Preventing Transmission of Lethal Disease: Removal Behaviour of Lasius fuliginosus (Hymenoptera: Formicidae) Towards Fungus Contaminated Aphids
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Species
2.2. Experimental Design
2.3. Data Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilson, E.O. The Insect Societies; Belknap Press of Harvard University Press: Cambridge, MA, USA, 1971; pp. 1–548. [Google Scholar]
- Brian, M.V. Social Insect Ecology and Behavioural Biology; Chapman & Hall: London, UK, 1983; pp. 1–377. [Google Scholar]
- Wilson, E.O.; Hölldobler, B. The rise of the ants: A phylogenetic and ecological explanation. Proc. Nat. Acad. Sci. USA 2005, 102, 7411–7414. [Google Scholar] [CrossRef] [PubMed]
- Jones, W.E.; Grace, J.K.; Tamashiro, M. Virulence of seven isolates of Beauveria bassiana and Metarhizium anisopliae to Coptotermes formosanus (Isoptera: Rhinotermitidae). Environ. Entomol. 1996, 25, 481–487. [Google Scholar] [CrossRef]
- Schmid-Hempel, P. Parasites in Social Insects; Princeton University Press: Princeton, NJ, USA, 1998; pp. 1–409. [Google Scholar]
- Boomsma, J.J.; Schmid-Hempel, P.; Hughes, W.H.O. Life histories and parasite pressure across the major groups of social insects. In Insect Evolutionary Ecology; Fellowes, M., Holloway, G., Rolff, J., Eds.; Proceedings of Royal Entomological Society: London, UK, 2005; pp. 139–175. [Google Scholar]
- Cremer, S.; Armitage, S.A.O.; Schmid-Hempel, P. Social Immunity. Curr. Biol. 2007, 17, R693–R702. [Google Scholar] [CrossRef] [PubMed]
