Identification and Expression Characterization of ATP-Binding Cassette (ABC) Transporter Genes in Melon Fly
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Identification, Characterization and Phylogenetic Analysis
2.3. RNA Isolation and cDNA Synthesis
2.4. Expression Profiles
2.5. Insecticide Exposures
2.6. Statistical Analysis
3. Results
3.1. Identification and Phylogenetic Analysis of ABC Transporter Genes
3.2. The Expression Pattern of ZcABCs
3.3. Transcriptional Responses of ZcABCs to Insecticides Exposures
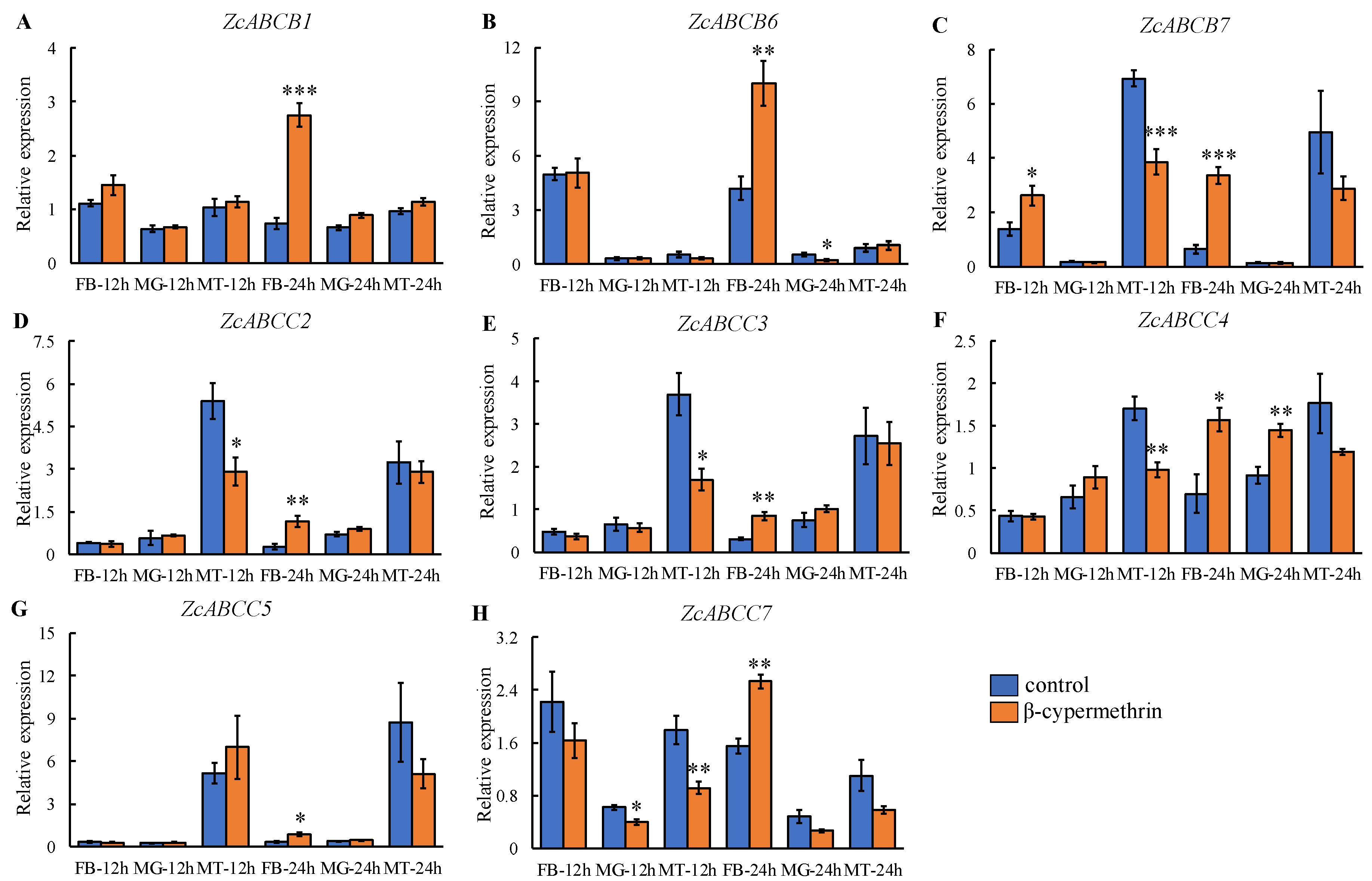
3.4. Transcriptional Response of ZcABCs to β-Cypermethrin Exposure in Tissues
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ford, R.C.; Beis, K. Learning the ABCs one at a time: Structure and mechanism of ABC transporters. Biochem. Soc. Trans. 2019, 47, 23–36. [Google Scholar] [CrossRef]
- Xiao, Q.Y.; Zhou, Y.T.; Lauschke, V.M. Ethnogeographic and inter-individual variability of human ABC transporters. Hum. Genet. 2020, 139, 623–646. [Google Scholar] [CrossRef]
- Dean, M.; Annilo, T. Evolution of the ATP-binding cassette (ABC) transporter superfamily in vertebrates. Annu. Rev. Genom. Hum. Genet. 2005, 6, 123–142. [Google Scholar] [CrossRef]
- Dermauw, W.; Van Leeuwen, T. The ABC gene family in arthropods: Comparative genomics and role in insecticide transport and resistance. Insect Biochem. Mol. Biol. 2014, 45, 89–110. [Google Scholar] [CrossRef]
- Bretschneider, A.; Heckel, D.G.; Vogel, H. Know your ABCs: Characterization and gene expression dynamics of ABC transporters in the polyphagous herbivore Helicoverpa armigera. Insect Biochem. Mol. Biol. 2016, 72, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hollenstein, K.; Dawson, R.J.P.; Locher, K.P. Structure and mechanism of ABC transporter proteins. Curr. Opin. Struct. Biol. 2007, 17, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Saurin, W.; Hofnung, M.; Dassa, E. Getting in or out: Early segregation between importers and exporters in the evolution of ATP-binding cassette (ABC) transporters. J. Mol. Evol. 1999, 48, 22–41. [Google Scholar] [CrossRef] [PubMed]
- Higgins, C.F. ABC transporters: From microorganisms to man. Annu. Rev. Cell Biol. 1992, 8, 67–113. [Google Scholar] [CrossRef]
