38, 938–949. [Google Scholar] [CrossRef]
- Moses, R.J. Experimental adipocere formation: Implications for adipocere formation on buried bone. J. Forensic Sci. 2012, 57, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, W.C.; Bass, W.M. Decomposition of buried bodies and methods that may aid in their location. J. Forensic Sci. 1985, 30, 836–852. [Google Scholar] [CrossRef] [PubMed]
- Simmons, T.; Cross, P.A.; Adlam, R.E.; Moffatt, C. The influence of insects on decomposition rate in buried and surface remains. J. Forensic Sci. 2010, 55, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Gaudry, E. The insects colonization of buried remains. In Current Concepts in Forensic Entomology, 1st ed.; Amendt, J., Goff, M.L., Campobasso, C.P., Grassberger, M., Eds.; Springer: New York, NY, USA, 2010; pp. 273–311. [Google Scholar]
- Lundt, H. Ökologische Untersuchungen über die tierische Besiedlung von Aas im Boden. Pedobiologia 1964, 4, 158–180. [Google Scholar]
- Payne, J.A.; King, E.W.; Beinhart, G. Arthropod succession and decomposition of buried pigs. Nature 1968, 219, 180–181. [Google Scholar] [CrossRef]
- Goff, M.L. Estimation of postmortem interval using arthropod development and successional patterns. Forensic Sci. Rev. 1993, 5, 81–94. [Google Scholar] [PubMed]
- Van Laerhoven, S.L.; Anderson, G.S. Insect succession on buried carrion in two biogeoclimatic zones of British Columbia. J. Forensic Sci. 1999, 44, 32–43. [Google Scholar]
- Bourel, B.; Tornel, G.; Hédouin, V.; Grosset, D. Entomofauna of buried bodies in northern France. Int. J. Legal Med. 2004, 118, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Huchet, J.B.; Greenberg, B. Flies, Mochicas and burial practices: A case study from Huaca de la Luna, Peru. J. Archaeol. Sci. 2010, 37, 2846–2856. [Google Scholar] [CrossRef]
- Szpila, K.; Voss, J.G.; Pape, T. A new dipteran forensic indicator in buried bodies. Med. Vet. Entomol. 2010, 24, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Gunn, A.; Bird, J. The ability of the blowflies Calliphora vomitoria (Linnaeus), Calliphora vicina (Rob-Desvoidy) and Lucilia sericata (Meigen) (Diptera: Calliphoridae) and the muscid flies Muscina stabulans (Fallen) and Muscina prolapsa (Harris) (Diptera: Muscidae) to colonise buried remains. Forensic Sci. Int. 2011, 207, 198–204. [Google Scholar]
- Martin-Vega, D.; Gomez-Gomez, A.; Baz, A. The “coffin fly” Conicera tibialis (Diptera: Phoridae) breeding on buried human remains after a postmortem interval of 18 years. J. Forensic Sci. 2011, 56, 1654–1656. [Google Scholar] [CrossRef]
- Pastula, E.C.; Merritt, R.W. Insect arrival pattern and succession on buried carrion in Michigan. J. Med. Entomol. 2013, 50, 432–439. [Google Scholar] [CrossRef]
- Huchet, J.B. Insect remains and their traces: Relevant fossil witnesses in the reconstruction of past funerary practices. Anthropologie 2014, 52, 329–346. [Google Scholar]
- Botham, J.L. Decomposition and arthropod succession on buried remains during winter and summer in Central South Africa: Forensic implications and predictive analyses. In Proceedings of the 19th Entomological Congress of the Entomological Society of Southern Africa, Grahamstown, South Africa, 12–15 July 2015. [Google Scholar]
- Bano, R.; Roy, S. Extraction of soil microarthropods: A low cost Berlese-Tullgren funnels extractor. Int. J. Fauna Biol. Stud. 2016, 3, 14–17. [Google Scholar]