- Cremer, S.; Pull, C.D.; Fürst, M.A. Social immunity: Emergence and evolution of colony-level disease protection. Annu. Rev. Entomol. 2018, 63, 105–123. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, X.Y.; Tang, Q.B.; Lei, C.L.; Huang, Q.Y. The mechanisms of social immunity against fungal infections in Eusocial Insects. Toxins 2019, 11, 244. [Google Scholar] [CrossRef]
- Tragust, S.; Mitteregger, B.; Barone, V.; Konrad, M.; Ugelvig, L.V.; Cremer, S. Ants disinfect fungus-exposed brood by oral uptake and spread of their poison. Curr. Biol. 2013, 23, 76–82. [Google Scholar] [CrossRef]
- Yek, S.H.; Mueller, U.G. The Metapleural Gland of Ants. Biol. Rev. 2011, 86, 774–791. [Google Scholar] [CrossRef]
- Christe, P.; Oppliger, A.; Bancalà, F.; Castella, G.; Chapuisat, M. Evidence for collective medication in ants. Ecol. Lett. 2003, 6, 19–22. [Google Scholar] [CrossRef]
- Castella, G.; Chapuisat, M.; Christe, P. Prophylaxis with resin in wood ants. Animal. Behaviour. 2008, 75, 1591–1596. [Google Scholar] [CrossRef]
- Walker, T.N.; Hughes, W.O.H. Adaptive social immunity in leaf-cutting ants. Biol. Lett. 2009, 5, 446–448. [Google Scholar] [CrossRef]
- Reber, A.; Purcell, J.; Buechel, S.D.; Buri, P.; Chapuisat, M. The expression and impact of antifungal grooming in ants. J. Evol. Biol. 2011, 24, 954–964. [Google Scholar] [CrossRef] [PubMed]
- Tranter, C.; LeFevre, L.; Evison, S.E.F.; Hughes, W.O.H. Threat detection: Contextual recognition and response to parasites by ants. Behav. Ecol. 2015, 26, 396–405. [Google Scholar] [CrossRef]
- Roy, H.E.; Steinkraus, D.; Eilenberg, E.; Pell, J.K.; Hajek, A. Bizarre interactions and endgames: Entomopathogenic fungi and their arthropod hosts. Annu. Rev. Entomol. 2006, 51, 331–357. [Google Scholar] [CrossRef] [PubMed]