- Dassa, E.; Bouige, P. The ABC of ABCs: A phylogenetic and functional classification of ABC systems in living organisms. Res. Microbiol. 2001, 152, 211–229. [Google Scholar] [CrossRef]
- Labbe, R.; Caveney, S.; Donly, C. Genetic analysis of the xenobiotic resistance-associated ABC gene subfamilies of the Lepidoptera. Insect Mol. Biol. 2011, 20, 243–256. [Google Scholar] [CrossRef]
- Epis, S.; Porretta, D.; Mastrantonio, V.; Comandatore, F.; Sassera, D.; Rossi, P.; Cafarchia, C.; Otranto, D.; Favia, G.; Genchi, C.; et al. ABC transporters are involved in defense against permethrin insecticide in the malaria vector Anopheles stephensi. Parasites Vectors 2014, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Pignatelli, P.; Ingham, V.A.; Balabanidou, V.; Vontas, J.; Lycett, G.; Ranson, H. The Anopheles gambiae ATP-binding cassette transporter family: Phylogenetic analysis and tissue localization provide clues on function and role in insecticide resistance. Insect Mol. Biol. 2018, 27, 110–122. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Liang, J.J.; Liu, S.N.; Wang, S.L.; Wu, Q.J.; Xie, W.; Zhang, Y.J. Changes in the expression of four ABC transporter genes in response to imidacloprid in Bemisia tabaci Q (Hemiptera: Aleyrodidae). Pest. Biochem. Physiol. 2019, 153, 136–143. [Google Scholar] [CrossRef] [PubMed]
- You, M.S.; Yue, Z.; He, W.Y.; Yang, X.H.; Yang, G.; Xie, M.; Zhan, D.L.; Baxter, S.W.; Vasseur, L.; Gurr, G.M.; et al. A heterozygous moth genome provides insights into herbivory and detoxification. Nat. Genet. 2013, 45, 220–225. [Google Scholar] [CrossRef]
- Mastrantonio, V.; Ferrari, M.; Epis, S.; Negri, A.; Scuccimarra, G.; Montagna, M.; Favia, G.; Porretta, D.; Urbanelli, S.; Bandi, C. Gene expression modulation of ABC transporter genes in response to permethrin in adults of the mosquito malaria vector Anopheles stephensi. Acta Trop. 2017, 171, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.O.; Zeng, X.C.; Wen, S.Y.; Gao, X.W.; Liu, X.M.; Tian, F.Y.; Shang, Q.L. Multiple ATP-binding cassette transporters genes are involved in thiamethoxam resistance in Aphis gossypii glover. Pest. Biochem. Physiol. 2020, 167, 8. [Google Scholar] [CrossRef] [PubMed]