- Le Quesne, W.J.; Payne, K.R. Handbooks for the Identification of British Insects—Cicadellidae (Typhlocybinae) with a Checklist of the British Auchenorhyncha (Hemiptera, Homoptera); Royal Entomological Society of London: London, UK, 1981; Volume 2, Part 2(c); p. 95. [Google Scholar]
- Disney, R.H.L. Handbooks for the Identification of British Insects. Scuttle Flies Diptera, Phoridae (except Megaselia); Royal Entomological Society of London: London, UK, 1983; Volume 10, Part 6; p. 81. [Google Scholar]
- Disney, R.H.L. Handbooks for the Identification of British Insects—Scuttle Flies Diptera, Phoridae (genus Megaselia); Royal Entomological Society of London: London, UK, 1989; Volume 10, Part 8; p. 155. [Google Scholar]
- Goulet, H.; Huber, J.T. Hymenoptera of the World: An Identification Guide to Families; Centre for Land and Biological Resources Research: Ottawa, ON, Canada, 1993; p. 668. [Google Scholar]
- Rozkosny, R.; Gregor, F.; Pont, A.C. The european fanniidae (Diptera). Acta Sci. Nat. Brno 1997, 31, 1–80. [Google Scholar]
- Thiele, H.U. Carabid Beetles in Their Environments. a Study on Habitat Selection by Adaptations in Physiology and Behavior; Springer: New York, NY, USA, 1977; p. 369. [Google Scholar]
- Pont, A.C.; Meier, R. The Sepsidae (Diptera) of Europe; Fauna entomologica Scandinavica; Brill: Leiden, The Netherlands, 2002; Volume 37, p. 196. [Google Scholar]
- Brandmayr, P.; Zetto, T.; Pizzolotto, R. I coleotteri Carabidi per la valutazione ambientale e la conservazione della biodiversità; APAT (Agenzia per la protezione dell’ambiente e per i servizi tecnici): Rome, Italy, 2005; Volume 34, p. 240, Manuali e Linee Guida. [Google Scholar]
- Oesterbroek, P. The European Families of Diptera—Identification, Diagnosis, Biology; KNNV Publishing: Utrech, Olanda, The Netherlands, 2006; p. 210. [Google Scholar]
- Latella, L.; Gobbi, M. La Fauna Del Suolo. Tassonomia Ecologia E Metodi DI Studio Dei Principali Gruppi DI Invertebrati Terrestri Italiani; MUSE—Museo delle Scienze: Trento, Italy, 2008; Volume 3, p. 191, Quaderni del Museo Tridentrino di Scienze Naturali. [Google Scholar]
- Szpila, K. Key for identification of European and Mediterranean blowflies (Diptera, Calliphoridae) of medical and veterinary importance—adult flies. In Forensic Entomology, an Introduction, 2nd ed.; Gennard, D., Ed.; Wiley-Blackwell: West Sussex, UK, 2012; pp. 77–81. [Google Scholar]
- Rochefort, S.; Giroux, M.; Savage, J.; Wheeler, T.A. Key to forensically important Piophilidae (Diptera) in the Nearctic Region. Can. J. Arthropod Identif. 2015, 27. [Google Scholar] [CrossRef]
- Chinery, M. Guida Degli Insetti D’Europa; Franco Muzzio Editore: Rome, Italy, 2016; p. 431. [Google Scholar]
- Gregor, F.; Rozkosny, R.; Bartàk, M.; Vanhara, J. Manual of Central European Muscidae (Diptera). Morphology, Taxonomy, Identification and Distribution; E. Schweizerbart Science Publisher: Stuttgart, Germany, 2016; Volume 162, p. 219. [Google Scholar]
- Ants (Hymenoptera: Formicidae) Collected in Cowling Arboretum and McKnight Prairie. Available online: https://acad.carleton.edu/curricular/BIOL/resources/ant/index.html (accessed on 18 December 2017).
- Ballerio, A.; Rey, A.; Uliana, M.; Rastelli, M.; Rastelli, S.; Romano, M.; Colacurcio, L. Coleotteri Scarabeoidea d’Italia. Available online: http://www.societaentomologicaitaliana.it/Coleotteri%20Scarabeoidea%20d’Italia%202014/scarabeidi/home.htm (accessed on 11 December 2017).