- Renucci, M.; Tirard, A.; Provost, E. Complex undertaking behavior in Temnothorax lichtensteini ant colonies: From corpse-burying behavior to necrophoric behavior. Insectes Sociaux 2011, 58, 9–16. [Google Scholar] [CrossRef]
- Diez, L.; Lejeune, P.; Detrain, C. Keep the nest clean: Survival advantages of corpse removal in ants. Biol. Lett. 2014, 10, 3–6. [Google Scholar] [CrossRef]
- Maák, I.; Camera, J.; Casacci, L.P.; Barbero, F.; Trigos-Peral, G.; Ślipiński, P.; Bonelli, S.; Zaccagno, M.; Witek, M. The influence of colony traits on the collective behaviour of Myrmica scabrinodis ants. Insect Conserv. Diver. 2019, 12, 481–491. [Google Scholar] [CrossRef]
- Frank, E.T.; Wehrhahn, M.; Linsenmair, K.E. Wound treatment and selective help in a termite-hunting ant. Proc. R. Soc. B. 2018, 285, 20172457. [Google Scholar] [CrossRef]
- Heinze, J.; Walter, B. Moribund Ants Leave Their Nests to Die in Social Isolation. Curr. Biol. 2010, 20, 249–252. [Google Scholar] [CrossRef]
- Bos, N.; Lefèvre, T.; Jensen, A.B.; d’Ettorre, P. Sick ants become unsociable. J. Evol. Biol. 2012, 25, 342–351. [Google Scholar] [CrossRef]
- Delabie, J.H.C. Trophobiosis between Formicidae and Hemiptera (Sternorrhyncha and Auchenorrhyncha): An overview. Neotrop. Entomol. 2001, 30, 501–516. [Google Scholar] [CrossRef]
- Stadler, B.; Dixon, A.F.G. Ecology and evolution of aphid–ant interactions. Annu. Rev. Ecol. Systemat. 2005, 36, 345–372. [Google Scholar] [CrossRef]
- Oliver, T.H.; Leather, S.R.; Cook, J.M. Macroevolutionary patterns in the origin of mutualisms involving ants. J. Evol. Biol. 2008, 21, 1597–1608. [Google Scholar] [CrossRef] [PubMed]