- Figueira-Mansur, J.; Ferreira-Pereira, A.; Mansur, J.F.; Franco, T.A.; Alvarenga, E.S.L.; Sorgine, M.H.F.; Neves, B.C.; Melo, A.C.A.; Leal, W.S.; Masuda, H.; et al. Silencing of P-glycoprotein increases mortality in temephos-treated Aedes aegypti larvae. Insect Mol. Biol. 2013, 22, 648–658. [Google Scholar] [CrossRef]
- Dalla Bona, A.C.; Chitolina, R.F.; Fermino, M.L.; Poncio, L.D.; Weiss, A.; Lima, J.B.P.; Paldi, N.; Bernardes, E.S.; Henen, J.; Maori, E. Larval application of sodium channel homologous dsRNA restores pyrethroid insecticide susceptibility in a resistant adult mosquito population. Parasites Vectors 2016, 9, 14. [Google Scholar] [CrossRef]
- Xu, C.J.; Li, C.Y.T.; Kong, A.N.T. Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch. Pharm. Res. 2005, 28, 249–268. [Google Scholar] [CrossRef]
- Despres, L.; David, J.P.; Gallet, C. The evolutionary ecology of insect resistance to plant chemicals. Trends Ecol. Evol. 2007, 22, 298–307. [Google Scholar] [CrossRef]
- Lin, M.G.; Wang, X.-J.; Zhang, Y.; Sun, R.F.; Zeng, L. Optimization of artificial diets for mass rearing of Bactrocera dorsalis, Bactrocera cucurbitae and Bactrocera tau. Chin. J. Appl. Entomol. 2013, 50, 1115–1125. [Google Scholar] [CrossRef]
- Koyama, J.; Kakinohana, H.; Miyatake, T. Eradication of the melon fly, Bactrocera cucurbitae, in Japan: Importance of behavior, ecology, genetics, and evolution. Annu. Rev. Entomol. 2004, 49, 331–349. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.H.; Khuhro, N.H.; Awais, M.; Memon, R.M.; Asif, M.U. Functional response of the pupal parasitoid, Dirhinus giffardii towards two fruit fly species, Bactrocera zonata and B. cucurbitae. Entomol. Gen. 2020, 40, 87–95. [Google Scholar] [CrossRef]
- Wei, D.; Xu, H.Q.; Chen, D.; Zhang, S.Y.; Li, W.J.; Smagghe, G.; Wang, J.J. Genome-wide gene expression profiling of the melon fly, Zeugodacus cucurbitae, during thirteen life stages. Sci. Data 2020, 7, 9. [Google Scholar] [CrossRef]
- Sim, S.B.; Geib, S.M. A Chromosome-scale assembly of the Bactrocera cucurbitae genome provides insight to the genetic basis of white pupae. G3-Genes Genomes Genet. 2017, 7, 1927–1940. [Google Scholar] [CrossRef]
- Letunic, I.; Doerks, T.; Bork, P. SMART: Recent updates, new developments and status in 2015. Nucleic Acids Res. 2015, 43, D257–D260. [Google Scholar] [CrossRef] [PubMed]
- Emanuelsson, O.; Nielsen, H.; Brunak, S.; von Heijne, G. Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J. Mol. Biol. 2000, 300, 1005–1016. [Google Scholar] [CrossRef]