- Mike’s Insect Keys. Available online: https://sites.google.com/view/mikes-insect-keys/mikes-insect-keys (accessed on 18 December 2017).
- Szpila, K. Key for the identification of third instars of European blowflies (Diptera: Calliphoridae) of forensic importance. In Current Concepts in Forensic Entomology; Amendt, J., Goff, M.L., Campobasso, C.P., Grassberger, M., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 43–56. [Google Scholar]
- Glime, J.M. Terrestrial insects: Holometabola—Diptera nematocera: Tipuloidea. Chapter 12–18. In Bryophyte ecology, Volume 2: Bryological Interaction; Glime, J.M., Ed.; International Association of Bryologists and Michigan Technological University: Houghton, MI, USA, 2016. [Google Scholar]
- Gunn, A. The colonisation of remains by the muscid flies Muscina stabulans (Fallén) and Muscina prolapsa (Harris) (Diptera: Muscidae). Forensic Sci. Int. 2016, 266, 349–356. [Google Scholar] [CrossRef]
- Turner, R.; Wiltshire, P. Experimental validation of forensic evidence: A study of the decomposition of buried pigs in heavy clay soil. Forensic Sci. Int. 1999, 101, 113–122. [Google Scholar] [CrossRef]
- Campobasso, C.P.; Henry, R.; Disney, L.; Introna, F. A case of Megaselia scalaris (Loew) (Dipt., Phoridae) breeding in a human corpse. Anil Aggrawal’s Internet J. Forensic Med. Toxicol. 2004, 5, 3–5. [Google Scholar]
- Francesconi, F.; Lupi, O. Myiasis. Clin. Microbiol. Rev. 2012, 25, 79–105. [Google Scholar] [CrossRef] [PubMed]
- Merritt, R.W.; Snider, R.; de Jong, J.L.; Benbow, M.E.; Kimbirauskas, R.K.; Kolar, R.E. Collembola of the grave: A cold case history involving arthropods 28 years after death. J. Forensic Sci. 2007, 52, 1359–1361. [Google Scholar] [CrossRef] [PubMed]
- Martin-Vega, D.; Baz, A. Sarcosaprophagous Diptera assemblages in natural habitats in central Spain: Spatial and seasonal changes in composition. Med. Vet. Entomol. 2013, 27, 64–76. [Google Scholar] [CrossRef]
- Greco, S.; Brandmayr, P.; Bonacci, T. Synanthropy and temporal variability of Calliphoridae living in Cosenza (Calabria, Southern Italy). J. Insect Sci. 2014, 14, 216. [Google Scholar] [CrossRef]
- Matuszewski, S.; Bajerlein, D.; Konwerski, S.; Szpila, K. An initial study of insect succession and carrion decomposition in various forest habitats of Central Europe. Forensic Sci. Int. 2008, 180, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Matuszewski, S.; Bajerlein, D.; Konwerski, S.; Szpila, K. Insect succession and carrion decomposition in selected forests of Central Europe. Part 2: Composition and residency patterns of carrion fauna. Forensic Sci. Int. 2010, 195, 42–51. [Google Scholar] [CrossRef]