- Steinkraus, D.C. Factors affecting transmission of fungal pathogens of aphids. J. Invertebr. Pathol. 2006, 92, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.G.; Chen, C.; Shang, S.W.; Ying, S.H.; Shen, Z.C.; Chen, X.X. Aphid dispersal flight disseminates fungal pathogens and parasitoids as natural control agents of aphids. Ecol. Entomol. 2007, 32, 97–104. [Google Scholar] [CrossRef]
- Baverstock, J.; Roy, H.E.; Pell, J.K. Entomopathogenic fungi and insect behaviour: From unsuspecting hosts to targeted vectors. BioControl 2010, 55, 89–102. [Google Scholar] [CrossRef]
- Phillips, D.; Willis, C.K.R. Defensive behaviour of ants in a mutualistic relationship with aphids. Behav. Ecol. Sociobiol. 2005, 59, 321–325. [Google Scholar] [CrossRef]
- Majerus, M.E.N.; Sloggett, J.J.; Godeau, J.-F.; Hemptinne, J.L. Interactions between ants and aphidophagous and coccidophagous ladybirds. Popul. Ecol. 2007, 49, 15–27. [Google Scholar] [CrossRef]
- Novgorodova, T.A.; Gavrilyuk, A.V. The degree of protection different ants (Hymenoptera: Formicidae) provide aphids (Hemiptera: Aphididae) against aphidophages. Eur. J. Entomol. 2012, 109, 187–196. [Google Scholar] [CrossRef]
- Nielsen, C.; Agrawal, A.A.; Hajek, A.E. Ants defend aphids against lethal disease. Biol. Lett. 2010, 6, 205–208. [Google Scholar] [CrossRef]
- Novgorodova, T.A.; Kryukov, V.Y. Quarantining behaviour of ants towards infected aphids as an antifungal mechanism in ant-aphid interactions. Entomol. Exp. Appl. 2017, 162, 293–301. [Google Scholar] [CrossRef]
- Novgorodova, T.A. Quarantining behaviour in ants: Are Myrmica aphid milkers able to detect and get rid of fungus-infected aphids? Entomol. Exp. Appl. 2020, 168, 869–877. [Google Scholar] [CrossRef]