- Julenius, K.; Molgaard, A.; Gupta, R.; Brunak, S. Prediction, conservation analysis, and structural characterization of mammalian mucin-type O-glycosylation sites. Glycobiology 2005, 15, 153–164. [Google Scholar] [CrossRef]
- Li, W.J.; Song, Y.J.; Han, H.L.; Xu, H.Q.; Wei, D.; Smagghe, G.; Wang, J.J. Genome-wide analysis of long non-coding RNAs in adult tissues of the melon fly, Zeugodacus cucurbitae (Coquillett). BMC Genom. 2020, 21, 600. [Google Scholar] [CrossRef] [PubMed]
- Hellemans, J.; Mortier, G.; De Paepe, A.; Speleman, F.; Vandesompele, J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007, 8, 14. [Google Scholar] [CrossRef]
- Ewart, G.D.; Howells, A.J. ABC transporters involved in transport of eye pigment precursors in Drosophila melanogaster. Methods Enzymol. 1998, 292, 213–224. [Google Scholar]
- Tian, L.X.; Song, T.X.; He, R.J.; Zeng, Y.; Xie, W.; Wu, Q.J.; Wang, S.L.; Zhou, X.G.; Zhang, Y.J. Genome-wide analysis of ATP-binding cassette (ABC) transporters in the sweetpotato whitefly, Bemisia tabaci. BMC Genom. 2017, 18, 16. [Google Scholar] [CrossRef]
- Sun, H.; Pu, J.; Chen, F.; Wang, J.; Han, Z. Multiple ATP-binding cassette transporters are involved in insecticide resistance in the small brown planthopper, Laodelphax striatellus. Insect Mol. Biol. 2017, 26, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Q.; Jiang, H.B.; Xiong, Y.; Peng, P.; Li, H.F.; Yuan, G.R.; Dou, W.; Wang, J.J. Genome-wide identification of ATP-binding cassette transporters and expression profiles in the Asian citrus psyllid, Diaphorina citri, exposed to imidacloprid. Comp. Biochem. Physiol. D Genom. Proteom. 2019, 30, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.M.; Zhou, S.; Tian, L.; Guo, E.N.; Luan, Y.X.; Zhang, J.Z.; Li, S. Genome-wide identification and characterization of ATP-binding cassette transporters in the silkworm, Bombyx mori. BMC Genom. 2011, 12, 14. [Google Scholar] [CrossRef]
- Xiao, L.F.; Zhang, W.; Jing, T.X.; Zhang, M.Y.; Miao, Z.Q.; Wei, D.D.; Yuan, G.R.; Wang, J.J. Genome-wide identification, phylogenetic analysis, and expression profiles of ATP-binding cassette transporter genes in the oriental fruit fly, Bactrocera dorsalis (Hendel) (Diptera: Tephritidae). Comp. Biochem. Physiol. D Genom. Proteom. 2018, 25, 1–8. [Google Scholar] [CrossRef] [PubMed]