- Dekeirsschieter, J.; Frederick, C.; Verheggen, F.J.; Drugmand, D.; Haubruge, E. Diversity of forensic rove beetles (Coleoptera, Staphylinidae) associated with decaying pig carcass in a forest biotope. J. Forensic Sci. 2013, 58, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Matuszewski, S. Estimating the preappearance interval from temperature in Creophilus maxillosus L. (Coleoptera: Staphylinidae). J. Forensic Sci. 2012, 57, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Turchetto, M.; Vanin, S. Forensic entomology and climate change. Forensic Sci. Int. 2001, 146 (Suppl.), 207–209. [Google Scholar] [CrossRef] [PubMed]
- Bugelli, V.; Gherardi, M.; Focardi, M.; Pinchi, V.; Vanin, S.; Campobasso, C.P. Decomposition pattern and insect colonization in two cases of suicide by hanging. Forensic Sci. Res. 2018, 3, 94–102. [Google Scholar] [CrossRef]
- Bonacci, T.; Vercillo, V.; Benecke, M. Dermestes frischii and D. undulatus (Coleoptera: Dermestidae) on a human corpse in Southern Italy: First report. Rom. J. Leg. Med. 2017, 25, 180–184. [Google Scholar] [CrossRef]
- Chin, H.C.; Hurahashi, H.; Marwi, M.A.; Jeffery, J.; Omar, B. Opportunistic insects associated with pig carrions in Malaysia. Sains Malays. 2011, 40, 601–604. [Google Scholar]
- Turner, B. Forensic entomology: Insects against crime. Sci. Prog. 1987, 71, 133–144. [Google Scholar]
| Infraorder/ Family | Genus/ Species | Stage of Decomposition | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Fresh | Bloated | Decay/Advanced Decay | Dry | ||||||
| SC | BE | SC | BE | SC | BE | SC | BE | ||
| Calliphoridae | Calliphora vicina | A(3) | -- | A(129); L(6) | L(92) | A(38); L(1) | -- | L(4) | -- |
| Calliphora vomitoria | A(16) | -- | A(258); L(14) | -- | A(93); L(534) | L(617 **) | L(3695) | -- | |
| Chrysomya albiceps | -- | -- | A(74) | -- | A(17) | -- | -- | -- | |
| Lucilia caesar | A(7) | -- | A(103) | -- | A(10) | -- | -- | -- | |
| Lucilia sericata | -- | -- | A(107); L(4) | -- | A(18) | L(7) | -- | -- | |
| Fanniidae | Fannia canicularis | -- | -- | A(26) | -- | A(24) | L(26) | -- | -- |
| Fannia lineata | -- | -- | -- | -- | A(1) | L(5) | -- | -- | |
| Muscidae | Hydrotaea aenescens | -- | -- | -- | -- | -- | L(123) | -- | -- |
| Hydrotaea capensis | -- | -- | -- | -- | A(1) | L(1373) | -- | -- | |
| Hydrotaea dentipes | A(1) | -- | A(149) | -- | A(147); L(50) | -- | L(87) | -- | |
| Hydrotaea ignava | -- | -- | -- | -- | A(1) | L(19) | -- | -- | |
| Musca domestica | A(10) | -- | A(235) | -- | A(45) | -- | -- | -- | |
| Muscina levida | -- | -- | A(1) | -- | A(1) | -- | -- | -- | |
| Muscina prolapsa | -- | -- | A(6) | -- | -- | L(26) | -- | -- | |
| Muscina stabulans | -- | -- | A(1) | -- | -- | L(118) | -- | -- | |
| Phaonia subventa | -- | -- | A(7) | -- | A(4) | -- | -- | -- | |
| Polietes meridionalis | -- | -- | A(5) | -- | -- | -- | -- | -- | |
| Azelia sp. | -- | -- | -- | -- | A(1) | -- | -- | -- | |
| Helina sp. | -- | -- | A(3) | -- | -- | -- | -- | -- | |
| Thricops sp. | -- | -- | A(10) | -- | -- | -- | -- | -- | |
| Phoridae | Megaselia scalaris | -- | -- | A(3) | A(1) | -- | A(3) | -- | A(1) |
| Conicera tibialis | -- | -- | -- | -- | -- | A(3 **) | -- | -- | |
| Chaetopleurophora sp. | -- | -- | A(2) | -- | -- | -- | -- | -- | |