- Anderson, C.; McShea, D.W. Individual versus social complexity, with particular reference to ant colonies. Biol. Rev. 2001, 76, 211–237. [Google Scholar] [CrossRef] [PubMed]
- Beckers, R.; Goss, S.; Deneubourg, J.L.; Pasteels, J.M. Colony size, communication and ant foraging strategy. Psyche 1989, 96, 239–256. [Google Scholar] [CrossRef]
- Thomas, M.L.; Elgar, M.A. Colony size affects division of labour in the ponerine ant Rhytidoponera metallica. Naturwissenschaften 2003, 90, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Holbrook, C.T.; Barden, P.M.; Fewell, J.H. Division of labor increases with colony size in the harvester ant Pogonomyrmex californicus. Behav. Ecol. 2011, 22, 960–966. [Google Scholar] [CrossRef]
- Czechowski, W.; Radchenko, A.; Czechowska, W. The ants (Hymenoptera, Formicidae) of Poland; Museum and Institute of Zoology PAS: Warsaw, Poland, 2002; pp. 1–200. [Google Scholar]
- Zakharov, A.A. Ants of Forest Communities, Their Life and Role in the Forest; KMK Scientific Press: Moscow, Russia, 2015; pp. 1–404. (In Russian) [Google Scholar]
- Radchenko, A.G. A Key to the identification of the ants of South Siberia. Trudy zapovednika “Daursky” 1994, 3, 95–118. (In Russian) [Google Scholar]
- Radchenko, A.G. Ants (Hymenoptera, Formicidae) of Ukraine; Institute of zoology by I.I. Shmalgausen NAS of Ukraine: Kiev, Ukraine, 2016; pp. 1–480. (In Russian) [Google Scholar]
- Aphids on the World’s Plants. An Online Identification and Information Guide. Available online: http://www.aphidsonworldsplants.info (accessed on 3 April 2020).