- He, Q.Y.; Yan, Z.T.; Si, F.L.; Zhou, Y.; Fu, W.B.; Chen, B. ATP-binding cassette (ABC) transporter genes involved in pyrethroid resistance in the Malaria cector Anopheles sinensis: Genome-wide identification, characteristics, phylogenetics, and expression profile. Int. J. Mol. Sci. 2019, 20, 1409. [Google Scholar] [CrossRef]
- Mackenzie, S.M.; Brooker, M.R.; Gill, T.R.; Cox, G.B.; Howells, A.J.; Ewart, G.D. Mutations in the white gene of Drosophila melanogaster affecting ABC transporters that determine eye colouration. Biochim. Biophys. Acta Biomembr. 1999, 1419, 173–185. [Google Scholar] [CrossRef]
- Yu, Z.T.; Wang, Y.W.; Zhao, X.M.; Liu, X.J.; Ma, E.B.; Moussian, B.; Zhang, J.Z. The ABC transporter ABCH-9C is needed for cuticle barrier construction in Locusta migratoria. Insect Biochem. Mol. Biol. 2017, 87, 90–99. [Google Scholar] [CrossRef]
- Broehan, G.; Kroeger, T.; Lorenzen, M.; Merzendorfer, H. Functional analysis of the ATP-binding cassette (ABC) transporter gene family of Tribolium castaneum. BMC Genom. 2013, 14, 18. [Google Scholar] [CrossRef]
- Jin, M.H.; Liao, C.Y.; Chakrabarty, S.; Zheng, W.G.; Wu, K.M.; Xiao, Y.T. Transcriptional response of ATP-binding cassette (ABC) transporters to insecticides in the cotton bollworm, Helicoverpa armigera. Pest. Biochem. Physiol. 2019, 154, 46–59. [Google Scholar] [CrossRef]
- Zhou, A.M.; Hassel, B.A.; Silverman, R.H. Expression cloning of 2-5a-dependent Rnase—A uniquely regulated mediator of interferon action. Cell 1993, 72, 753–765. [Google Scholar] [CrossRef]
- Denecke, S.; Fusetto, R.; Batterham, P. Describing the role of Drosophila melanogaster ABC transporters in insecticide biology using CRISPR-Cas9 knockouts. Insect Biochem. Mol. Biol. 2017, 91, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.J.; Sun, D.; Kang, S.; Zhou, J.L.; Gong, L.J.; Qin, J.Y.; Guo, L.; Zhu, L.H.; Bai, Y.; Luo, L.; et al. CRISPR/Cas9-mediated knockout of both the PxABCC2 and PxABCC3 genes confers high-level resistance to Bacillus thuringiensis Cry1Ac toxin in the diamondback moth, Plutella xylostella (L.). Insect Biochem. Mol. Biol. 2019, 107, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.M.; Toe, H.K.; Sanou, A.; Namountougou, M.; Hughes, A.; Diabate, A.; Dabire, R.; Simard, F.; Ranson, H. Additional selection for insecticide resistance in urban Malaria Vectors: DDT Resistance in Anopheles arabiensis from Bobo-Dioulasso, Burkina Faso. PLoS ONE 2012, 7, e0045995. [Google Scholar] [CrossRef]