| Piophilidae | Piophila casei | -- | -- | A(7) | -- | A(6) | -- | -- | -- |
| Stearibia nigriceps | -- | -- | A(46) | -- | A(10) | -- | -- | -- | |
| Prochyliza sp. | -- | -- | A(3) | -- | -- | -- | -- | -- | |
| Sepsidae | Sepsis duplicata | -- | -- | A(11) | -- | A(3) | -- | -- | -- |
| Sepsis cynipsea | -- | -- | A(2) | -- | -- | -- | -- | -- | |
| Anthomyiidae | Anthomyia sp. | -- | -- | A(2) | -- | A(1) | -- | -- | -- |
| Sarcophagidae | Sarcophaga sp. | -- | -- | A(1) | -- | -- | -- | -- | -- |
| Cecidomyidae | -- | -- | -- | -- | -- | -- | A(2) | -- | -- |
| Drosophilidae | -- | -- | -- | -- | A(1) | -- | A(4) | -- | -- |
| Dryomyzidae | -- | -- | -- | A(4) | -- | -- | -- | -- | -- |
| Ephydridae | -- | -- | -- | -- | A(2 *) | -- | -- | -- | -- |
| Heleomyzidae | -- | A(1) | -- | -- | -- | -- | -- | -- | -- |
| Lauxaniidae | -- | A(1) | -- | -- | -- | -- | -- | -- | -- |
| Psychodidae | -- | -- | -- | -- | A(1) | -- | A(12 **) | -- | -- |
| Scatopsidae | -- | -- | -- | -- | A(2) | -- | A(2) | -- | -- |
| Sciaridae | -- | -- | -- | -- | A(7 *) | -- | A(30 **) | -- | A(13 *) |
| Simulidae | -- | -- | -- | A(2 *) | -- | A(8 *) | A(1) | A(1 *) | -- |
| Sphaeroceridae | -- | -- | -- | -- | A(1 *) | -- | A(36 *) | -- | -- |
| Tipulomorpha | -- | -- | -- | -- | -- | -- | L(13 *) | -- | -- |
| Family | Genus/ Species | Stage of Decomposition | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Fresh | Bloated | Decay/Advanced Decay | Dry | ||||||
| SC | BE | SC | BE | SC | BE | SC | BE | ||
| Cleridae | Necrobia ruficollis | -- | -- | -- | -- | -- | A(2) | -- | -- |
| Necrobia violacea | -- | -- | -- | -- | -- | A(4) | -- | -- | |
| Dermestidae | Dermestes frischi | -- | -- | -- | -- | -- | A(9); L(10) | -- | A(2) |
| Geotrupidae | Anoplotrupes stercorosus | -- | -- | A(1) | -- | -- | -- | -- | -- |
| Histeridae | Margarinotus brunneus | -- | -- | -- | -- | -- | A(6) | -- | A(3) |
| Margarinotus ventralis | -- | -- | -- | -- | -- | A(1) | -- | A(1) | |
| Saprinus semistriatus | -- | -- | -- | -- | -- | A(10) | -- | A(2) | |
| Hydrophilidae | Sphaeridium lunatum | -- | -- | -- | -- | -- | A(1) | -- | -- |
| Nitidulidae | Nitidula flavomaculata | -- | -- | -- | -- | A(11) | -- | A(19) | -- |
| Silphidae | Necrodes littoralis | -- | -- | -- | -- | A(1) | -- | -- | -- |
| Thanatophilus rugosus | -- | -- | -- | -- | -- | A(1) | -- | -- | |
| Staphylinidae | Creophilus maxillosus | -- | -- | -- | -- | A(14) | A(5) | A(3) | -- |
| Anotylus sp. | -- | -- | -- | A(3 **) | -- | A(3) | -- | -- | |
| Carpelimus sp. | A(1) | -- | A(10) | -- | -- | -- | -- | -- | |
| Heterothops sp. | -- | -- | -- | -- | -- | A(4) | -- | -- | |
| Paederus sp. | -- | -- | -- | -- | A(1) | -- | A(2) | -- | |
| Platystethus sp. | -- | -- | -- | -- | -- | A(9) | -- | -- | |
| Philonthus sp. | -- | -- | -- | A(1 *) | -- | A(1) | -- | -- | |
| Quedius sp. | -- | -- | -- | A(1) | -- | A(4) | -- | A(1) | |