- Novgorodova, T.A. Organization of honeydew collection by foragers in different ant species (Hymenoptera, Formicidae): Effect of colony size and species specificity. Eur. J. Entomol. 2015, 112, 688. [Google Scholar] [CrossRef]
- Novgorodova, T.A. Ant-aphid interactions in multispecies ant communities: Some ecological and ethological aspects. Eur. J. Entomol. 2005, 102, 495–502. [Google Scholar] [CrossRef]
- Polovinko, G.P.; Yaroslavtseva, O.N.; Teshebaeva, Z.A.; Kryukov, V.Y. Dominating Species of entomophilous Ascomycetes anamorphs in West Siberia, Primorsky Krai, and Kyrgyzstan. Contemp. Probl. Ecol. 2010, 3, 515–521. [Google Scholar] [CrossRef]
- Kryukov, V.Y.; Yaroslavtseva, O.N.; Elisaphenko, E.A.; Mitkovets, P.V.; Lednev, G.R.; Duisembekov, B.A.; Zakianb, S.M.; Glupov, V.V. Change in the temperature preferences of Beauveria bassiana sensu lato isolates in the latitude gradient of Siberia and Kazakhstan. Microbiology 2012, 81, 453–459. [Google Scholar] [CrossRef]
- De Zarzuela, M.F.M.; Leite, L.G.; Marcondes, J.E.; De Carvalho Campos, A.E. Entomopathogens isolated from invasive ants and tests of their pathogenicity. Psyche 2012, 2012, 975069. [Google Scholar] [CrossRef]
- Bos, N.; Sundström, L.; Fuchs, S.; Freitak, D. Ants medicate to fight disease. Evolution 2015, 69, 2979–2984. [Google Scholar] [CrossRef] [PubMed]
- Kryukov, V.Y.; Khodyrev, V.P.; Yaroslavtseva, O.N.; Kamenova, A.S.; Duisembekov, B.A.; Glupov, V.V. Synergistic action of entomopathogenic Hyphomycetes and the bacteria Bacillus thuringiensis ssp. morrisoni in the infection of colorado potato beetle Leptinotarsa decemlineata. Appl. Biochem. Microbiol. 2009, 45, 511–516. [Google Scholar]