- Strycharz, J.P.; Lao, A.; Li, H.M.; Qiu, X.H.; Lee, S.H.; Sun, W.L.; Yoon, K.S.; Doherty, J.J.; Pittendrigh, B.R.; Clark, J.M. Resistance in the highly DDT-resistant 91-R strain of Drosophila melanogaster involves decreased penetration, increased metabolism, and direct excretion. Pest. Biochem. Physiol. 2013, 107, 207–217. [Google Scholar] [CrossRef]
- Li, Z.; Cai, T.W.; Qin, Y.; Zhang, Y.H.; Jin, R.H.; Mao, K.K.; Liao, X.; Wan, H.; Li, J.H. Transcriptional response of ATP-binding cassette (ABC) transporters to insecticide in the brown planthopper, Nilaparvata lugens (Stal). Insects 2020, 11, 280. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.T.; Budding, M.; Griffin, M.S.; Croop, J.M. Isolation and characterization of Drosophila multidrug resistance gene homologs. Mol. Cell. Biol. 1991, 11, 3940–3948. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vache, C.; Camares, O.; Cardoso-Ferreira, M.C.; Dastugue, B.; Creveaux, I.; Vaury, C.; Bamdad, M. A potential genomic biomarker for the detection of polycyclic aromatic hydrocarbon pollutants: Multidrug resistance gene 49 in Drosophila melanogaster. Environ. Toxicol. Chem. 2007, 26, 1418–1424. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Johnson, Z.L.; Wasserman, M.R.; Levring, J.; Chen, J.; Liu, S.X. Characterization of the kinetic cycle of an ABC transporter by single-molecule and cryo-EM analyses. eLife 2020, 9, e56451. [Google Scholar] [CrossRef] [PubMed]
- Koenig, C.; Bretschneider, A.; Heckel, D.G.; Grosse-Wilde, E.; Hansson, B.S.; Vogel, H. The plastic response of Manduca sexta to host and non-host plants. Insect Biochem. Mol. Biol. 2015, 63, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.K.; Yang, X.M.; Wu, Z.L.; Shen, Q.W.; Miao, L.J.; Zheng, Y.; Qian, K.; Wang, J.J. Identification and transcriptional response of ATP-binding cassette transporters to chlorantraniliprole in the rice striped stem borer, Chilo suppressalis. Pest Manag. Sci. 2019, 76, 3626–3635. [Google Scholar] [CrossRef] [PubMed]
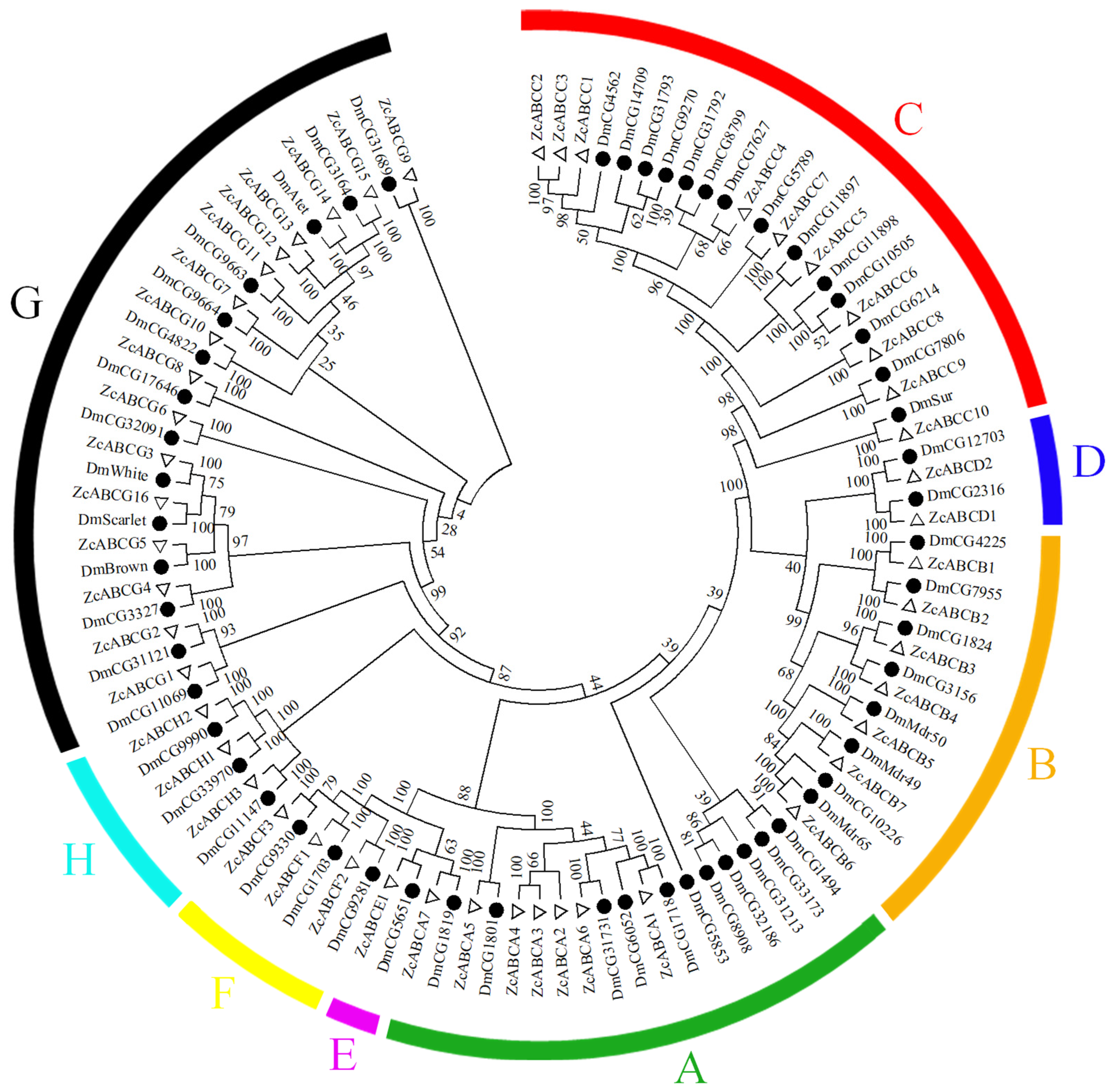