| Aleocharinae | -- | -- | -- | A(16) | -- | A(10) | -- | -- | |
| Piestinae | -- | -- | -- | -- | -- | A(1) | -- | -- | |
| Carabidae | Abax sp. | -- | -- | -- | A(1) | -- | -- | -- | -- |
| Acinopus sp. | -- | -- | -- | A(1) | -- | -- | -- | -- | |
| Ophonus cribricollis | -- | -- | -- | A(1) | -- | -- | -- | -- | |
| Ophonus sp. | -- | -- | -- | A(1) | -- | -- | -- | -- | |
| Trechus quadristriatus | -- | -- | -- | A(1) | -- | -- | -- | -- | |
| Buprestidae | -- | -- | -- | A(1) | -- | -- | -- | -- | -- |
| Chrysomelidae | -- | -- | -- | A(1) | -- | -- | -- | -- | -- |
| Curculionidae | -- | -- | -- | A(1) | -- | A(1) | -- | -- | -- |
| Scarabeidae | -- | -- | -- | A(1) | -- | -- | A(3) | -- | -- |
| Order | Family | Genus/ Species | Stage of Decomposition | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fresh | Bloated | Decay/Advanced Decay | Dry | |||||||
| SC | BE | SC | BE | SC | BE | SC | BE | |||
| Araneae | Amaurobiidae | -- | -- | -- | -- | -- | A(2) | -- | -- | -- |
| Lycosidae | -- | -- | -- | -- | A(7) | -- | -- | -- | -- | |
| Thomisidae | -- | -- | -- | -- | -- | A(1) | -- | -- | -- | |
| Salticidae | -- | -- | -- | -- | -- | A(1) | -- | -- | -- | |
| Dermaptera | Forficulidae | Forficula auricularia | -- | -- | -- | A(1) | -- | -- | -- | -- |
| Hymenoptera | Braconidae | -- | -- | -- | A(18) | A(1 *) | A(8) | A(1) | -- | -- |
| Formicidae | Messor sp. | -- | -- | -- | A(1) | A(1) | -- | -- | A(1) | |
| Camponotus sp. | -- | -- | -- | A(2) | -- | -- | -- | -- | ||
| Pheidole sp. | -- | -- | -- | -- | -- | A(36) | -- | -- | ||
| Pteromalidae | Nasoniavitripennis | -- | -- | -- | -- | -- | A(19 **) | -- | A(2) | |
| Rhynchota | Cicadellidae | Eupteryx sp. | -- | -- | A(1) | -- | -- | -- | -- | -- |
| Delphacidae | -- | -- | -- | A(1) | -- | -- | -- | -- | -- | |
| Geophilomorpha | -- | -- | -- | -- | -- | A(1) | -- | -- | -- | -- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonacci, T.; Mendicino, F.; Bonelli, D.; Carlomagno, F.; Curia, G.; Scapoli, C.; Pezzi, M. Investigations on Arthropods Associated with Decay Stages of Buried Animals in Italy. Insects 2021, 12, 311. https://doi.org/10.3390/insects12040311
Bonacci T, Mendicino F, Bonelli D, Carlomagno F, Curia G, Scapoli C, Pezzi M. Investigations on Arthropods Associated with Decay Stages of Buried Animals in Italy. Insects. 2021; 12(4):311. https://doi.org/10.3390/insects12040311
Chicago/Turabian StyleBonacci, Teresa, Federica Mendicino, Domenico Bonelli, Francesco Carlomagno, Giuseppe Curia, Chiara Scapoli, and Marco Pezzi. 2021. "Investigations on Arthropods Associated with Decay Stages of Buried Animals in Italy" Insects 12, no. 4: 311. https://doi.org/10.3390/insects12040311
APA StyleBonacci, T., Mendicino, F., Bonelli, D., Carlomagno, F., Curia, G., Scapoli, C., & Pezzi, M. (2021). Investigations on Arthropods Associated with Decay Stages of Buried Animals in Italy. Insects, 12(4), 311. https://doi.org/10.3390/insects12040311