- Kryukov, V.Y.; Yaroslavtseva, O.N.; Levchenko, M.V.; Lednyov, G.R.; Glupov, V.V. Phenotypic variability of environmental isolates of the entomopathogenic fungus Beauveria bassiana. Microbiology 2010, 79, 265–269. [Google Scholar] [CrossRef]
- Krukov, V.Y.; Tomilova, O.G.; Luzina, O.A.; Yaroslavtseva, O.N.; Akhanaev, Y.B.; Tyurin, M.V.; Duisembekov, B.A.; Salakhutdinov, N.F.; Glupov, V.V. Effects of fluorine-containing usnic acid and fungus Beauveria bassiana on the survival and immune-physiological reactions of Colorado potato beetle larvae. Pest. Manag. Sci. 2018, 74, 598–606. [Google Scholar] [CrossRef]
- Pereira, H.; Detrain, C. Pathogen avoidance and prey discrimination in ants. R. Soc. Open Sci. 2020, 7, 191705. [Google Scholar] [CrossRef]
- Bird, A.B.; Hesketh, H.; Cross, J.V.; Copland, M. The common black ant, Lasius niger (Hymenoptera: Formicidae), as a vector of the entomopathogen Lecanicillium longisporum to rosy apple aphid, Dysaphis plantaginea (Homoptera: Aphididae). Biocontr. Sci. Technol. 2004, 14, 757–767. [Google Scholar] [CrossRef]
- Konorov, E.A.; Nikitin, M.A.; Mikhailov, K.V.; Lysenkov, S.N.; Belenky, M.; Chang, P.L.; Nuzhdin, S.V.; Scobeyeva, V.A. Genomic exaptation enables Lasius niger adaptation to urban environments. BMC Evol. Biol. 2017, 17, 39. [Google Scholar] [CrossRef]
- Diez, L.; Urbain, L.; Lejeune, P.; Detrain, C. Emergency measures: Adaptive response to pathogen intrusion in the ant nest. Behav. Process. 2015, 116, 80–86. [Google Scholar] [CrossRef]
- Leclerc, J.B.; Detrain, C. Impact of colony size on survival and sanitary strategies in fungus-infected ant colonies. Behav. Ecol. Sociobiol. 2018, 72, 3. [Google Scholar] [CrossRef]
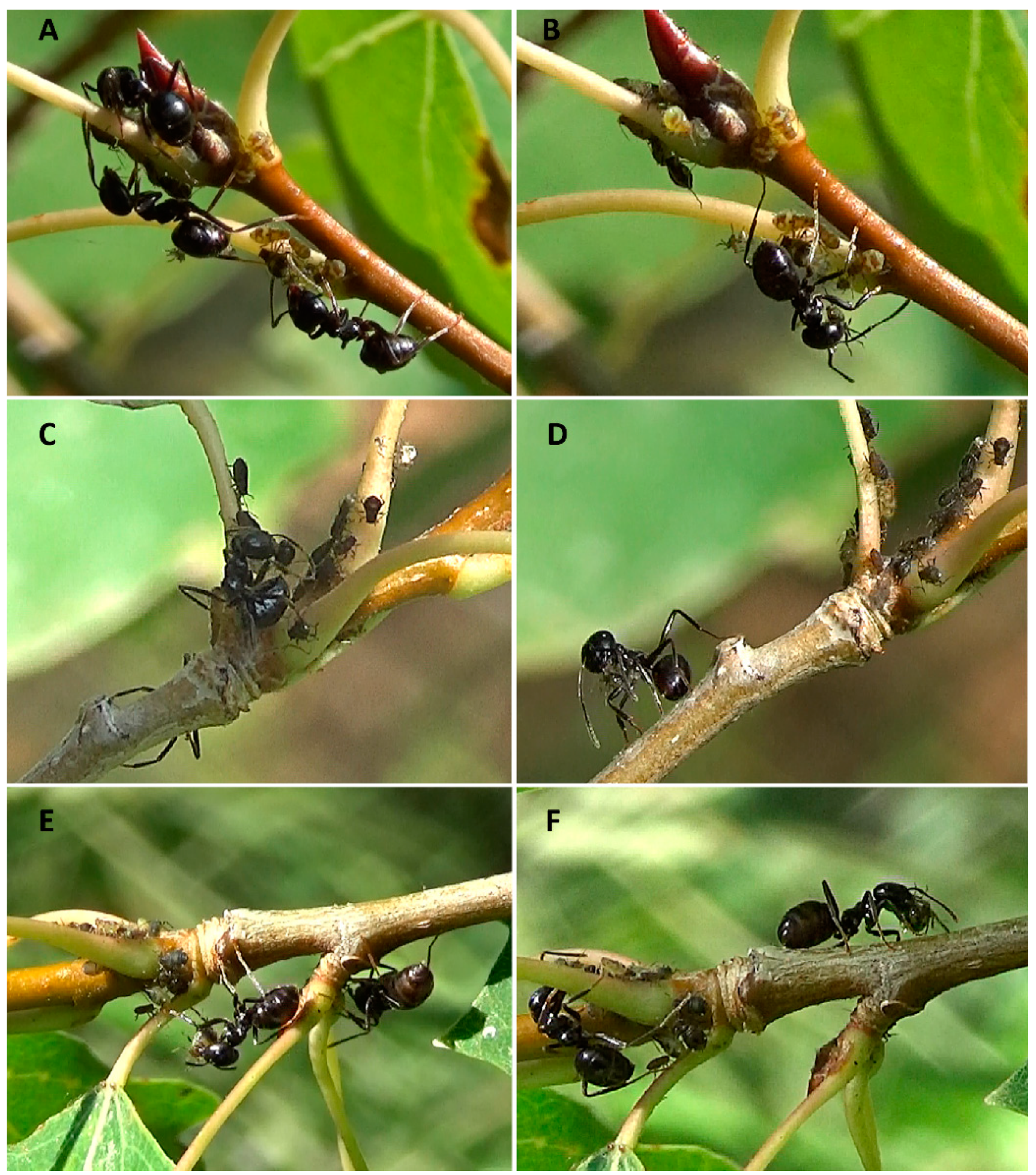