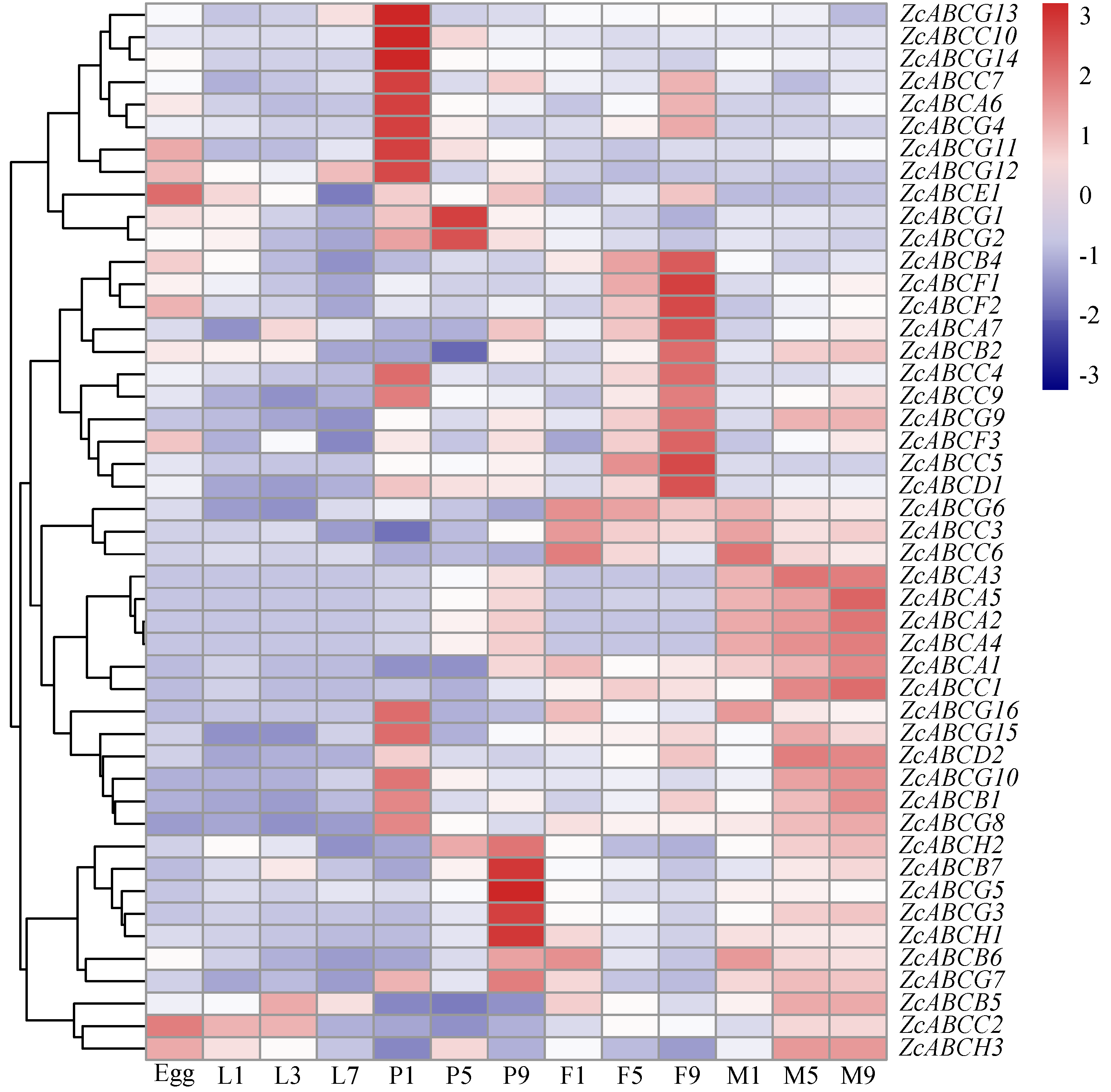
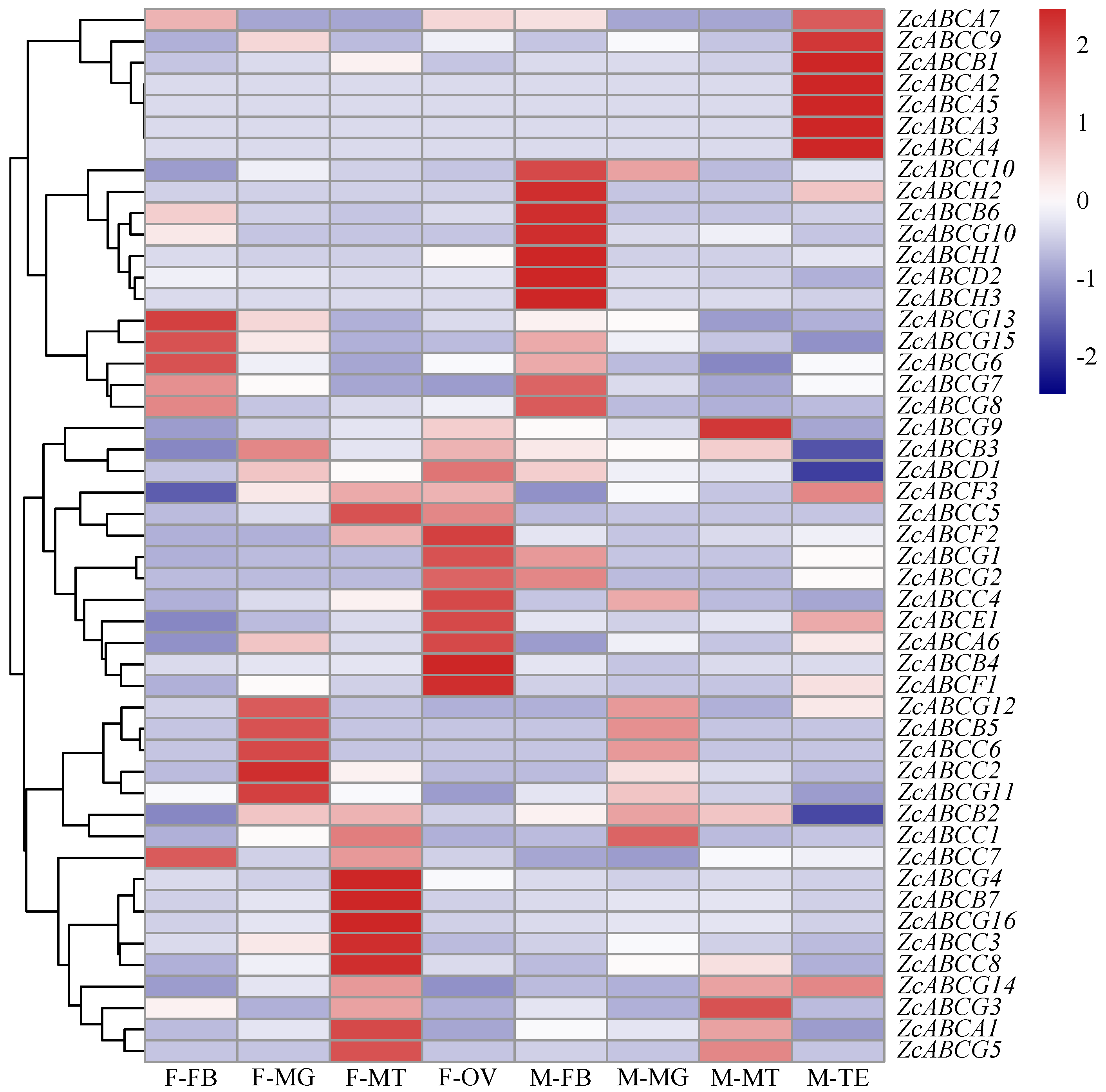
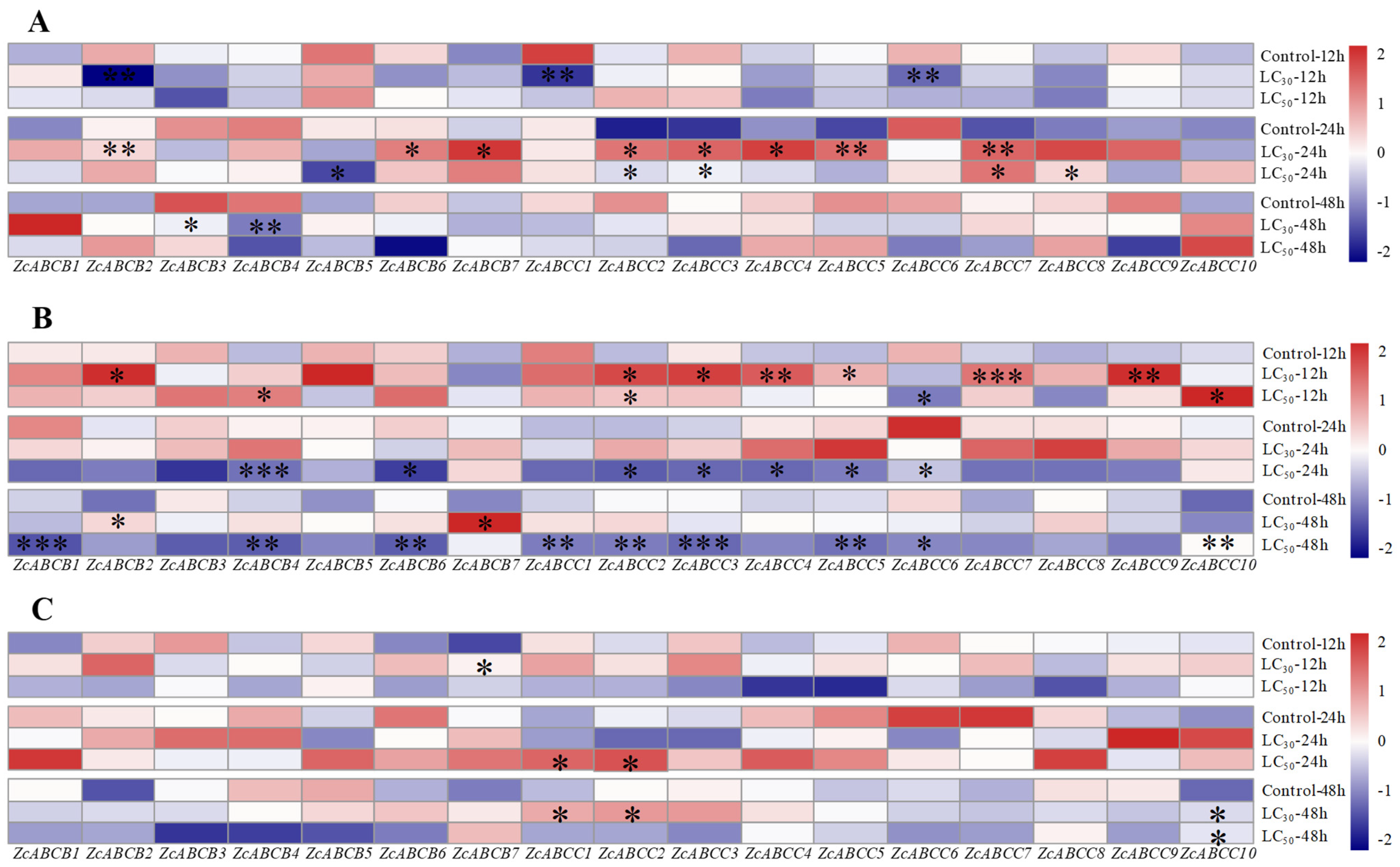
| Subfamily | Gene | Gene IDc | Length (AA) | Topology a | N-Glc b | O-Glc c |
|---|---|---|---|---|---|---|
| A | ZcABCA1 | LOC105218935 | 1768 | (5/7TMD-NBD)2 | 14 | 13 |
| A | ZcABCA2 | LOC105214217 | 1210 | (6TMD-NBD)2 | 8 | 1 |
| A | ZcABCA3 | LOC105214171 | 458 | NBD | 1 | 1 |
| A | ZcABCA4 | LOC105214218 | 1685 | (7/6TMD-NBD)2 | 11 | 6 |
| A | ZcABCA5 | LOC105210581 | 1610 | (7/6TMD-NBD)2 | 3 | 15 |
| A | ZcABCA6 | LOC105219391 | 1568 | (6TMD-NBD)2 | 9 | 15 |
| A | ZcABCA7 | LOC105215594 | 1957 | (6/7TMD-NBD)2 | 12 | 6 |
| B | ZcABCB1 | LOC105218240 | 850 | 11TMD-NBD | 7 | 1 |
| B | ZcABCB2 | LOC105221102 | 719 | 6TMD-NBD | 5 | 4 |
| B | ZcABCB3 | LOC105218701 | 732 | 3TMD-NBD | 3 | 9 |
| B | ZcABCB4 | LOC105217120 | 673 | 11TMD-NBD | 6 | 2 |
| B | ZcABCB5 | LOC105213990 | 1295 | (6/5TMD-NBD)2 | 3 | 9 |
| B | ZcABCB6 | LOC105212618 | 1296 | (7/6TMD-NBD)2 | 7 | 7 |
| B | ZcABCB7 | LOC105212186 | 1302 | (6/5TMD-NBD)2 | 9 | 4 |
| C | ZcABCC1 | LOC105214114 | 1352 | (6/5TMD-NBD)2 | 4 | 4 |
| C | ZcABCC2 | LOC105214112 | 1353 | (7/5TMD-NBD)2 | 9 | 4 |
| C | ZcABCC3 | LOC105214120 | 1346 | (7/5TMD-NBD)2 | 7 | 5 |
| C | ZcABCC4 | LOC105214115 | 1365 | (7/5TMD-NBD)2 | 5 | 11 |
| C | ZcABCC5 | LOC105209430 | 1358 | (4/5TMD-NBD)2 | 9 | 8 |
| C | ZcABCC6 | LOC105214620 | 1295 | (3/4TMD-NBD)2 | 12 | 8 |
| C | ZcABCC7 | LOC105217830 | 1408 | (6/5TMD-NBD)2 | 5 | 5 |
| C | ZcABCC8 | LOC105218206 | 1552 | 7TMD-(5TMD-NBD)2 | 7 | 4 |
| C | ZcABCC9 | LOC105210838 | 1494 | 5TMD-(6TMD-NBD)2 | 10 | 1 |
| C | ZcABCC10 | LOC105217701 | 1313 | 4TMD-NBD | 15 | 59 |
| D | ZcABCD1 | LOC105218326 | 737 | 4TMD-NBD | 2 | 2 |
| D | ZcABCD2 | LOC105208882 | 658 | 3TMD-NBD | 4 | 6 |
| E | ZcABCE1 | LOC105221159 | 611 | NBD-NBD | 1 | 6 |
| F | ZcABCF1 | LOC105212708 | 897 | NBD-NBD | 8 | 22 |
| F | ZcABCF2 | LOC105216261 | 613 | NBD-NBD | 4 | 8 |
| F | ZcABCF3 | LOC105208450 | 708 | NBD-NBD | 4 | 8 |
| G | ZcABCG1 | LOC105220708 | 607 | NBD-7TMD | 3 | 9 |
| G | ZcABCG2 | LOC105220707 | 891 | NBD-7TMD | 7 | 36 |
| G | ZcABCG3 | LOC105215537 | 679 | NBD-5TMD | 4 | 21 |
| G | ZcABCG4 | LOC105209688 | 1124 | NBD-5TMD | 9 | 85 |
| G | ZcABCG5 | LOC105217559 | 666 | NBD-7TMD | 2 | 7 |