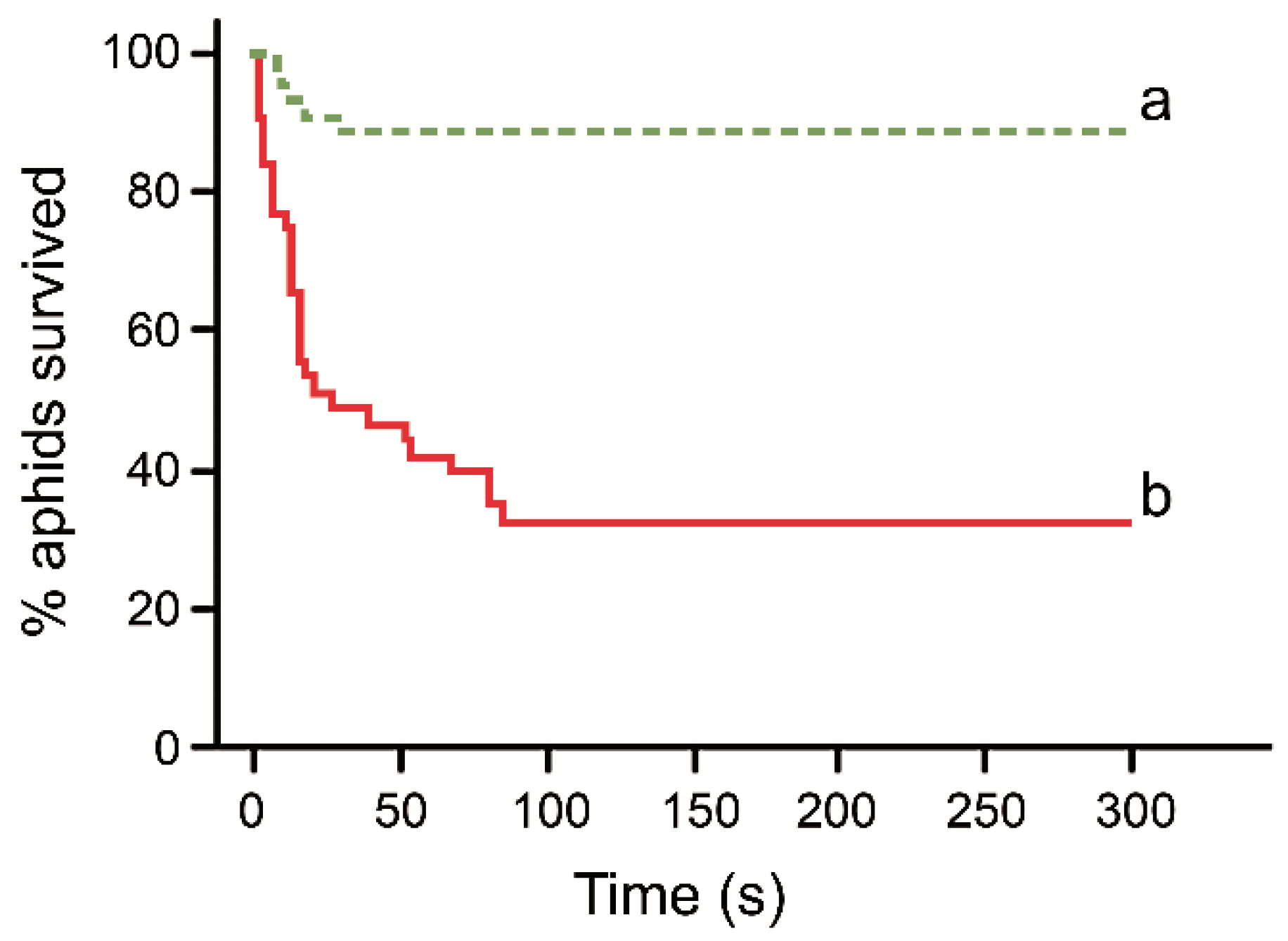
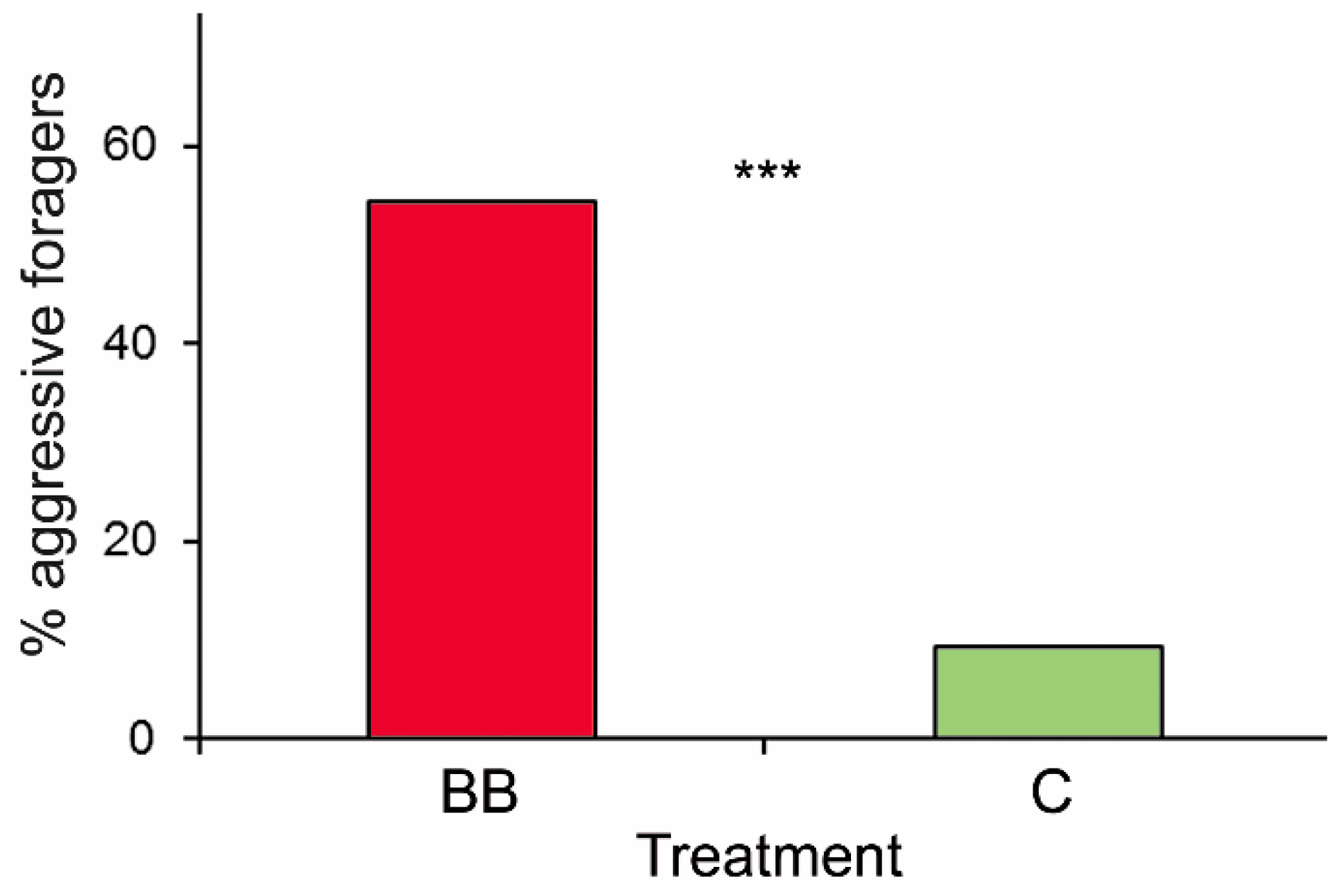
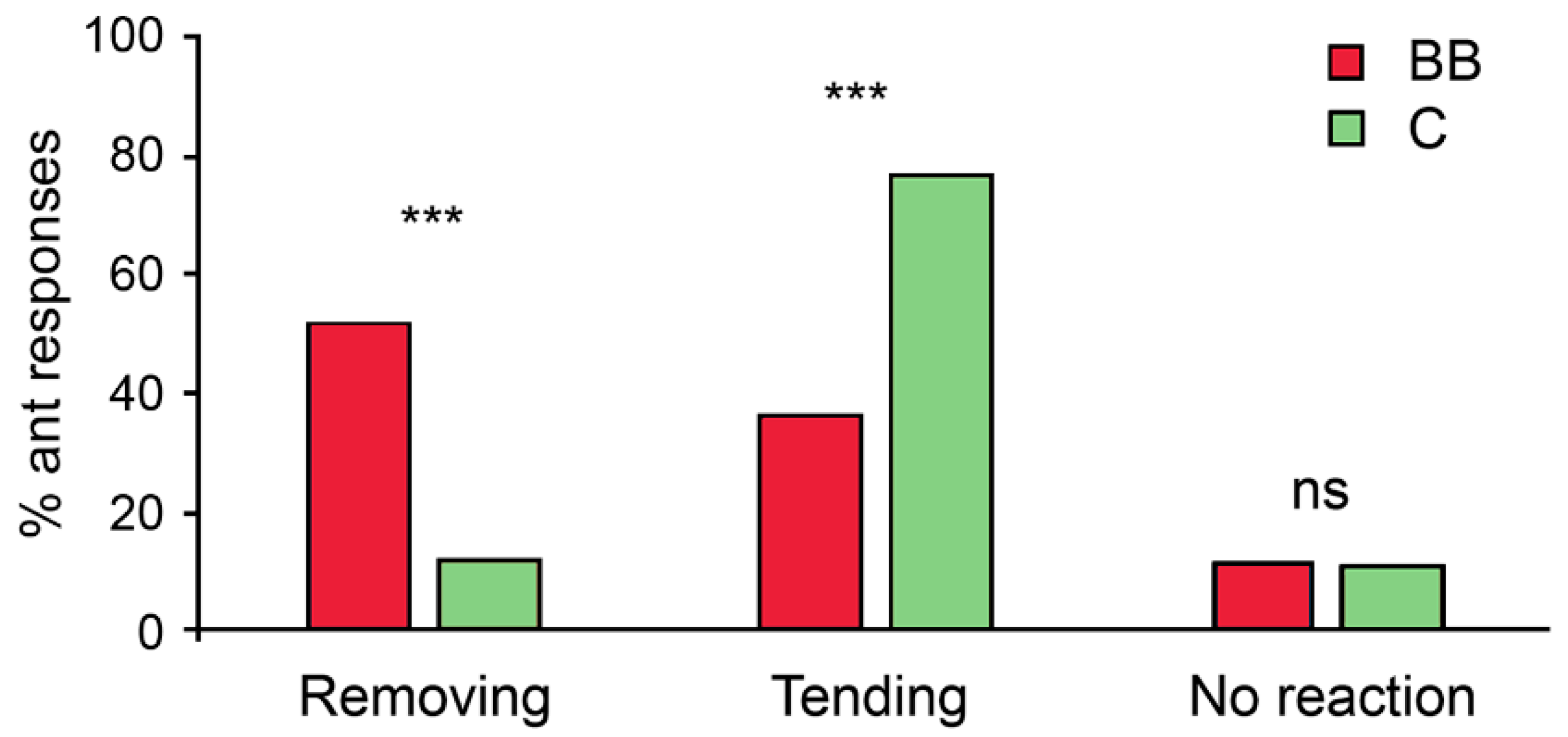
| Response Variable | Explanatory Variables | d.f. | Wald Stat. | p-Value |
|---|---|---|---|---|
| Removal of experimental aphids | Treatment | 1 | 26.22 | <0.0001 |
| Ant colony | 2 | 0.55 | 0.76 | |
| Order | 1 | 0.01 | 0.94 | |
| Treatment × Ant colony | 2 | 0.43 | 0.81 | |
| Treatment × Order | 1 | 0.73 | 0.39 | |
| The percentage of ants acting aggressively towards experimental aphids | Treatment | 1 | 26.04 | <0.0001 |
| NAM | 1 | 0.60 | 0.44 | |
| NAPH | 1 | 1.11 | 0.29 | |
| Ant colony | 2 | 0.37 | 0.83 | |
| Treatment × Ant colony | 2 | 0.52 | 0.77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novgorodova, T. Preventing Transmission of Lethal Disease: Removal Behaviour of Lasius fuliginosus (Hymenoptera: Formicidae) Towards Fungus Contaminated Aphids. Insects 2021, 12, 99. https://doi.org/10.3390/insects12020099
Novgorodova T. Preventing Transmission of Lethal Disease: Removal Behaviour of Lasius fuliginosus (Hymenoptera: Formicidae) Towards Fungus Contaminated Aphids. Insects. 2021; 12(2):99. https://doi.org/10.3390/insects12020099
Chicago/Turabian StyleNovgorodova, Tatiana. 2021. "Preventing Transmission of Lethal Disease: Removal Behaviour of Lasius fuliginosus (Hymenoptera: Formicidae) Towards Fungus Contaminated Aphids" Insects 12, no. 2: 99. https://doi.org/10.3390/insects12020099
APA StyleNovgorodova, T. (2021). Preventing Transmission of Lethal Disease: Removal Behaviour of Lasius fuliginosus (Hymenoptera: Formicidae) Towards Fungus Contaminated Aphids. Insects, 12(2), 99. https://doi.org/10.3390/insects12020099