| G | ZcABCG6 | LOC105215923 | 641 | NBD-7TMD | 3 | 12 |
| G | ZcABCG7 | LOC105209629 | 638 | NBD-7TMD | 6 | 15 |
| G | ZcABCG8 | LOC105209627 | 724 | NBD-6TMD | 2 | 0 |
| G | ZcABCG9 | LOC105218359 | 615 | NBD-5TMD | 4 | 6 |
| G | ZcABCG10 | LOC105213062 | 688 | NBD-6TMD | 3 | 0 |
| G | ZcABCG11 | LOC105209626 | 806 | NBD-6TMD | 5 | 46 |
| G | ZcABCG12 | LOC105217960 | 619 | NBD-6TMD | 6 | 6 |
| G | ZcABCG13 | LOC105217961 | 1388 | NBD-5TMD-NBD-8TMD | 9 | 26 |
| G | ZcABCG14 | LOC105213059 | 862 | NBD-7TMD | 7 | 31 |
| G | ZcABCG15 | LOC105213060 | 730 | NBD-6TMD | 7 | 2 |
| G | ZcABCG16 | LOC105212186 | 743 | NBD-5TMD | 4 | 28 |
| H | ZcABCH1 | LOC105217189 | 775 | NBD-7TMD | 10 | 15 |
| H | ZcABCH2 | LOC105217218 | 764 | NBD-7TMD | 10 | 12 |
| H | ZcABCH3 | LOC105218730 | 725 | NBD-7TMD | 4 | 9 |
| Insecticides | n | Slope(±SE) | LC30 (mg/L) | 95% CI a | LC50 (mg/L) | 95% CI | χ2 b |
|---|---|---|---|---|---|---|---|
| β-Cypermethrin | 300 | 5.376 ± 0.541 | 100.580 | 91.336–109.824 | 125.908 | 115.291–138.841 | 0.443 |
| Abamectin | 360 | 2.682 ± 0.234 | 9.344 | 6.734–11.963 | 14.658 | 11.405–18.723 | 5.1793 |
| Dinotefuran | 331 | 4.045 ± 0.375 | 219.616 | 118.042–296.924 | 296.010 | 196.121–398.702 | 8.8061 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.-Q.; Ma, M.; Ma, Y.-P.; Zhang, S.-Y.; Li, W.-J.; Wei, D.; Wang, J.-J. Identification and Expression Characterization of ATP-Binding Cassette (ABC) Transporter Genes in Melon Fly. Insects 2021, 12, 270. https://doi.org/10.3390/insects12030270
Xu H-Q, Ma M, Ma Y-P, Zhang S-Y, Li W-J, Wei D, Wang J-J. Identification and Expression Characterization of ATP-Binding Cassette (ABC) Transporter Genes in Melon Fly. Insects. 2021; 12(3):270. https://doi.org/10.3390/insects12030270
Chicago/Turabian StyleXu, Hui-Qian, Meng Ma, Yun-Peng Ma, Su-Yun Zhang, Wei-Jun Li, Dong Wei, and Jin-Jun Wang. 2021. "Identification and Expression Characterization of ATP-Binding Cassette (ABC) Transporter Genes in Melon Fly" Insects 12, no. 3: 270. https://doi.org/10.3390/insects12030270
APA StyleXu, H.-Q., Ma, M., Ma, Y.-P., Zhang, S.-Y., Li, W.-J., Wei, D., & Wang, J.-J. (2021). Identification and Expression Characterization of ATP-Binding Cassette (ABC) Transporter Genes in Melon Fly. Insects, 12(3), 270. https://doi.org/10.3390/insects12030270







